Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation
Abstract
:1. Introduction
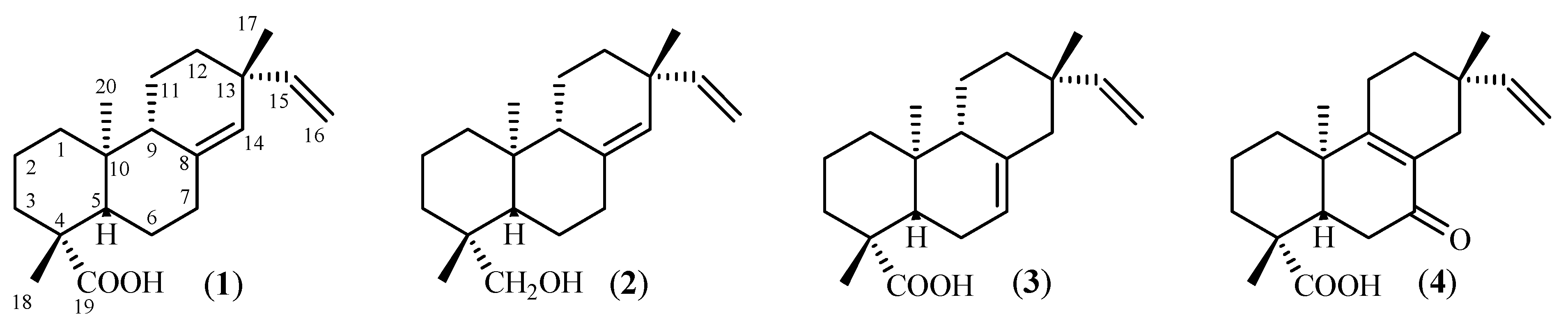
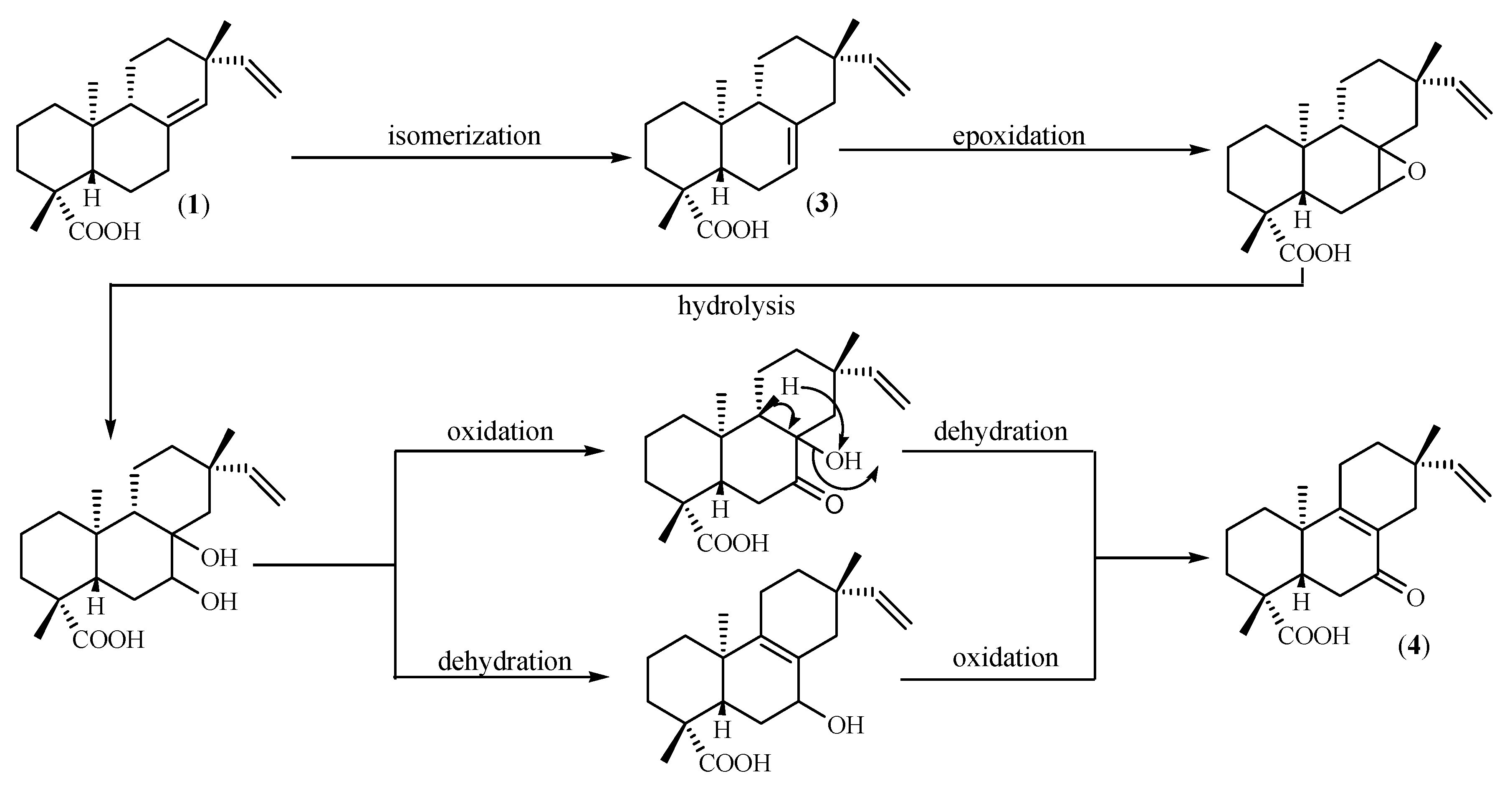
2. Results and Discussion
3. Experimental
3.1. General
3.2. Isolation and identification of PA
3.3. Microorganisms
3.4. Microbial transformation procedures
3.5. Isolation of diterpenes
3.6. Bacterial strains and antimicrobial testing
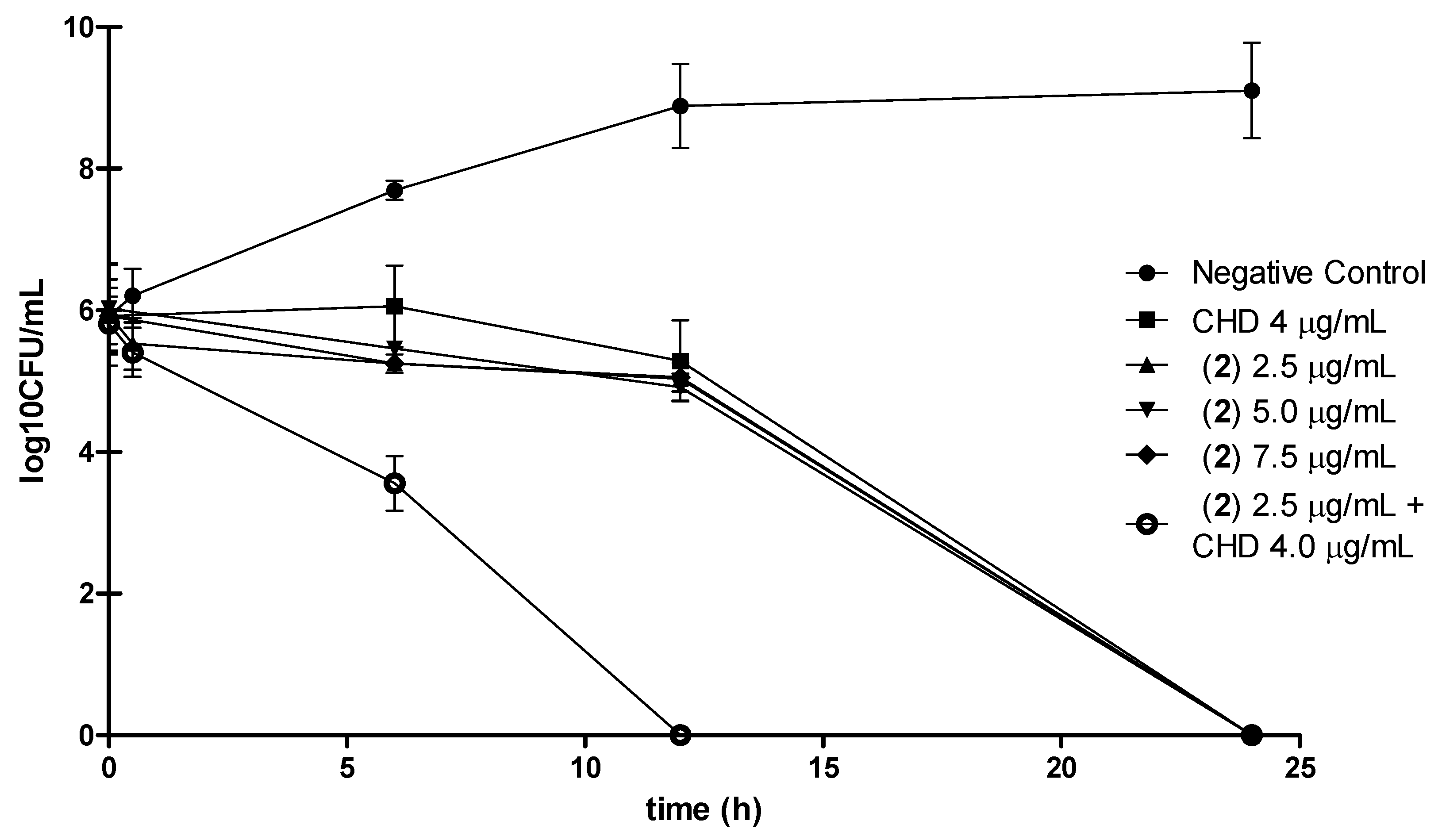
3.7. Time-kill curves
3.8. Synergistic antimicrobial activity
4. Conclusions
Acknowledgements
References
- Chung, J.Y.; Choo, J.H.; Lee, M.H.; Hwang, J. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine 2006, 13, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, J.; Zhou, X. Anticaries effect of compounds extracted from Galla chinensis in a multispecies biofilm model. Oral Microbiol. Immunol. 2008, 23, 459–465. [Google Scholar] [CrossRef] [PubMed]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008, 119, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.B.; Martins, C.H.G.; Souza, M.G.M.; Furtado, N.A.J.C.; Heleno, V.C.G.; Sousa, J.P.B.; Rocha, E.M.P.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.S.; Ambrósio, S.R. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother. Res. 2010. In Press. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Vacca Smith, A.M.; Bowen, W.H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K. Effects of Apis mellifera propolis on the activities of streptococcal glucosyltransferases in solution and adsorbed onto saliva-coated hydroxyapatite. Caries Res. 2000, 34, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, S.R.; Furtado, N.A.J.C.; De Oliveira, D.C.R.; Da Costa, F.B.; Martins, C.H.G.; De Carvalho, T.C.; Porto, T.S.; Veneziani, R.C.S. Antimicrobial activity of kaurane diterpenes against oral pathogens. Z. Naturforsch. 2008, 63c, 326–330. [Google Scholar] [CrossRef]
- Furiga, A.; Lonvaud-Funel, A.; Dorignac, G.; Badet, C. In vitro anti-bacterial and anti-adherence effects of natural polyphenolic compounds on oral bacteria. J. Appl. Microbiol. 2008, 105, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.; Dodds, M.; Tian, M. Naturally occurring phenolic antibacterial compounds show effectiveness against oral bacteria by a quantitative structure-activity relationship study. J. Agr. Food Chem. 2008, 56, 11151–11156. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Rangel, R.; Furtado, N.A.J.C.; De Carvalho, T.C.; Martins, C.H.G.; Veneziani, R.C.S.; Da Costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; Ambrosio, S.R. Pimarane-type Diterpenes: Antimicrobial activity against oral pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, S.R.; Tirapelli, C.R.; Da Costa, F.B.; De Oliveira, A.M. Kaurane and pimarane-type diterpenes from the Viguiera species inhibit vascular smooth muscle contractility. Life Sci. 2006, 79, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.R.; Ambrosio, S.R.; Da Costa, F.B.; De Oliveira, A.M. Diterpenes: a therapeutic promise for cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 1–8. [Google Scholar] [PubMed]
- Chou, B.H.; Yang, L.M.; Chang, S.F.; Hsu, F.L.; Lo, C.H.; Lin, W.K.; Wang, L.H.; Liu, P.C.; Lin, S.J. Fungal transformation of isosteviol lactone and its biological evaluation for inhibiting the AP-1 transcription factor. Phytochemistry 2009, 70, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Marquina, S.; Parra, J.L.; Gonzalez, M.; Zamilpa, A.; Escalante, J.; Trejo-Hernandez, M.R.; Alvarez, L. Hydroxylation of the diterpenes ent-kaur-16-en-19-oic and ent-beyer-15-en-19-oic acids by the fungus Aspergillus niger. Phytochemistry 2009, 70, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, T.C.; Polizeli, A.M.; Turatti, I.C.C.; Severiano, M.E.; De Carvalho, C.E.; Ambrosio, S.R.; Crotti, A.E.M.; De Figueiredo, U.S.; Vieira, P.C.; Furtado, N.A.J.C. Screening of filamentous fungi to identify biocatalysts for lupeol biotransformation. Molecules 2010, 15, 6140–6151. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Pleszczynska, M.; Wiater, A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticariogenic activity. Molecules 2010, 15, 4639–4651. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Uto, S.; Nakayama, M.; Hayashi, S.; Yamasaki, K.; Kasai, R.; Tanaka, O. (-)-Thermarol, a new ent-pimarane-class diterpene diol from Jungermannia thermarum (Liverwort). Tetrahedron Lett. 1976, 28, 2451–2454. [Google Scholar] [CrossRef]
- Herz, W.; Kulanthaivel, P. Ent-pimaranes, ent-kauranes, heliangolides and other constituents of 3 Helianthus species. Phytochemistry 1984, 23, 1453–1459. [Google Scholar] [CrossRef]
- Miyazawa, M.; Uemura, T.; Kameoka, H. Biotransformation of sesquiterpenoids, (+)-aromadendrene and (-)-alloaromadendrene by Glomerella cingulata. Phytochemistry 1995, 40, 793–796. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kawai, K.; Nakajima, S. Asymmetric reduction of 2-methyl-2-aryloxyacetic acids by Glomerella cingulata. Agr. Biol. Chem. Tokyo 1984, 48, 1373–1374. [Google Scholar] [CrossRef]
- He, A.M.; Li, T.; Daniels, L.; Fotheringham, I.; Rosazza, J.P.N. Nocardia sp carboxylic acid reductase: Cloning, expression, and characterization of a new aldehyde oxidoreductase family. Appl. Environ. Microb. 2004, 70, 1874–1881. [Google Scholar] [CrossRef]
- Haridy, M.S.A.; Ahmed, A.A.; Doe, M. Microbiological transformation of two labdane diterpenes, the main constituents of Madia species, by two fungi. Phytochemistry 2006, 67, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S. Phytochemicals for bacterial resistance - Strengths, weaknesses and opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Cruz, J.F.; Zhu, M.; Kinghorn, A.D.; Wu, C.D. Antimicrobial constituents of Thompson seedless raisins (Vitis vinifera) against selected oral pathogens. Phytochem. Lett. 2008, 1, 151–154. [Google Scholar] [CrossRef]
- Botelho, M.A.; Nogueira, N.A.P.; Bastos, G.M.; Fonseca, S.G.C.; Lemos, T.L.G.; Matos, F.J.A.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G.A.C. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 2007, 40, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Coimbra, H.S.; Pereira, A.C.; Almeida, V.A.; Lima, T.C.; Costa, E.S.; Vinholis, A.H.; Royo, V.A.; Silva, R.; Filho, A.A.; Cunha, W.R.; Furtado, N.A.J.C.; Martins, C.H.; Carvalho, T.C.; Bastos, J.K. Evaluation of Piper cubeba extract, (-)-cubebin and its semi-synthetic derivatives against oral pathogens. Phytother. Res. 2007, 21, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Himejima, M.; Tsujimoto, K.; Muroi, H.; Ichikawa, N. Antibacterial activity of crinitol and its potentiation. J. Nat. Prod. 1992, 55, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Muroi, H.; Himejima, M. Antibacterial activity of totarol and its potentiation. J. Nat. Prod. 1992, 55, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H.; Okuda, K. An investigation of diterpenes from the leaves of Rabdosia trichocarpa and their antibacterial activity against oral microorganisms. Chem. Pharm. Bull. (Tokyo) 1994, 42, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Pan, Q.; Shi, Y.; Williams, I.D.; Sung, H.H.; Zhang, Q.; Liang, J.Y.; Ip, N.Y.; Min, Z.D. Ent-rosane and labdane diterpenoids from Sagittaria sagittifolia and their antibacterial activity against three oral pathogens. J. Nat. Prod. 2006, 69, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Shi, Y.; Yu, B.; Williams, I.D.; Sung, H.H.; Zhang, Q.; Liang, J.Y.; Ip, N.Y.; Min, Z.D. Antibacterial diterpenoids from Sagittaria pygmaea. Planta Med. 2007, 73, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Souza, M.G.; Silva, M.L.; Filho, A.A.; Martins, C.H.; Crotti, A.E.; Pauletti, P.M.; Groppo, M.; Cunha, W.R. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: relevance of carnosic acid and carnosol. Chem. Biodivers. 2010, 7, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- D’lArrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [PubMed]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vasquez, L. A structure-activity study of antibacterial diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of diterpenoid alkaloid mixtures using vacuum liquid chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic tecnique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Schorr, K.; Da Costa, F.B. Terpenoids of Viguiera arenaria (Asteraceae). Biochem. Syst. Ecol. 2004, 32, 221–224. [Google Scholar] [CrossRef]
- Mihashi, S.; Yanagisawa, I.; Tanaka, O.; Shibata, S. Further study on diterpenes of Aralia spp. Tetrahedron Lett. 1969, 21, 1683–1686. [Google Scholar] [CrossRef]
- Jackson, M.; Karwowski, J.P.; Humphrey, P.E.; Kohl, W.L.; Barlow, G.J.; Tanaka, S.K. Calbistrins, novel antifungal agents produced by Penicillium restrictum. 1. Production, taxonomy of the producing organism and biological activity. J. Antibiot. 1993, 46, 34–38. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |
| Compound | Microorganism | |||||
|---|---|---|---|---|---|---|
| L. casei | S. mitis | S. mutans | S. sanguinis | S. sobrinus | S. salivarius | |
| 1* | 3.0 | 4.0 | 4.5 | 2.5 | 4.0 | 5.0 |
| 2 | 2.5 (5.0) | 1.5 (3.0) | 1.5 (2.5) | 3.5 (7.0) | 4.0 (7.0) | 3.5 (7.0) |
| 3 | 5.0 (12.0) | 4.0 (7.0) | 6.0 (9.0) | 8.0 (15.0) | 7.5 (7.5) | 5.0 (10.0) |
| 4 | 160.0 (**) | 200.0 (**) | 200.0 (**) | 120.0 (200.0) | 200.0 (**) | 180.0 (**) |
| PC | 0.9 | 3.6 | 0.9 | 3.6 | 0.9 | 0.9 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Severiano, M.E.; Simao, M.R.; Porto, T.S.; Martins, C.H.G.; Veneziani, R.C.S.; Furtado, N.A.J.C.; Arakawa, N.S.; Said, S.; Oliveira, D.C.R.d.; Cunha, W.R.; et al. Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation. Molecules 2010, 15, 8553-8566. https://doi.org/10.3390/molecules15128553
Severiano ME, Simao MR, Porto TS, Martins CHG, Veneziani RCS, Furtado NAJC, Arakawa NS, Said S, Oliveira DCRd, Cunha WR, et al. Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation. Molecules. 2010; 15(12):8553-8566. https://doi.org/10.3390/molecules15128553
Chicago/Turabian StyleSeveriano, Marcela E., Marilia R. Simao, Thiago S. Porto, Carlos H. G. Martins, Rodrigo C. S. Veneziani, Niege A. J. C. Furtado, Nilton S. Arakawa, Suraia Said, Dioneia C. R. de Oliveira, Wilson R. Cunha, and et al. 2010. "Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation" Molecules 15, no. 12: 8553-8566. https://doi.org/10.3390/molecules15128553
APA StyleSeveriano, M. E., Simao, M. R., Porto, T. S., Martins, C. H. G., Veneziani, R. C. S., Furtado, N. A. J. C., Arakawa, N. S., Said, S., Oliveira, D. C. R. d., Cunha, W. R., Gregorio, L. E., & Ambrosio, S. R. (2010). Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation. Molecules, 15(12), 8553-8566. https://doi.org/10.3390/molecules15128553




