Domoic Acid - A New Toxin in the Croatian Adriatic Shellfish Toxin Profile
Abstract
:1. Introduction
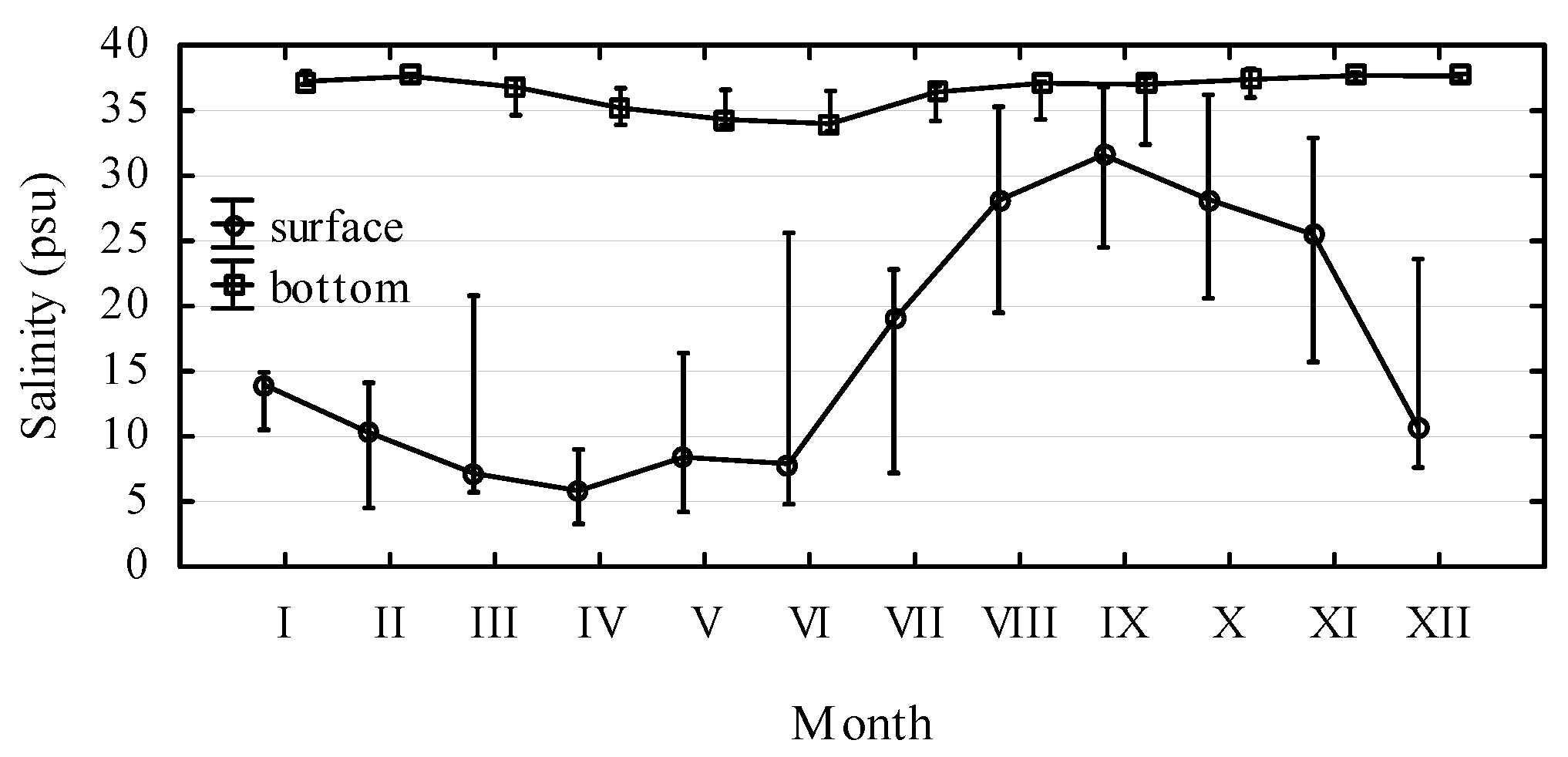
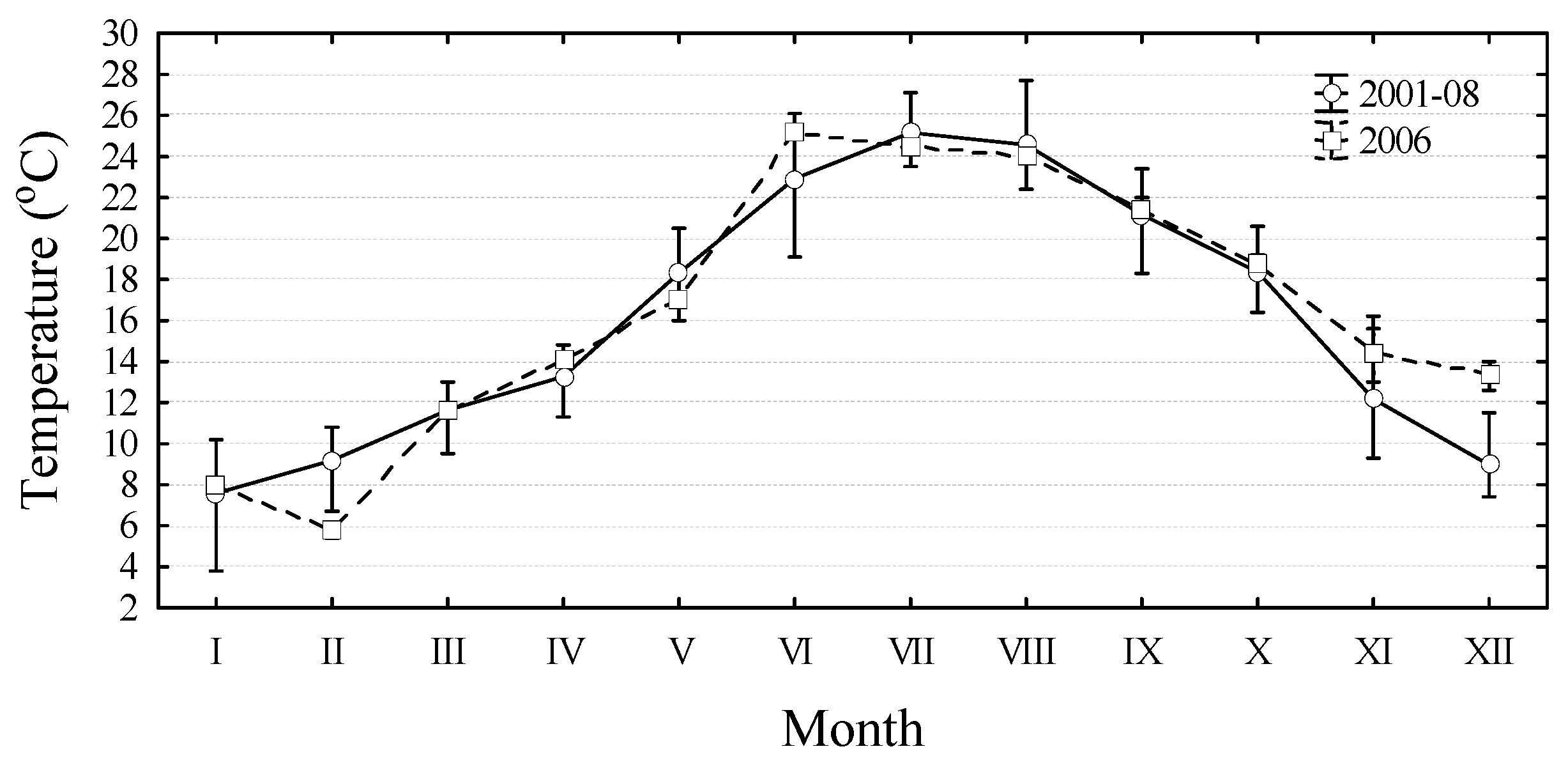
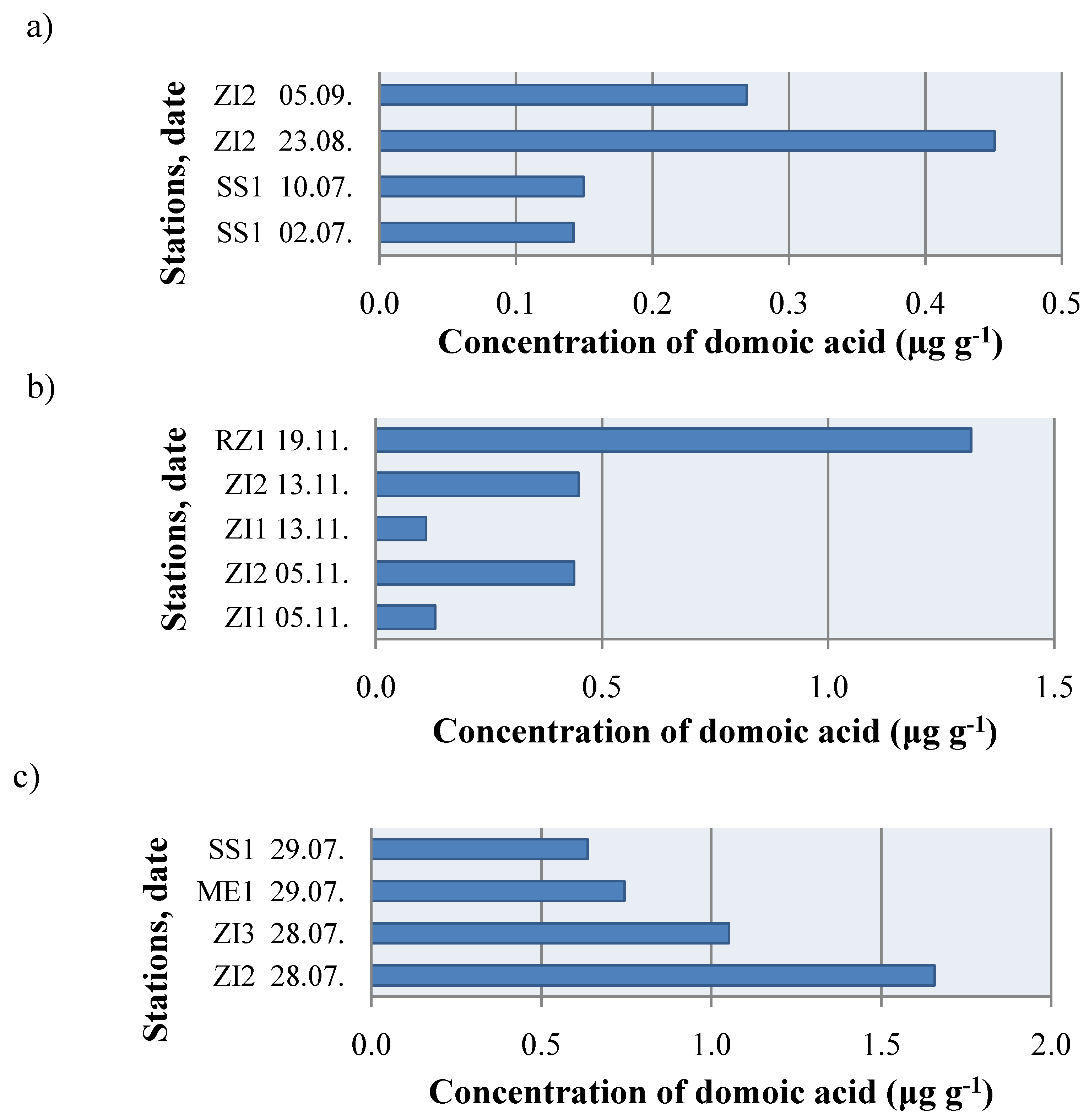
2. Results and Discussion
| Research area of the Adriatic Sea | Mean | Min. | Max. | Std.dev. | |
|---|---|---|---|---|---|
| Surface | North | 17.3 | 7.6 | 26.7 | 5.8 |
| Central | 16.8 | 5.4 | 27.1 | 6.1 | |
| South | 17.8 | 6.5 | 27.3 | 6.0 | |
| Bottom | North | 16.7 | 8.1 | 24.9 | 5.2 |
| Central | 17.4 | 12.0 | 22.1 | 2.9 | |
| South | 17.6 | 6.7 | 25.4 | 4.5 | |
| Research area of the Adriatic Sea | Mean | Min. | Max. | Std.dev. | |
|---|---|---|---|---|---|
| Surface | North | 33.5 | 3.7 | 37.7 | 7.1 |
| Central | 15.2 | 1.9 | 38.3 | 9.1 | |
| South | 33.7 | 24.2 | 37.9 | 2.8 | |
| Bottom | North | 36.6 | 28.8 | 37.9 | 1.4 |
| Central | 36.5 | 32.4 | 38.2 | 1.3 | |
| South | 36.2 | 29.8 | 37.9 | 1.2 | |
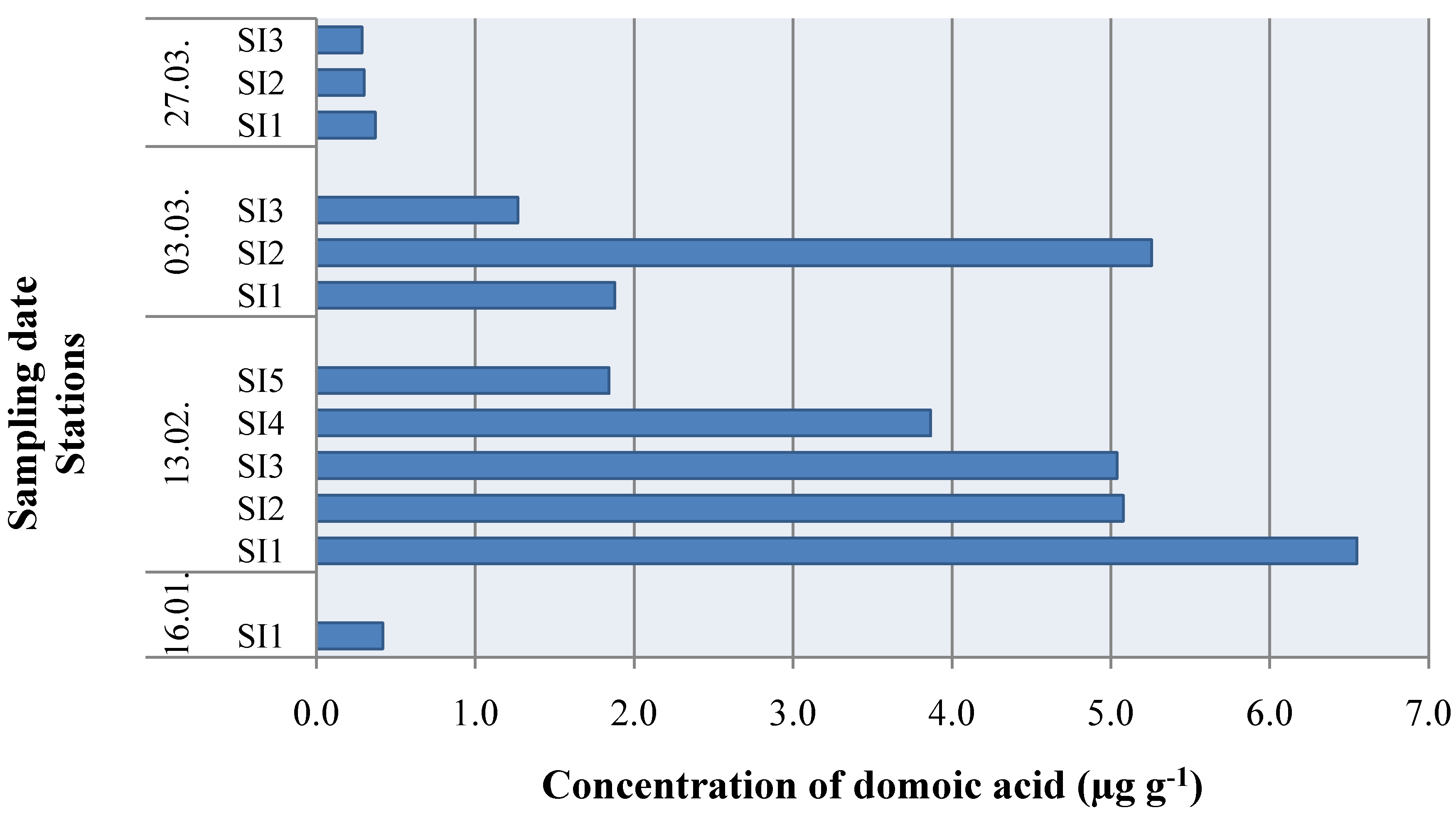
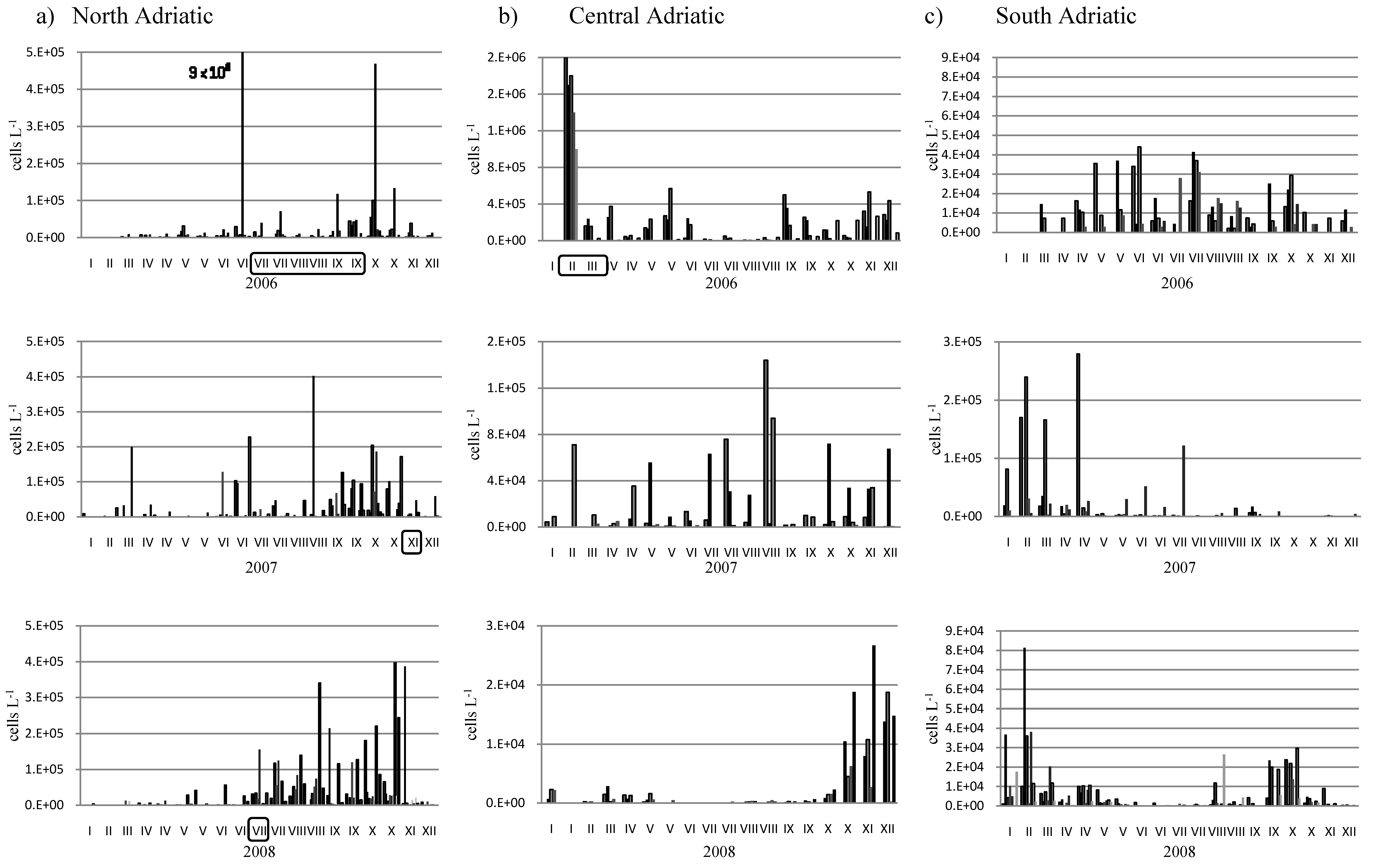
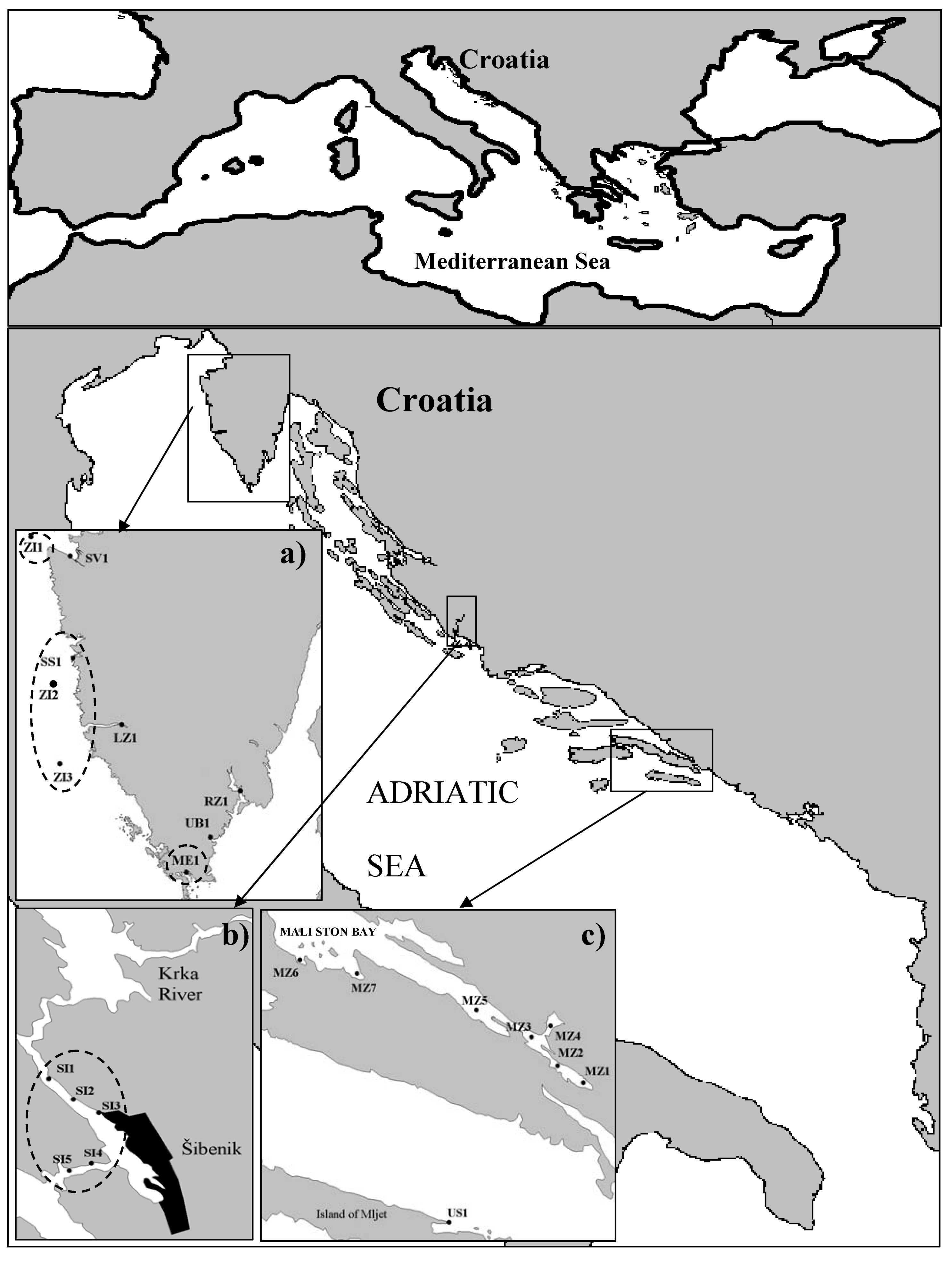
3. Experimental
3.1. Collection of shellfish samples
3.2. Preparation of shellfish samples
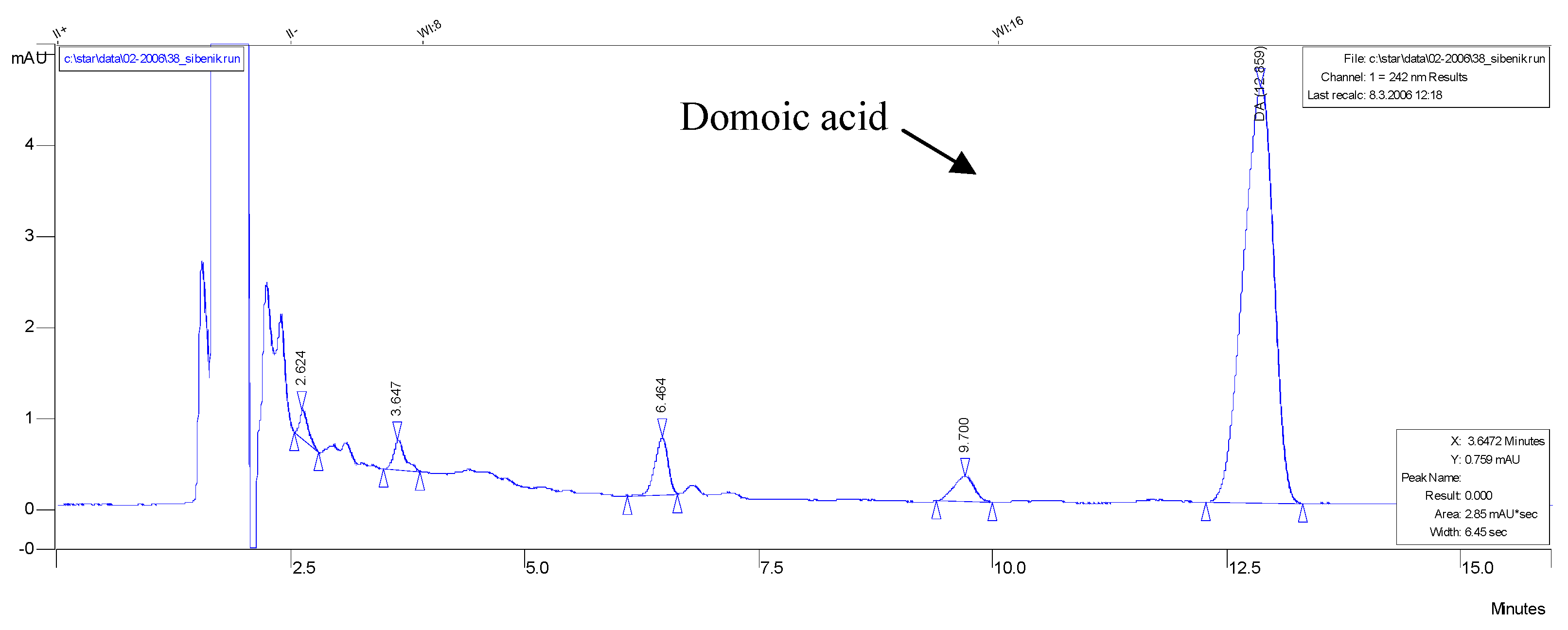
3.3. HPLC analysis
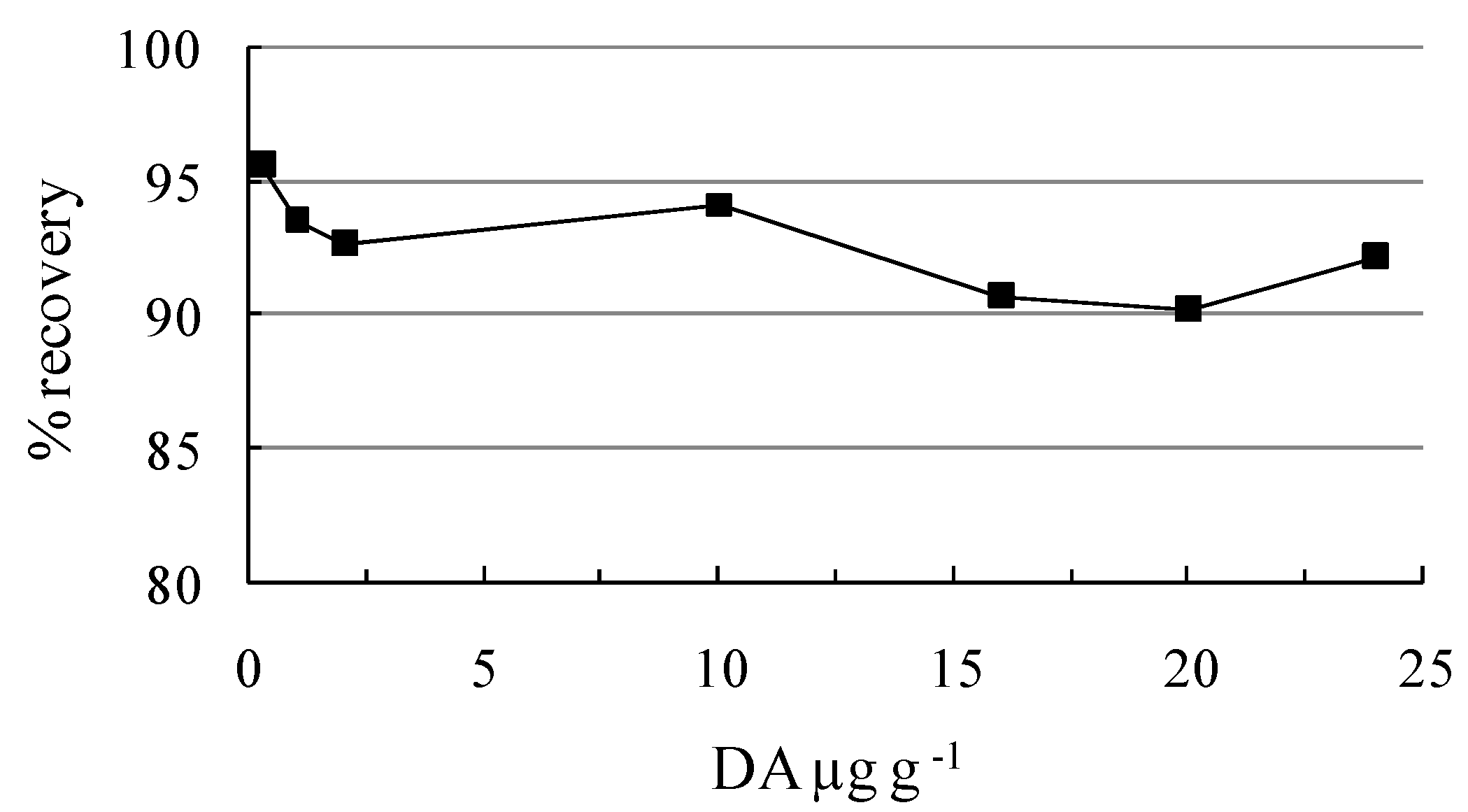
| Certified Concentrations (µg g-1) | Amount found (µg g-1) | Coefficient of variation (%) | Recovery (%) |
|---|---|---|---|
| 41 ± 2 | 40.76 | 0.13 | 99.40 |
| 41 ± 2 | 40.61 | 0.10 | 99.05 |
| 41 ± 2 | 40.27 | 0.23 | 98.23 |
| 41 ± 2 | 39.42 | 0.74 | 96.14 |
| 41 ± 2 | 39.91 | 0.32 | 97.34 |
3.4. Phytoplankton, temperature and salinity water sample analysis
4. Conclusions
Acknowledgments
- Sample Availability: Not available.
References
- Wright, J.L.C.; Boyd, R.K.; de Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P.; Pathak, V.; Quiliam, M.A.; Regan, M.A.; Sim, P.G.; Thibault, P.; Walter, J.A.; Richard, D.J.A.; Dewar, C. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar]
- Todd, E.C.D. Domoic acid and amnesic shellfish poisoning—a review. J. Food Protect. 1993, 56, 69–83. [Google Scholar]
- Bates, S.S.; Bird, C.J.; Defreitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Dodense, P.; Pocklington, R.; Quilliam, M.A.; Sim, P.G.; Smith, J.C.; Rao, D.V.S.; Todd, C.D.; Walter, J.A.; Wrigh, J.L.C. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edwards Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar]
- Amzil, Z.; Fresnel, J.; LeGal, D.; Billard, C. Domoic acid accumulation in French shellfish shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. Pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- Cusack, C.K.; Bates, S.S.; Quilliam, M.A.; Patching, J.W.; Raine, R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002, 38, 1106–1112. [Google Scholar] [CrossRef]
- Costa, P.R.; Garrido, S. Domoic acid accumulation in the sardine Sardina pilchardus and its relationship to Pseudo-nitzschia diatom ingestion. Mar. Ecol. Prog. Ser. 2004, 284, 261–268. [Google Scholar] [CrossRef]
- Fire, S.E.; Wanga, Z.; Leighfield, T.A.; Morton, S.L.; McFee, W.E.; McLellan, W.A.; Litaker, R.W.; Tester, P.A.; Hohn, A.A.; Lovewell, G.; Harms, C.; Rotstein, D.S.; Barco, S.G.; Costidis, A.; Sheppard, B.; Bossart, G.D.; Stolen, M.; Durden, W.N.; Van Dolah, F.M. Domoic acid exposure in pygmy and dwarf sperm whales (Kogia spp.) from southeastern and mid-Atlantic U.S. waters. Harmful Algae 2009, 8, 658–664. [Google Scholar]
- Pan, Y.; Parsons, M.L.; Busman, M.; Moeller, P.D.R.; Dortch, Q.; Powell, C.L.; Doucette, G.J. Pseudo-nitzschia sp. Cf. pseudodelicatissima-a confirmed producer of domoic acid from the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2001, 220, 83–92. [Google Scholar] [CrossRef]
- Trainer, V.L. Harmful Algal Blooms in the PICES Region of the North Pacific; Taylor, F.J., Trainer, V.L., Eds.; Scientific Report 23; PICES: Sidney, Austrilia, 2002; pp. 89–118.
- Fehling, J.; Green, D.H.; Davison, K.; Bolch, C.J.; Bates, S.S. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in Scottish waters. J. Phycol. 2004, 40, 622–630. [Google Scholar] [CrossRef]
- Besiktepe, S.; Ryabushko, L.; Ediger, D.; Yilmaz, D.; Zenginer, A.; Ryabushko, V.; Lee, R. Domoic acid production by Pseudo-nitzschia calliantha Lundholm, Moestrup at Hasle (bacillariophyta) isolated from the Black Sea. Harmful Algae 2008, 7, 438–442. [Google Scholar] [CrossRef]
- Baugh, K.A.; Bush, J.M.; Bill, B.D.; Lefebvre, K.A.; Trainer, V.L. Estimates of specific toxicity in several Pseudo-nitzschia species from the Washington coast, based on culture and field studies. Afr. J. Mar. Sci. 2006, 28, 403–407. [Google Scholar]
- Bates, S.S. Domoic acid producing diatoms: another genus added! J. Phycol. 2000, 36, 978–985. [Google Scholar] [CrossRef]
- Kotaki, Y.; Lundholm, N.; Onodera, H.; Kobayashi, K.; Bajarias, F.; Furio, E.; Iwataki, M.; Fukuyo, Y.; Kodama, M. Wide distributionof Nitzschia navis-varingica, a new domoic acid-producing benthic diatom found in Vietnam. Fisheries Science 2004, 70, 28–32. [Google Scholar] [CrossRef]
- Debonnel, G.; Weiss, M.; de Montigny, C. Reduced neuroexcitatory effect of domoic acid following mossy fiber denervation of the rat dorsal hippocampus: further evidence that toxicity of domoic acid involves kainate receptor activation. Can. J. Physiol. Pharmacol. 1989, 67, 904–908. [Google Scholar] [CrossRef]
- Cavas, T.; Könen, S. In vivo genotoxicity testing of the amnesic shellfish poison (domoic acid) in piscine erythrocytes using the micronucleus test and the comet assay. Aquat. Toxicol. 2008, 90, 154–159. [Google Scholar] [CrossRef]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Munday, R.; Holland, P.T.; McNabb, P.; Selwood, I.A.; Rhodes, L.L. Comparative toxicity to mice of domoic acid and isodomoic acids A, B and C. Toxicon 2008, 52, 954–956. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. Domoic acid in Portuguese shellfish and fish. Toxicon 2001, 39, 893–904. [Google Scholar] [CrossRef]
- Ciminello, P.; Dell'Aversano, C.; Fattorusso, E.; Forino, M.; Magano, S.G.; Tartaglione, L.; Quilliam, A. M.; Tubaro, A.; Polleti, R. Hydrophilic interaction liquid chromatography/mass spectrometry for determination of domoic acid in Adriatic shellfish. Rapid Commun. Mass Spectrom. 2005, 19, 2030–2038. [Google Scholar] [CrossRef]
- Kaniou-Grigoriadou, I.; Mouratidou, T.; Katikou, P. Investigation on the presence of the domoic acid in Greek shellfish. Harmful Algae 2005, 4, 717–723. [Google Scholar] [CrossRef]
- James, K.J.; Gillman, M.; Amandi, M.F.; López-Rivera, A.; Puente, P.F.; Lehane, M.; Mitrović, S.; Furey, A. Amnesic shellfish poisoning toxin in bivalve molluscs in Ireland. Toxicon 2005, 46, 852–858. [Google Scholar] [CrossRef]
- Bogan, Y.M.; Kennedy, D.J.; Harkin, A.L.; Gillespie, J.; Vause, B.J.; Beukers-Stewart, B.D.; Hess, P.; Slater, J.W. Variation in domoic acid concentration in king scallop (Pecten maximus) from fishing grounds around the Isle of Man. Harmful Algae 2007, 6, 81–92. [Google Scholar] [CrossRef]
- Leira, F.J.; Vieites, J.M.; Botana, L.M.; Vyeites, M.R. Domoic Acid Levels of Naturally Contaminated Scallops as Affected by Canning. J. Food Sci. 1998, 63, 1081–1083. [Google Scholar]
- Bargu, S.; Powell, C.L.; Coale, S.L.; Busman, M.; Doucette, G.J.; Silver, M.W. Krill: a potential vestor for domoic acid in marine foods webs. Mar. Ecol.Prog.Ser. 2002, 237, 209–216. [Google Scholar] [CrossRef]
- Bargu, S.; Lefebvre, K.; Silver, M.W. Effect of dissolved domoic acid on the grazing rate of krill Euohausia pacifica. Mar. Ecol. Prog.Ser. 2006, 312, 169–176. [Google Scholar] [CrossRef]
- Lopez-Rivera, A.; Pinto, M.; Insinilla, A.; Isla, B.S.; Uribe, E.; Alvarez, G.; Lehane, M.; Furey, A.; James, K.J. The occurence of domoic acid linked to a toxic diatom bloom in a new potential vector: The tunicate Pyura chilensis (piure). Toxicon 2009, 54, 754–762. [Google Scholar] [CrossRef]
- Costa, P.R.; Rosa, R.; Duarte-Silva, A.; Brotas, V.; Sampayo, M.A.M. Accumulation, transformation and tissue distribution of domoic acid, the amnesic shellfish poisoning toxin, in the common cuttlefish, Sepia officinalis. Aquat. Toxicol. 2005, 74, 82–91. [Google Scholar] [CrossRef]
- Beltran, A.S.; Palafox-Uribe, M.; Garajales-Montiel, J.; Cruz-Villacorta, A.; Ochoa, J.L. Sea bird mortality at Cabo San Lucas Mexico: evidence that toxin diatom blooms are spreading. Toxicon 1997, 35, 447–453. [Google Scholar] [CrossRef]
- Scholin, C.A.; Gulland, F.; Doucette, J.G.; Benson, S.; Busman, M.; Chavez, P.F.; Cordaro, J.; DeLong, R.; De Vogelaere, A.; Harvey, J.; Haulena, M.; Lefebvre, K.; Lipscomb, T.; Loscutoff, S.; Lowenstine, L.J.; Marin, III R.; Miller, P.E.; McLellan, W.A.; Moeller, P.D.R.; Powell, C.L.; Rowles, T.; Silvagni, P.; Silver, M.; Spraker, T.; Trainer, V.; Van Dolah, F.M. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar]
- Bejrano, A.C.; Gulland, F.M.; Goldstein, T.; St Leger, J.; Hunter, M.; Schwacke, L.H.; VanDolah, F.M.; Rowles, TK. Demographic and spatio-temporal signature of the biotoxin domoic acid in Califronia sea lion (Zalophus californius) stranding records. Mar. Mamm. Sci. 2008, 24, 899–912. [Google Scholar]
- Lefebvre, K.A.; Noren, D.P.; Schultz, I.R.; Bogard, S.M.; Wilson, J.; Eberhart, B.T.L. Uptake, tissue distribution and excretion of domoic acid after oral exposure in coho salmon (Oncorhynchus kisutch). Aquat. Toxicol. 2007, 81, 266–274. [Google Scholar] [CrossRef]
- Honer, R.A.; Kussake, M.B.; Moynihan, B.P.; Skinner, R.N.; Wekell, J.C. Retention of domoic acid by Pacific razor clams Siliqua patula (Dixon, 1789) – preliminary study. J. Shellfish Res. 1993, 12, 451–456. [Google Scholar]
- Anonymous. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Commun. 2002, L 226, 22–82.
- Walz, P.M.; Garrison, D.L.; Graham, W.M.; Cattey, M.A.; Tjeerdema, R.S.; Silver, M.W. Domoic acid-producing diatom blooms in Monterey Bay, California: 1991-1993. Nat. Toxins 1993, 2, 271–279. [Google Scholar]
- Burić, Z.; Viličić, D.; Caput Mihalić, K.; Carić, M.; Kralj, K.; Ljubešić, N. Pseudo-nitzschia blooms in the Zrmanja River estuary (eastern Adriatic Sea). Diatom Res. 2008, 23, 51–63. [Google Scholar] [CrossRef]
- Marić, D.; Burić, Z.; Godrijan, J.; Bosak, S.; Djakovac, T.; Peharec, P. The occurrence of potentially toxic Pseudo-nitzschia calliantha in the costal waters of the Northern Adriatic Sea. In 20th International Diatom Symposium; Jasprica, N., Car, A., Čalić, M., Eds.; University of Dubrovnik: Dubrovnik, Croatia, 2008; p. 182. [Google Scholar]
- Caroppo, C.; Congestri, R.; Bracchini, L.; Albertano, P. On the presence of Pseudo-nitzschia calliantha Lundholm, Moestrup et Hasle and Pseudo-nitzschia delicatissima (Cleve) Heiden in the Southern Adriatic Sea (Meditrrranean Sea, Italy). J. Plankton Res. 2005, 27, 763–774. [Google Scholar] [CrossRef]
- Spatharis, S.; Danielidis, B.D.; Tsirtsis, G. Recurrent Pseudo-nitzschia calliantha (Bacillariophyceae) and Alexandrium insuetum (Dinophyceae) winter blooms induced by agricultural runoff. Harmful Algae 2007, 6, 811–822. [Google Scholar] [CrossRef]
- Cabrini, M.; Cok, S.; Pecchiar, I.; Fonda Umani, S.; Andri, M. Carbon partitioning among the first trophic levels in the North Western Adriatic basin. Chem. Ecol. 2002, 18, 95–105. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risk: a review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Goldstein, T.; Mazet, J.A.; Zabka, T.S.; Langlois, G.; Colegrove, K.M.; Silver, M.; Bargu, S.; Van Dolah, F.; Leighfield, T.; Conrad, P.A.; Barakos, J.; Williams, D.C.; Dennison, S.; Haulena, M.; Gulland, F.M. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. In Proc. Roy. Soc. Biol. Sci. Ser. B 2008, 275, 267–276. [Google Scholar] [CrossRef]
- Anonymous. Council Directive 79/923/EEC of 30 October 1979 on the quality required of shellfish waters. Off. J. Eur. Commun. 1979, L0923, 1–9.
- Anonymous. Council Directive 91/492/EEC laying down the health conditions for the production and the placing on the market of live bivalve molluscs. Off. J. Eur. Commun. 1991, L268, 15–34.
- Ninčević-Gladan, Ž.; Skejić, S.; Bužančić, M.; Marasović, I.; Arapov, J.; Ujević, I.; Bojanić, N.; Grbec, B.; Kušpilić, G.; Vidjak, O. Seasonal variability in Dinophysis spp. Abundance and diarrhetic shelfish poisoning outbreaks along the eastern Adriatic coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid extraction and cleanup fot liquid chromatographic determination of domoic acid in unsalted seafood. J. AOAC Int. 1995, 78, 543–553. [Google Scholar]
- Utermöhl, H. Zur Vervollkommung der quantitative Phytoplankton Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ujević, I.; Ninčević-Gladan, Ž.; Roje, R.; Skejić, S.; Arapov, J.; Marasović, I. Domoic Acid - A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules 2010, 15, 6835-6849. https://doi.org/10.3390/molecules15106835
Ujević I, Ninčević-Gladan Ž, Roje R, Skejić S, Arapov J, Marasović I. Domoic Acid - A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules. 2010; 15(10):6835-6849. https://doi.org/10.3390/molecules15106835
Chicago/Turabian StyleUjević, Ivana, Živana Ninčević-Gladan, Romana Roje, Sanda Skejić, Jasna Arapov, and Ivona Marasović. 2010. "Domoic Acid - A New Toxin in the Croatian Adriatic Shellfish Toxin Profile" Molecules 15, no. 10: 6835-6849. https://doi.org/10.3390/molecules15106835
APA StyleUjević, I., Ninčević-Gladan, Ž., Roje, R., Skejić, S., Arapov, J., & Marasović, I. (2010). Domoic Acid - A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules, 15(10), 6835-6849. https://doi.org/10.3390/molecules15106835





