Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide
Abstract
:1. Introduction
2. Results and Discussion
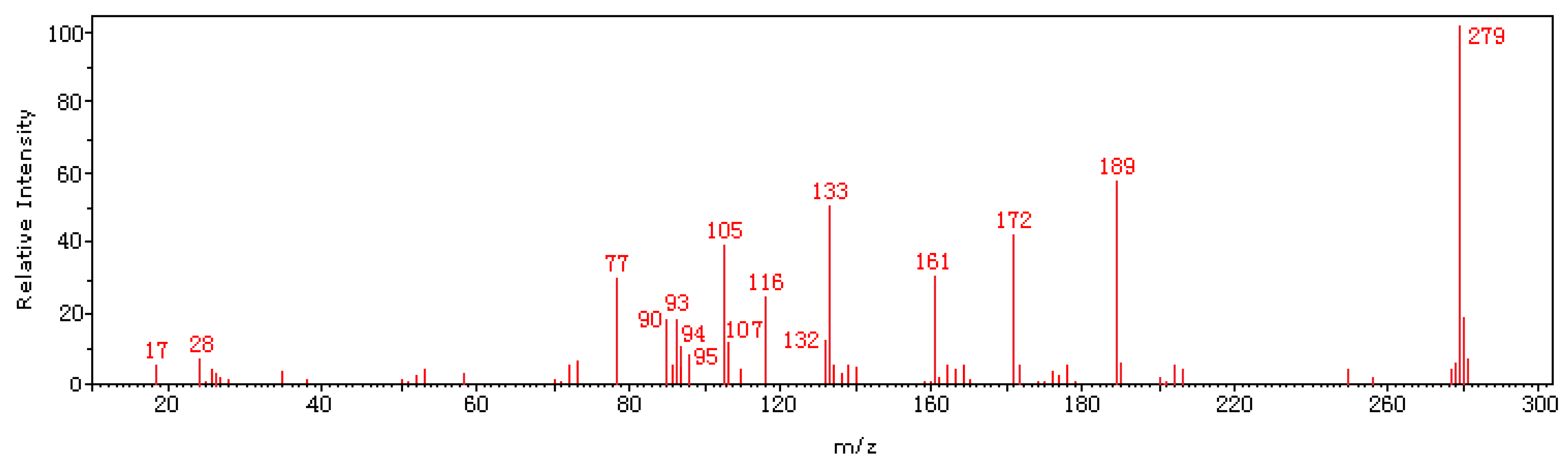
2.1. Synthesis and characterization
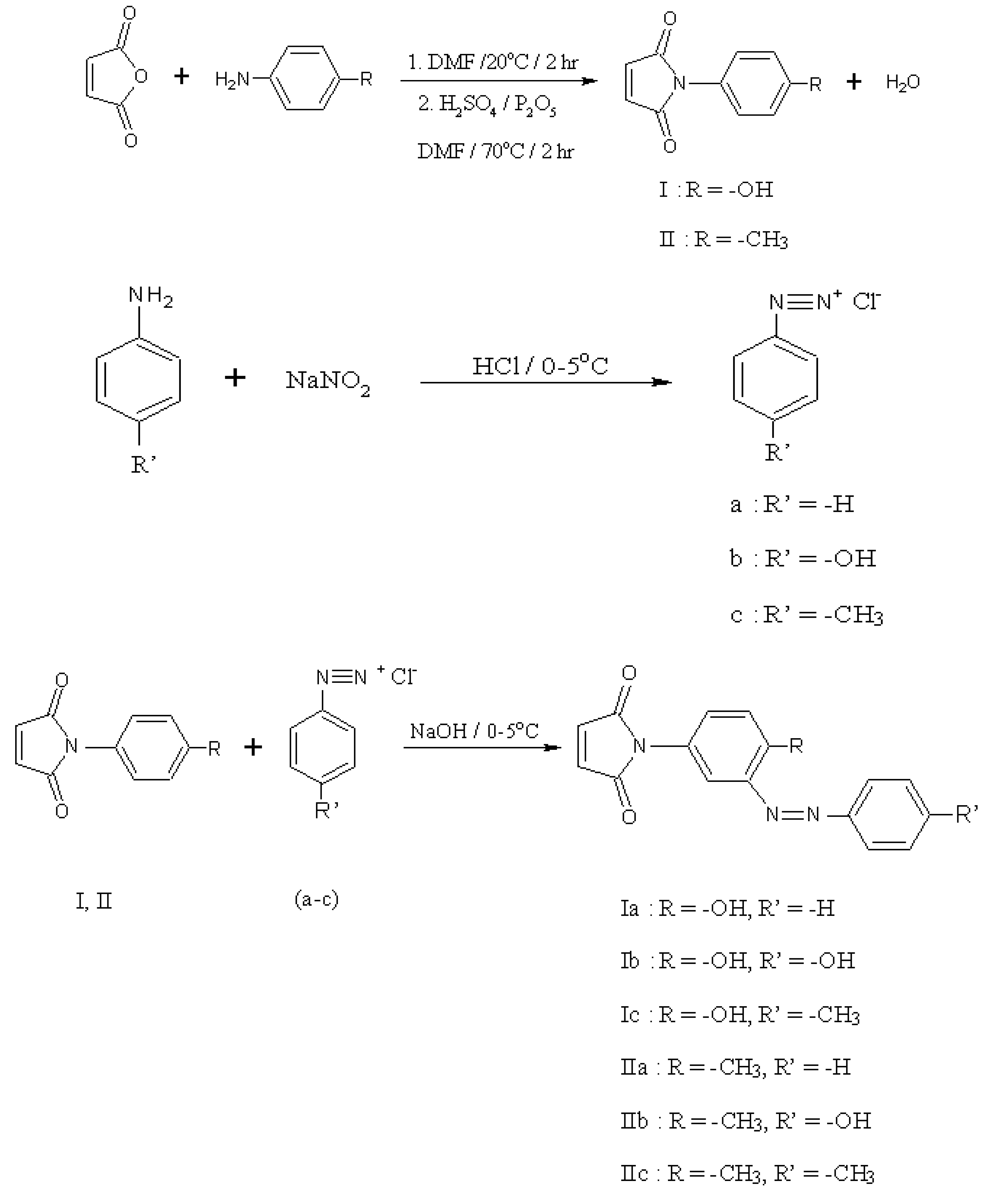
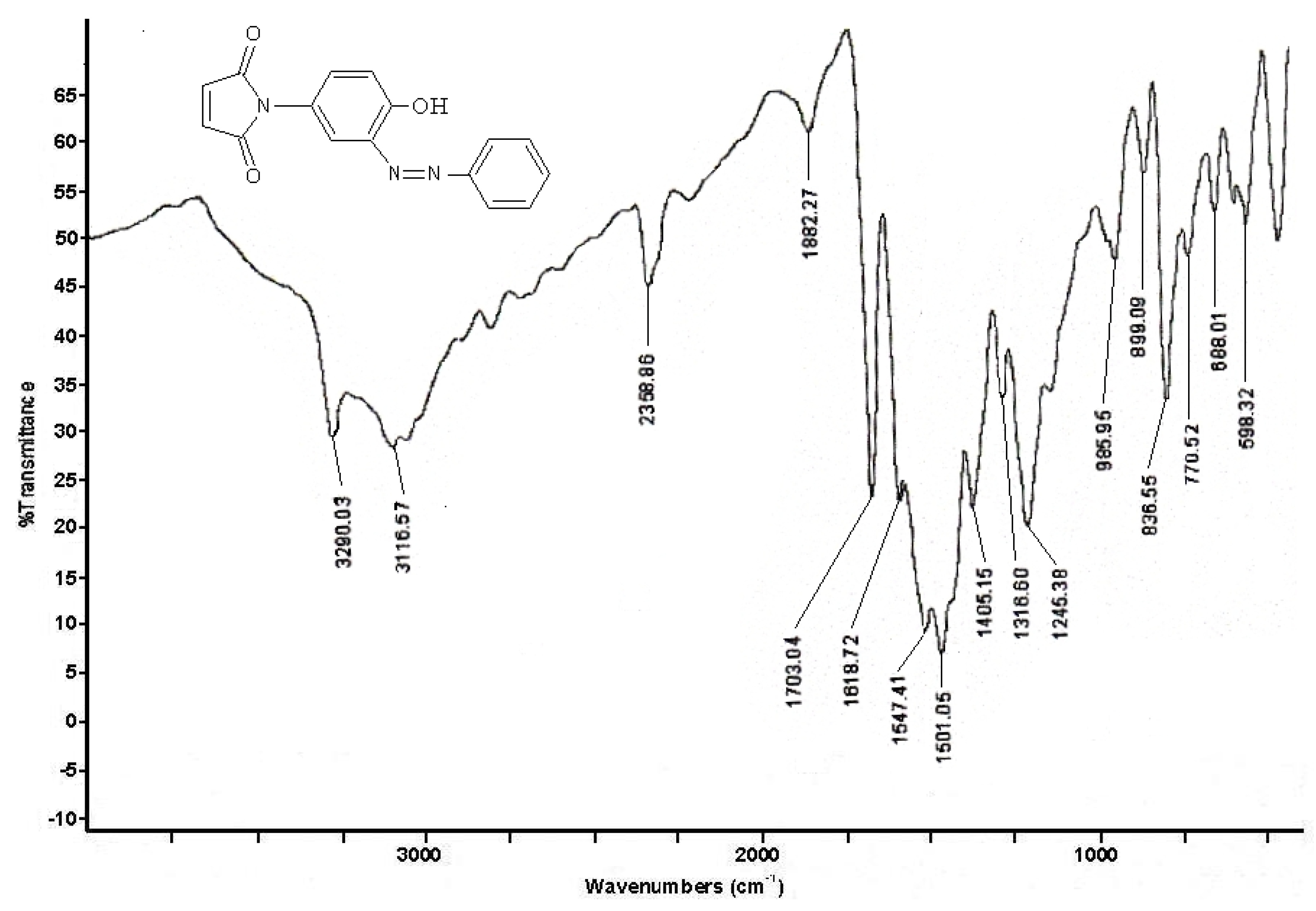
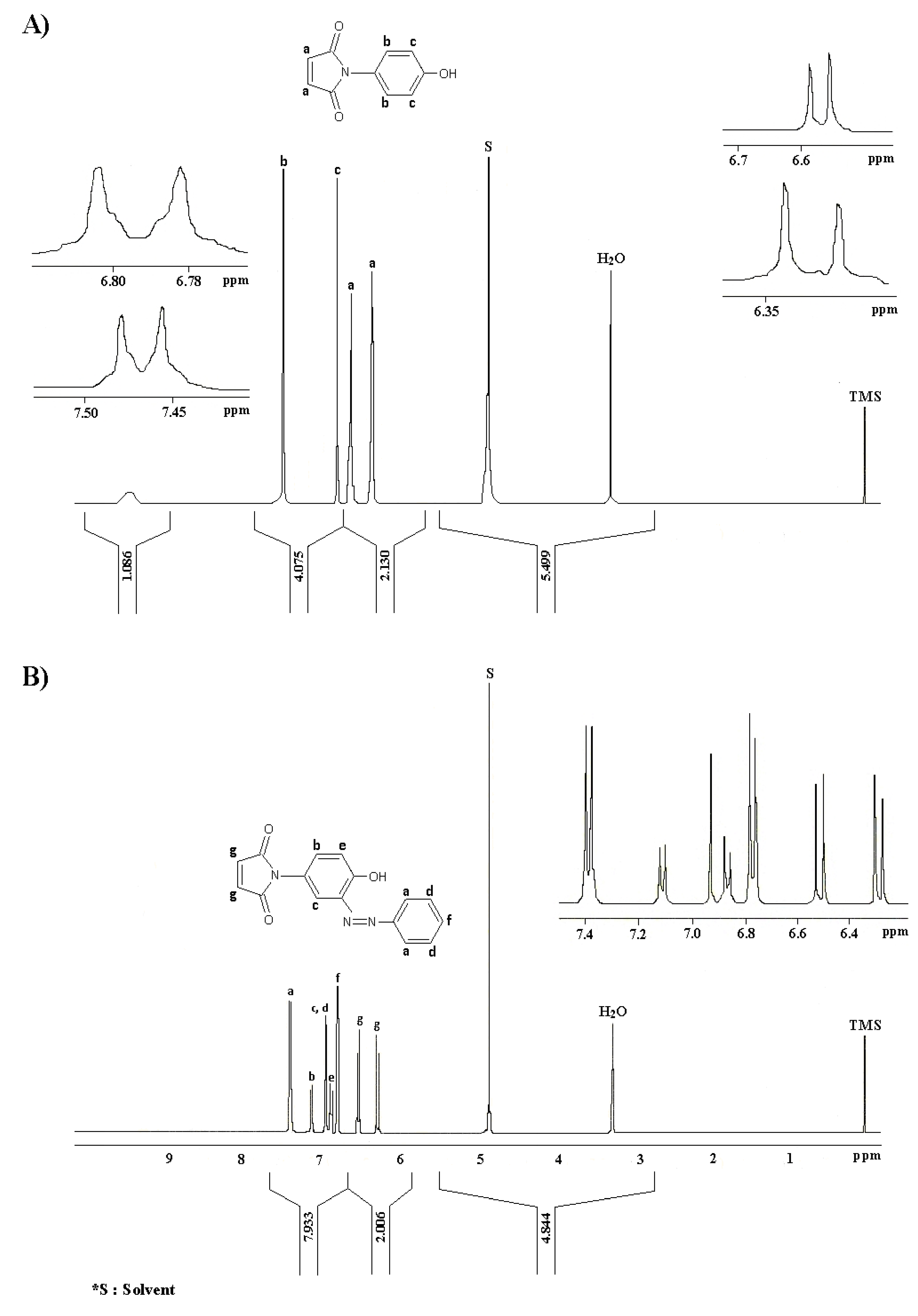
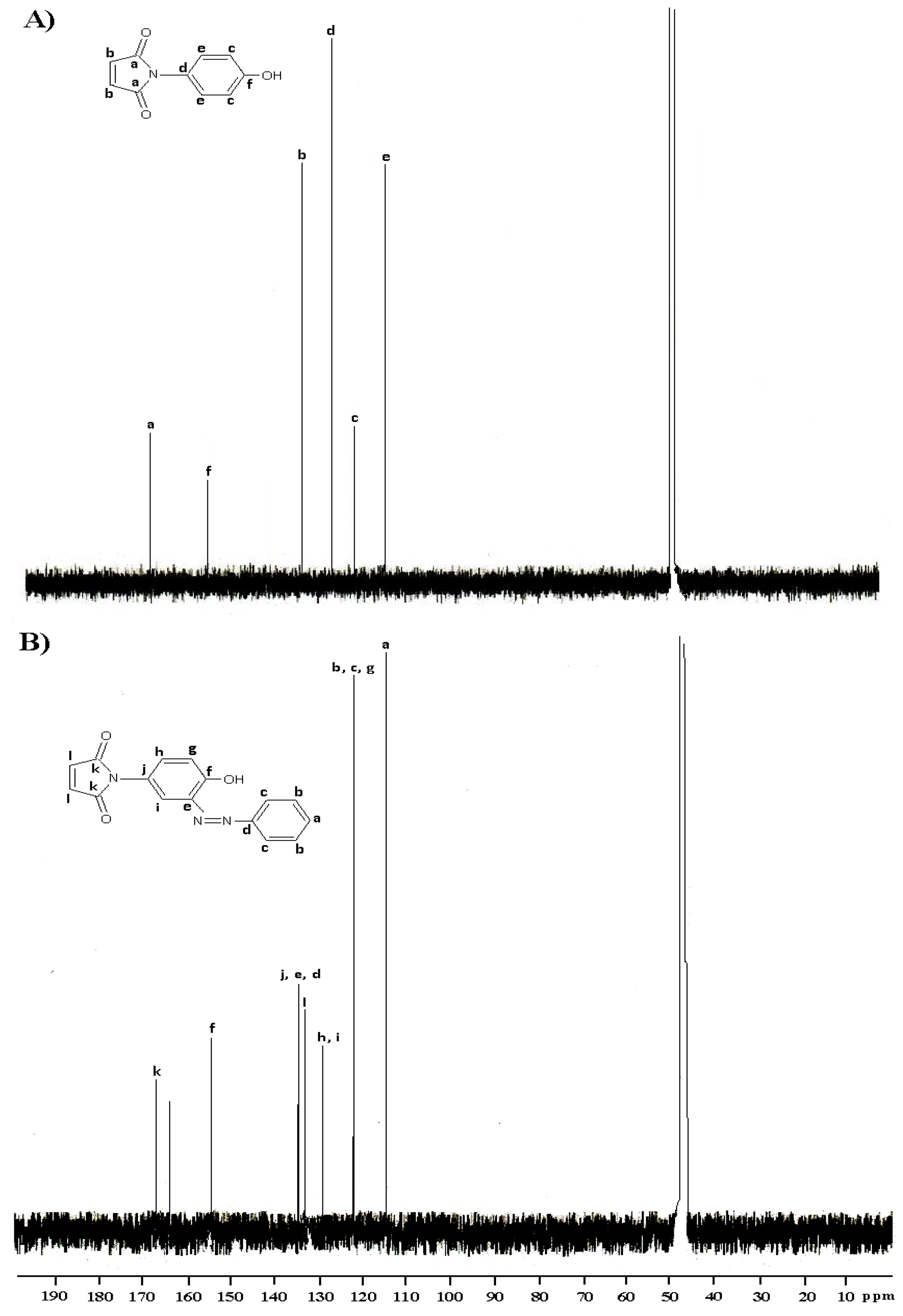
3. Experimental
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of N-(4-hydroxyphenyl)maleimide (I)
3.4. Synthesis of N-(4-methylphenyl)maleimide (II)
3.5. General procedure for preparation of the heterocyclic azo compounds I(a-c) and II(a-c)
4. Conclusions
Acknowledgements
- Sample Availability: Contact the authors.
References
- Bojarski, A.J.; Mokrosz, M.J.; Duszynska, B.; Bugno, R. New imide 5-HT1A receptor ligands – modification of terminal fragment geometry. Molecules 2004, 9, 170–177. [Google Scholar] [CrossRef]
- Alaa, A.M.; Aziz, A. Novel and versatile methodology for synthesis of cyclic imides and evaluation of their cytotoxic, DNA binding, apoptotic inducing activities and molecular modeling study. Eur. J. Med. Chem. 2007, 42, 614–626. [Google Scholar]
- Langmuir, M.E.; Yang, J.R.; Moussa, A.M.; Laura, R.; Lecompte, K.A. New naphthopyranone based fluorescent thiol probes. Tetrahedron Lett. 1995, 36, 3989–3992. [Google Scholar]
- Ohkubo, M.; Nishimura, T.; Jona, H.; Honma, T.; Morishima, H. Practical synthesis of Indolopyrrolocarbazoles. Tetrahedron Lett. 1996, 52, 8099–8112. [Google Scholar]
- Hamper, B.C.; Dukesherer, D.R.; South, M.S. Solid-phase synthesis of proline analogs via a three component 1,3-dipolar cycloaddition. Tetrahedron Lett. 1996, 37, 3671–3674. [Google Scholar] [CrossRef]
- Iijima, T.; Suzuki, N.; Fukuda, W.; Tomoi, M.J. Toughening of aromatic diamine-cured epoxy resins by modification with n-phenylmaleimide-styrene-p-hydroxystyrene terpolymers. Eur. Polym. J. 1995, 31, 775–783. [Google Scholar]
- Bharel, R.; Choudhary, V.; Varma, I.K. Thermal and mechanical properties of copolymers of methyl methacrylate with N-phenyl maleimide. J. Appl. Polym. Sci. 1993, 49, 31–38. [Google Scholar]
- Walter, W.W.; Michael, H.A. Polyimides. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Handley, G.J.; Nelson, E.R.; Somers, T.C. Compounds derived from β-substituted glutaric acids: glutarimides, glutaramic acids, 1,5-pentanediols. Aust. J. Chem. 1960, 13, 127–144. [Google Scholar]
- Polonaski, T.; Milewska, M.J.; Gdaniec, M. Synthesis, structure and chiroptical spectra of the bicyclic α-diketones, imides and dithioimides related to santenone. Tetrahedron: Asymmetry 2000, 11, 3113–3122. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ehrenkaufer, R.L.E. Chemistry of imides. II. Cyclic imides and some unusual products from some diacid chlorides and lithium nitride. J. Org. Chem. 1971, 36, 44–45. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Hsu, K.Y. Maleimide-epoxy resins: Preparation, thermal properties, and flame retardance. Polymer 2003, 44, 565–573. [Google Scholar] [CrossRef]
- Reddy, P.Y.; Kondo, S.; Toru, T.; Ueno, Y. Lewis acid and hexamethyldisilazane-promoted efficient synthesis of N-Alkyl- and N-Arylimide derivatives. J. Org. Chem. 1997, 62, 2652–2654. [Google Scholar] [CrossRef]
- Muller, G.W.; Konnecke, W.E.; Smith, A.M.; Khetani, V.D. A concise two-step synthesis of thalidomide. Org. Process Res. Dev. 1999, 3, 139–140. [Google Scholar] [CrossRef]
- Bon, E.; Reau, R.; Bertand, G.; Bigg, D.C.H. Aluminum trichloride-promoted aminolysis of cyclic imides and oxazolidinones. Tetrahedron Lett. 1996, 37, 1217–1220. [Google Scholar]
- Benjamin, E.; Hijji, Y. The synthesis of unsubstituted cyclic imides using hydroxylamine under microwave irradiation. Molecules 2008, 13, 157–169. [Google Scholar] [CrossRef]
- Jaskowska, J.; Kowalski, P. N-alkylation of imides using phase transfer catalysts under solvent-free conditions. J. Heterocyclic Chem. 2008, 45, 1371–1375. [Google Scholar] [CrossRef]
- Kavitha, K.; Vangala, R.R.; Khagga, M.; Sarbani, P. Lewis acid free high speed synthesis of nimesulide-based novel n-substituted cyclic imides. J. Braz. Chem. Soc. 2010, 21, 1060–1064. [Google Scholar] [CrossRef]
- Allcock, H.R.; Sarah, D.; Karen, M.K. Poly(organophosphazenes) with chromophores as substituent groups. Macromolecules 1978, 11, 357–359. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry, 4th ed.; Pearson Education International: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Rao, C.N.R. Ultra-Violet and Visible Spectroscopy: Chemical Applications, 2nd ed.; Butterworths: London, UK, 1967. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mohammed, I.A.; Mustapha, A. Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide. Molecules 2010, 15, 7498-7508. https://doi.org/10.3390/molecules15107498
Mohammed IA, Mustapha A. Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide. Molecules. 2010; 15(10):7498-7508. https://doi.org/10.3390/molecules15107498
Chicago/Turabian StyleMohammed, Issam Ahmed, and Asniza Mustapha. 2010. "Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide" Molecules 15, no. 10: 7498-7508. https://doi.org/10.3390/molecules15107498
APA StyleMohammed, I. A., & Mustapha, A. (2010). Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide. Molecules, 15(10), 7498-7508. https://doi.org/10.3390/molecules15107498



