Abstract
This study provides new data on the various carotenoids found in bambangan (Mangifera pajang Kosterm.) peel and pulp extracts, such as all-trans-α- and β-carotene, cis-β-carotene, 9-cis-β-carotene, and cryptoxanthin. Chemical and biological antioxidant assays were determined to evaluate the antioxidant capacity of bambangan peel and pulp extracts. Bambangan pulp had higher α- and β-carotene contents (7.96 ± 1.53 and 20.04 ± 1.01 mg/100 g) than its peel (4.2 ± 0.14 and 13.09 ± 0.28 mg/100 g); the cryptoxanthin contents of bambangan peel and pulp were 0.60 and 1.18 mg/100 g, respectively. The antioxidant activity results determined by chemical assay using the 2,2-diphenyl-2-picrylhydrazyl (DPPH) method showed that bambangan peel extract had higher DPPH radical scavenging activity than its pulp. In the biological assays bambangan peel and pulp had protective effects against hemoglobin and LDL oxidation at an extract concentration of 1 ppm. Bambangan peel is a therefore a potential source of natural antioxidants and could be utilized as a functional ingredient.
1. Introduction
Bambangan (Mangifera pajang Kosterm.) is an indigenous fruit of the Borneo region. The tree can grow up to 20 m in height and bears up to hundreds of semi-oval shaped fruits which are brown colored and can weigh up to 2 kg. The pulp of bambangan fruit represents 50–67% of the fruit’s total weight, is fibrous and juicy, with a specific aromatic flavor and strong smell, and can be eaten fresh. Bambangan peel is a major by-product of its pulp and juice processing industries. It is fibrous and may contain various bioactive compounds such as polyphenols and carotenoids, like those found in mango peel [1]. In Borneo, bambangan peel is commonly used for cooking curry.
Among the various types of carotenoids, α- and β-carotene are commonly found in fruits. Carotenes are terpenes with the general formula C40Hx and have an unsaturated and long aliphatic hydrocarbon chain. Yellow-orange colored carotene occurs in several isomeric forms, such as α-, β-, γ‑, δ-, ε-, or ζ-carotene. The most common types of carotenoid are α- and β-carotene, which have several geometrical isomer forms. Isomers of β-carotene such as all-trans-β-carotene, 9-cis-β-carotene and 13-cis-β-carotene are commonly found in foods (Figure 1). Lutein and zeaxanthin are typical carotenoids abundantly found in vegetables. However, fruits have low concentration of lutein and zeaxanthin. Carotenoids are potential antioxidants and free radical scavengers. A previous study reported that carotenoids had protective effects against oxidation of liposomes [2]. Moreover, many researchers have argued that β-carotene had acceptable degree of antioxidant capacity and cardio-protective effect [3,4].
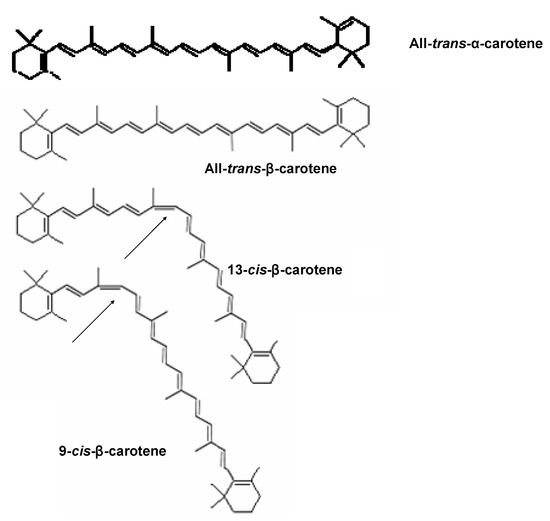
Figure 1.
The structure of all-trans-α- and β-carotene and two other geometrical isomersof β-carotene. Source: ESA [5].
The amount of research on bambangan fruit has increased rapidly over the past few years,. Recent evidence suggests that bambangan fruit has acceptable amounts of β-carotene [6]. Previously, we have determined the isoflavone contents of bambangan fruit [7], while Abu Bakar et al. [8] have reported on its antioxidant activity, but little attention has been paid to the different carotenoids of this fruit. Therefore, the aim of this study is to determine and compare the carotenoid contents and isomers and the antioxidant capacity of bambangan peel and pulp extracts.
2. Results
2.1. High Performance Liquid Chromatography (HPLC) Analysis of Carotenoids in Bambangan Peel and Pulp Extracts
HPLC analysis was performed to determine the carotenoid contents in bambangan peel and pulp extracts. As a C18 column is not appropriate for determination of cis-isomers, therefore a C30 column was used for separation of these β-carotene isomers [9]. The percentages of recovery for α- and β-carotene were in the range of 80−105%. The LOD values for α- and β-carotene were 1.39 and 5.46 ppm, while LOQ values were 6.26 and 24.55 ppm, respectively. With the exception of several unresolved peaks a total of eight carotenoids were effectively resolved in the chromatograms of bambangan peel and pulp extracts. The separation factor (α) values for all peaks were greater than 1 (Table 1 and Table 2), which indicate a good selectivity of mobile phase to carotenoid composition.

Table 1.
Retention time (min), factor (k'), separation factor (α), peak purity (%) and other identification parameters for geometry isomers of carotenoids in bambangan peel extract.
| Peak no. | Carotenoid | Retention time | k' | α | Peak purity | Concentration* | λmax (nm) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 7.27 ± 0.3 | 3.33 | 1.38 (1,2) | 93.5 | − | 398 | 420 | 440 |
| 2 | Unknown | 9.39 ± 0.2 | 4.59 | 1.41 (2,4) | 99.0 | − | 424 | 444 | 470 |
| 3 | − | − | − | − | − | − | − | − | − |
| 4 | Unknown | 12.59 ± 0.3 | 6.49 | 1.84 (4,5) | 99.7 | − | 402 | 425 | 446 |
| 5 | Unknown | 21.72 ± 0.1 | 11.93 | 1.15 (5,6) | 99.0 | − | 402 | 428 | 446 |
| 6 | Unknown | 24.63 ± 0.2 | 13.66 | 1.08 (6,7) | 99.9 | − | 404 | 428 | 450 |
| 7 | Unknown | 26.55 ± 0.3 | 14.80 | 1.06 (7,8) | 99.5 | − | 404 | 428 | 450 |
| 8 | Cryptoxanthin | 28.10 ± 0.4 | 15.73 | 1.03 (8,9) | 91.6 | 0.60 ± 0.001 | 424 | 444 | 472 |
| 9 | Cis-cryptoxanthin | 28.81 ± 0.1 | 16.15 | 1.09 (9,10) | 99.0 | 0.07 ± 0.001 | 424 | 446 | 470 |
| 10 | Cis-β-carotene | 31.35 ± 0.3 | 17.66 | 1.24 (10,11) | 96.6 | 2.87 ± 0.52 | 424 | 446 | 472 |
| 11 | All-trans-α-carotene | 38.53 ± 0.2 | 21.93 | 1.12 (11,12) | 99.0 | 4.20 ± 0.14 | 428 | 450 | 476 |
| 12 | Cis-β-carotene | 42.97 ± 0.1 | 24.58 | 1.04 (12,13) | 99.0 | 3.64 ± 0.48 | 428 | 453 | 476 |
| 13 | All-trans-β-carotene | 44.49 ± 0.1 | 25.48 | 1.09 (13,14) | 99.9 | 13.09 ± 0.28 | 428 | 453 | 478 |
| 14 | Unknown | 48.36 ± 0.2 | 27.79 | 1.04 (14,15) | 99.9 | − | 404 | 458 | 478 |
| 15 | 9-Cis-β-carotene | 50.11 ± 0.2 | 28.83 | 1.04 (14,15) | 99.4 | 2.53 ± 0.66 | 428 | 444 | 468 |
* The concentration of individual carotenoid is expressed as mean ± SD in mg/100 g.
Moreover, the retention factor (k') values for all peaks ranged from 3.21 to 28.83, which also suggests that a suitable solvent strength was maintained. In addition, the purity factor for all peaks, except for peak number 4, was higher than 90% (Table 2). All peaks were identified based on the comparison of the retention time and absorption spectra between the studied samples and carotenoid standards. The Q-ratio was calculated for further confirmation of β-carotene cis-isomers, which was in the range of 0.1−0.23.

Table 2.
Retention time (min), factor (k'), separation factor (α), peak purity (%) and other identification parameters for geometry isomers of carotenoids in bambangan pulp extract.
| Peak no. | Carotenoid | Retention time | k' | α | Peak purity | Concentration* | λmax (nm) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 7.32 ± 0.2 | 3.21 | 1.36 (1,2) | 96.3 | − | 400 | 402 | 444 |
| 2 | Unknown | 9.34 ± 0.3 | 4.37 | 1.17 (2,3) | 99.0 | − | 416 | 442 | 468 |
| 3 | Ζeta-carotene | 11.72 ± 0.3 | 5.13 | 1.23(3,4) | 99.0 | − | 398 | 422 | 444 |
| 4 | Unknown | 12.68 ± 0.2 | 6.29 | 2.09 (4,6) | 85.2 | − | 400 | 422 | 446 |
| 5 | − | − | − | − | − | − | − | − | − |
| 6 | Unknown | 24.62 ± 0.1 | 13.15 | 1.09 (6,7) | 99.6 | − | 424 | 444 | 464 |
| 7 | Unknown | 26.75 ± 0.3 | 14.37 | 1.06 (7,8) | 99.9 | − | 424 | 444 | 464 |
| 8 | Cryptoxanthin | 28.23 ± 0.2 | 15.22 | 1.12 (8,10) | 99.7 | 1.18 ± 0.01 | 424 | 444 | 472 |
| 9 | − | − | − | − | − | − | − | − | − |
| 10 | Cis-β-carotene | 31.51 ± 0.2 | 17.11 | 1.24 (10,11) | 99.9 | 3.04 ± 0.13 | 424 | 444 | 470 |
| 11 | All-trans-α-carotene | 38.79 ± 0.2 | 21.29 | 1.07 (11,12) | 99.5 | 7.96 ± 1.53 | 424 | 450 | 470 |
| 12 | Cis-β-carotene | 41.43 ± 0.3 | 22.81 | 1.08 (12,13) | 99.0 | 3.74 ± 0.37 | 420 | 444 | 474 |
| 13 | All-trans-β-carotene | 44.69 ± 0.1 | 24.68 | 1.09 (13,14) | 99.9 | 20.04 ± 1.01 | 428 | 453 | 478 |
| 14 | Unknown | 48.62 ± 0.3 | 26.94 | 1.05 (14,15) | 99.0 | − | 402 | 453 | 487 |
| 15 | 9-Cis-β-carotene | 50.90 ± 0.2 | 28.25 | 1.05 (14,15) | 99.0 | 2.72 ± 0.10 | 426 | 444 | 472 |
* The concentration of individual carotenoid is expressed as mean ± SD in mg/100 g.
In this study, a total of 15 peaks were found in bambangan peel extract (Figure 2A). Peaks 8 and 9 were identified as cryptoxanthin and cis-cryptoxanthin, peaks 10 and 12 were identified as cis-β-carotene, while peaks 11−13 were identified as α- and β-carotene, as further confirmed by spiking with α- and β-carotene standards. Moreover, peak 15 was identified as 9-cis-β-carotene, based on its bsorption spectra. The chromatogram profile of bambangan pulp was similar to that of peel (Figure 2), except for the zeta-carotene (peak 3) in bambangan pulp. cis-Cryptoxanthin was not detected in pulp along with other unidentified peaks. Additionally, the peaks of zeta-carotene and cis-cryptoxanthin were tentatively identified based on the absorption spectra. The UV-visible spectra for the identified carotenoids are in agreement with Rodriguez-Amaya and Kimura [10] The peaks of cryptoxanthin, α- and β-carotene were quantified by standard calibrations based on peak area, while all identified cis-β-carotenes were quantified using β-carotene standard calibration curve.
The results of this study showed that bambangan peel extract had 4.2 ± 0.14 and 13.09 ± 0.28 mg/100 g of α- and β-carotene (Table 1), while pulp contained 7.96 ± 1.53 and 20.04 ± 1.01 mg/100 g of α- and β-carotene respectively (Table 2). Therefore, α- and β-carotene contents of bambangan peel extract were significantly lower than pulp (p < 0.05). For bambangan peel and pulp extracts, 2.53 and 2.72 mg/100 g of 9-cis-β-carotene were found. Besides, other cis-isomers of β-carotene were identified in peel and pulp extracts ranged from 2.87–3.74 mg/100 g of fruit.
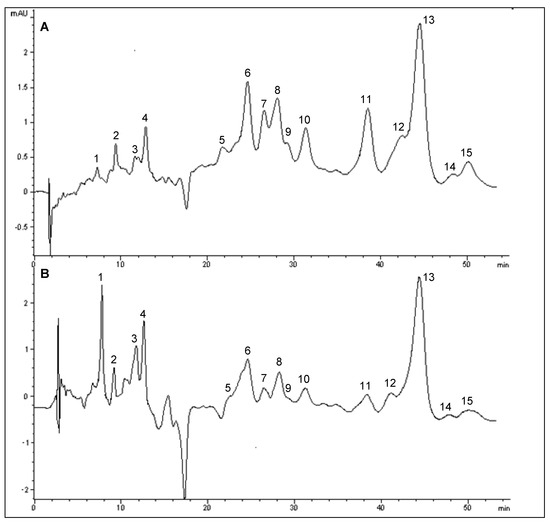
Figure 2.
HPLC chromatogram of carotenoids in bambangan peel (A) and pulp (B) extracts.
2.2. Antioxidant Activity of Chemical Assay
Due to low solubility of carotenoids in hexane, low concentrations (0.1–2.0 ppm of β-carotene equivalents) of carotenoid-rich sample extracts were used for the chemical and biological assays. A low concentration of carotenoid compound, especially β-carotene, was able to fully dissolve in absolute methanol [10,11,12]. By applying a semi-aqueous phase system, carotenoids were able to scavenge free radicals as antioxidant [13,14].
The scavenging activity (%) against DPPH radicals for bambangan peel and pulp extracts is shown in Figure 3. Results showed that the percentage scavenging activity of peel and pulp extracts at 0.1 ppm were not statistically different. However, a significant different was found between bambangan peel and pulp extracts at higher concentrations (0.5–2.0 ppm) (p < 0.05). The scavenging activity of the peel extract was concentration dependent. Compared to bambangan peel extract, the scavenging activity of pulp extract decreased when the concentration reaches an optimum level of 1.5 ppm. This finding is in agreement with Jimenez-Escrig et al. [11] that the scavenging effects of the superoxide anion free radical decreased with increasing extract concentration. In addition, the scavenging activity was significantly higher in bambangan peel and pulp extracts compared to β-carotene standard.
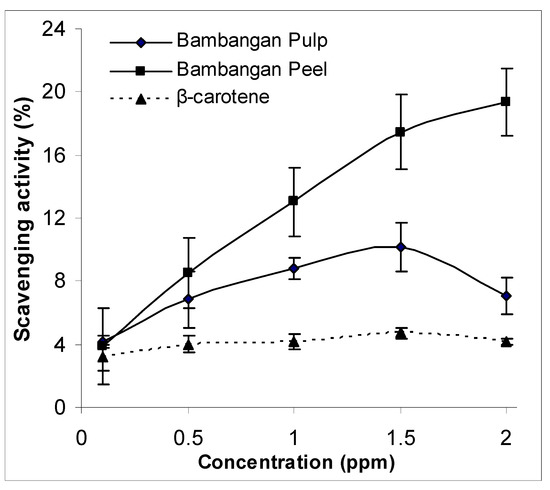
Figure 3.
DPPH scavenging activity between bambangan peel and pulp extracts.
2.3. Antioxidant Activity of Biological Assays
The reliability of chemical assays for antioxidant determination, especially those involved in hydrogen transfer pathways, is questionable [15]. For this reason, biological assays were performed to determine the antioxidant activity of bambangan peels and pulp extracts. In this study, hemoglobin and LDL oxidation assays were chosen for determination as they are mimicking human biological system [16].
The result showed that compared to pulp bambangan peel extracts had protective effect against hemoglobin oxidation. MDA equivalents were used to express the biological assay results. The MDA equivalent of hemoglobin oxidation treated with bambangan peels extract was significantly lower (p < 0.05) than pulp extract at 2 ppm concentration (Figure 4A). The MDA equivalents of H2O2-induced hemoglobin oxidation treated with three different extract concentrations (0.1, 1 and 2 ppm) significantly decreased (p < 0.05) compared to H2O2-induced control. Therefore, both bambangan peels and pulp extracts had protective effect against H2O2-induced oxidation.
The results showed that both bambangan peels and pulp extracts had protective effect against copper-induced LDL oxidation using the three studied concentration (0.1, 1 and 2 ppm), in which MDA equivalent levels significantly reduced compared to copper-induced control (Figure 4B). The results suggest that bambangan peels and pulp extracts have protective effects against LDL oxidation at 1 ppm concentration. Moreover, the MDA equivalents did not show any significant differences between bambangan peels and pulp extract at three different concentrations. However, low extract concentration is best in the free radical scavenging model, where higher concentrations of carotenoid may exacerbate oxidation [17].
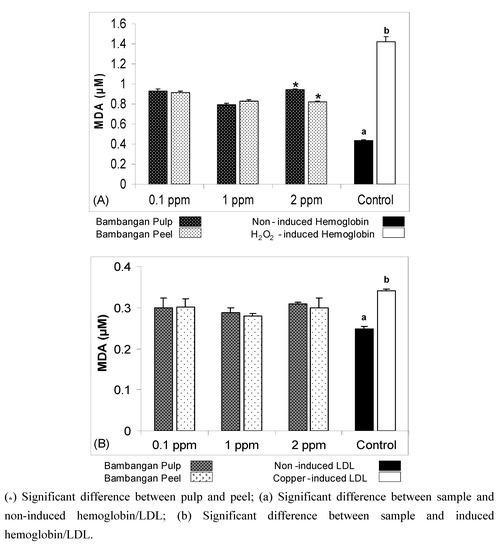
Figure 4.
Comparison of malondialdehyde (MDA) equivalents between LDL oxidation treated with bambangan peels and pulp extracts (A); copper induced oxidation treated with bambangan peels and pulp extracts (B).
3. Discussion
The carotenoid contents of bambangan fruit found in this study were higher than those of other Mangifera fruits. The result was supported by Idris and Idris [6], who reported that Mangifera indica and M. pentandra fruits had 10 and 3 mg of β-carotene per 100 g (dry basis). The fruits of M. foetida contain 2.6–4.8 mg of total carotenoids per 100 g [18]. However, the carotenoid contents of the studied bambangan peel mainly consisted of carotenes and trace amounts of xanthophyll. Besides, bambangan peel and pulp extracts were confirmed to have carotenoids, such as α- and β-carotene, cryptoxanthin and carotenoid isomers, along with unidentified compounds. Moreover, α- and β-carotene contents of bambangan pulp were higher than its peel. In addition, the occurrence of cryptoxanthin in bambangan pulp is relatively new, compared to mango pulp [9].
The DPPH radical scavenging assay is one of the chemical assays involving an electron transfer pathway, and commonly used to determine the activity of antioxidant compounds. Apparently, this finding suggests that carotenoids have lower scavenging activity than most of the polar compounds measured using the DPPH assay. Another interesting finding was that the studied bambangan peel extract had a low carotenoid content, but it had high scavenging activity. If compared to bambangan pulp extract, it had higher carotenoid content; the fact suggests that β-carotene is a weaker antioxidant as compared to non-carotenoid compounds, which also has lower scavenging activity. Besides, bambangan peel extract had higher scavenging ability than β-carotene. As shown in the result, the scavenging activity decreased when the carotenoid-rich extract reaches an optimum level of 1.5 ppm. At low concentrations, carotenoids, especially β-carotene, have strong antioxidant effects. However, a high concentration of carotenoid in biological system has been reported to have no protective effect against LDL oxidation, since they might act as pro-oxidants [19].
There are several possible explanations for LDL oxidation being one of the major risk factors for cardiovascular disease. Lau [20] has discussed how natural antioxidants that help to suppress LDL oxidation, could also reduce the risk of cardiovascular disease; while Heinecke [21] has reported on lipoprotein oxidation in cardiovascular disease. However, our study found that moderate concentration of bambangan peel and pulp extracts have protective effect against copper-induced LDL oxidation. Thus, it can be suggested that the concentration of the bambangan extracts is an important aspect of this study. Furthermore, a high extract concentration will reduce the scavenging activity, while low amounts of bioactive substances may have minimal or no protective effects. This was supported by Li et al. [22] who claimed that a high dose of lotus germ oil has pro-oxidation effects. In the biological system, the studied bambangan peel extract was able to significantly reduce the secondary oxidation product, MDA, produced from H2O2-induced hemoglobin oxidation, as compared to its pulp extract. Ajila et al. [23] support our findings in that mango peel extract exhibited a high antioxidant activity. Our study was also in agreement with Ajila and Prasada Rao [1] who reported that mango peel extracts protected erythrocytes against oxidative stress.
In Nature, fruit peel has the ability to protect the pulp from various types of environmental changes, including heat and oxidative stresses that might due to chemical and biological reactions. A laboratory test carried out by Sapitnitskaya et al. [24] showed that grapefruit peel tissues allow the fruit to respond adequately to chilling. Similarly, antioxidant substances found in bambangan peel enable to protect the pulp against environmental changes. These substances could be other types of carotenoids and polyphenolic compounds. Besides, the carotenoid content of the studied bambangan peel was lower than its pulp. The possible substances that might contribute to the protective effect could be xanthophyll and polyphenolic compounds, which were not determined. Moreover, Gil et al. [25] reported that the antioxidant activities of phenolic compounds were greater than those of carotenoids. A future study with more focus on polyphenolics and antioxidant capacity of bambangan peel is needed, which should also include various extraction methods.
4. Materials and Methods
4.1. Chemicals and Standards
Carotene standards (α- and β-carotene, HPLC grade), bovine serum albumin (BSA), 2,2-diphenyl-2-picrylhydrazyl (DPPH), 60% iodixanol (OptiPrep), trichloroacetic acid and thiobarbituric acid (for TBA assay), sodium azide, Tris-HCl buffer, phosphate buffer tablet and Hepes were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA); methanol (HPLC grade), dichloromethane (HPLC grade), isopropanol (HPLC grade) and n-hexane were purchased from Fisher Scientific (Waltham, MA, USA). Sodium chloride, sodium carbonate, sodium hydroxide, potassium sodium tartrate, copper (II) chloride (CuCl2), hexane (extraction solvent), Folin-Ciocalteu reagent (for protein determination) and hydrogen peroxide (H2O2) were obtained from Merck (Darmstadt, Germany); while HCl for TBA assay was purchased from Scharlau (Barcelona, Spain) and ethylenediaminetetraacetic acid (EDTA) was obtained from Calbiochem (EMD Chemicals, NJ, USA).
4.2. Extraction of Sample
Fresh bambangan fruits and information on their commercial ripening stage were obtained from Sarawak, Malaysia. The fruit was manually peeled to separate it from the pulp, and its kernel was removed. Then the pulp and peel were stored at −80 °C before freeze drying using a bench top freeze dryer (Virtis, NY, USA). Lyophilized samples were ground into fine particles using a grinder and sieved (particle size of 20 mesh). The sample powders were stored at −20 °C before extraction. Lyophilized pulp and peel (1.0 g) were mixed with hexane (20 mL). The mixtures were stirred using a Unimax 1010 DT shaking incubator (Heidolph, Schwabach, Germany). After 5 min, the hexane layers were separated from the residue and re-extracted again with hexane until the layer become colorless. The pooled hexane fraction was separated from the residue by centrifugation at 1,000 g for 10 min. The hexane was evaporated from the extract using a Büchi rotary evaporator (Flawil, Switzerland). The resulting extracts were completely dried by purging with nitrogen gas and then stored at −80 °C in amber bottles. The extracts were re-dissolved in dichloromethane and absolute methanol for HPLC and antioxidant analysis, respectively.
4.3. HPLC Determination of Carotenoids
Identification and quantification of carotenoids in bambangan peel and pulp extracts were carried out using the HPLC method as described by Tai and Chen [26] with some modifications. HPLC analysis was carried out using a Hewlett Packard (HP1100) system (Agilent Technologies, Palo Alto, CA, USA), coupled with diode array detector. A 150 × 4.6 mm, 3 µm C30 analytical column (Waters Co., Milford, MA, USA) was used in this analysis. The mobile phase consisted of methanol- dichloromethane-isopropanol (89:1:10, v/v/v). The carotenoid separation was performed by isocratic elution at a flow rate of 1.0 mL/min. The injection volume was 20 μL, with the column temperature at 25 °C. The detection was carried out at absorption of 453 nm.
4.4. Identification of Carotenoids
The carotenoid contents of bambangan peel and pulp were identified using the HPLC system software by comparing the retention time (RT) and absorption spectra of unknown peaks with reference standards. In addition, identification was done by spiking carotenoid standards into the samples. Furthermore, the cis-isomers of carotenoids were identified based on the spectral characteristics and Q-ratio values reported by previous literature [9,10]. Quantification was done by external standard calibration based on the peak area.
4.5. Preparation of Standard Curve and Recovery
Several concentrations of all-trans-α- and β-carotene solution (3–80 ppm) were injected into HPLC, and the linear regression equation for each standard curve was obtained. The regression equation and correlation coefficient (r2) were calculated using Microsoft Excel, and the r2 for all standards was more than 0.99. The limit of detection (LOD) and limit of quantification (LOQ) were measured based on a method described by Holcombe [27]. LOD and LOQ are referred to the smallest amount or concentration of a compound that can be estimated or quantified with acceptable reliability [28]. Three concentrations of α-carotene (2.9, 5.7, 20.3 ppm) and β-carotene (7.1, 22.2, 79.7 ppm) were prepared for the recovery tests.
4.6. Antioxidant Capacity
The antioxidant capacity of bambangan peel and pulp extracts were determined by chemical (2,2-diphenyl-2-picrylhydrazyl) and biological (hemoglobin and copper-induced oxidation) assays.
4.6.1. 2,2-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
The scavenging activity of bambangan peel and pulp extracts was performed based on DPPH radical scavenging assay as described by Lai et al. [29] with some modifications. The analysis was carried out by addition of carotenoid-rich sample extract obtained from peel and pulp of bambangan (0.5–2.0 ppm of β-carotene equivalents dissolved in methanol) to 0.1 M Tris-HCl buffer (0.8 mL, pH 7.4) and 0.5 mM DPPH solution (1.0 mL). The mixture was well shaken and kept for 20 min at room temperature. The absorbance was read at 517 nm against a blank. The percentage scavenging activity was calculated using the following equation:
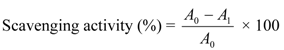 where A0 is the absorbance of the control, and A1 is the absorbance of the sample extract.
where A0 is the absorbance of the control, and A1 is the absorbance of the sample extract.
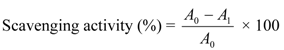
4.6.2. RBC preparation and LDL Isolation
Fasting venous blood (10 mL) from healthy volunteers (aged 20–30 years) were collected in EDTA tubes (0.4 g/L). As described by Chu and Liu [30], the tube was centrifuged at 1,600 g for 20 min at 15 °C to separate red blood cells (RBC) from the blood. The RBC was washed with phosphate buffer saline (PBS) three times. LDL was isolated from prepared plasma by sequential ultracentrifugation at 16 °C at 33,000 g for 3 h using an Optima L-100 K ultracentrifuge (Beckman, Brea, CA, USA) with slow acceleration to 305 g. The procedure was based on a method developed by Graham et al. [31] with some modifications. Plasma (4 mL) was mixed with 60% iodixanol (1 mL) and 4 mL was transferred to Optiseal tube. The mixture was overlaid with 20% of iodixanol before the tube was filled up with Hepes-buffer saline. After ultracentrifugation, LDL-containing fractions were removed from the lipoprotein layer, which can be seen as orange-brownish colored layer. The protein content of LDL-containing fraction was determined by Lowry’s method [32] using bovine serum albumin as the standard.
4.6.3. Copper-induced LDL Oxidation
Low density lipoprotein (LDL) oxidation was carried out based on a method described by Tsoukatos et al. [33] with some modifications. Pooled LDL-containing fractions were suspended in PBS (1.7 mL, pH 7.4) in a final volume of 2 mL containing 80 g/L LDL protein and 4 µmol/L CuCl2, and incubated at 37 °C for 3 h with and without addition of carotenoid-rich sample extracts (0.1, 1 and 2 ppm of of β-carotene equivalents in methanol, 0.1 mL). The control analysis was performed without addition of CuCl2 and sample extract. The oxidation was terminated by addition of EDTA (0.01%, final concentration). Malondialdehyde (MDA) produced from LDL oxidation was measured using TBA assay as described by Buege and Aust [34].
4.6.4. Hemoglobin Oxidation Assay
Hemoglobin oxidation was performed using a method previously described by Rodríguez et al. [35] with slight modifications. The experiment was carried out within a day of blood withdrawal. The red blood cells (RBCs) were gently re-suspended with PBS to obtain 5% of RBC and pre-incubated at 37 °C for 10 min in the presence of 1 mmol/L sodium azide (to inhibit microbial growth). Subsequently, 1.6 mL of RBC was transferred to test tube for oxidation analysis. All test tubes except control tube were added with 10 mmol/L of H2O2 and with or without the addition of carotenoid-rich sample extract (0.1, 1 and 2 ppm of of β-carotene equivalents in methanol, 0.2 mL). After 60 min incubation at 37 °C, the mixture was kept for 60 sec in an ice bath and centrifuged at 1853g for 10 min at 4 °C. The MDA equivalents were measured using TBA assay as described before.
4.7. Statistical Analysis
Data were presented as mean ± standard deviation of three determinations. Data were subjected to independent sample t-test and analysis of variance (ANOVA). The percentage of scavenging activity was compared between the bambangan peel and pulp extracts, and with standard (β-carotene) for chemical assay. The mean values of MDA produced were compared between the sample extracts and control. The significant level was set at p < 0.05. SPSS for Windows version 15.0 was used for statistical analysis.
5. Conclusions
This study examined the carotenoid content and antioxidant capacity of bambangan peel and pulp. The result has shown that bambangan peel extract had an acceptable level of antioxidant capacity in the studied chemical and biological assays. The evidence from this study suggests that carotenoid-rich bambangan peel can be a potential functional food. Further investigations are needed to determine other polyphenolic compounds in bambangan peel and pulp, and their corresponding biological activity.
Acknowledgements
The authors would like to acknowledge Universiti Putra Malaysia for financial support under the Research University Grant Scheme (RUGS) (Project No: 02-01-09-0703RU). We are grateful to the Department of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia for laboratory facilities to carry out this study.
- Samples Availability: Samples of the compounds (carotenoids mixture) are available from the authors.
References
- Ajila, C.M.; Prasada Rao, U.J.S. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem. Toxicol. 2008, 46, 303–309. [Google Scholar] [CrossRef]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophy. Acta 1997, 1336, 575–586. [Google Scholar]
- Mayne, S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB Journal 1996, 10, 690–701. [Google Scholar]
- Tavanil, A.; La Vecchia, C. β-carotene and risk of coronary heart disease. A review of observational and intervention studies. Biomed. Pharmacotherap. 1999, 53, 409–416. [Google Scholar] [CrossRef]
- ESA. Carotenoid Isomers; ESA Application Note, 5600A. ESA Inc: Chelmsford, MA, USA, 2009. Available online: http://www.esainc.com/docs/spool/70-4927P_Carotenoid_Isomers.pdf/, accessed on 7 October 2009.
- Idris, K.; Idris, S. Nutrient composition of Malaysian indigenous fruits; Abstract of the Nutrition Society of Malaysia 18th Scientific Conference. Kuala Lumpur, Malaysia; 22–23 March 2003. [Google Scholar]
- Khoo, H.E.; Ismail, A. Determination of daidzein and genistein contents in Mangifera fruits. Mal. J. Nutr. 2008, 14, 189–198. [Google Scholar]
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Chen, J.P.; Tai, C.Y.; Chen, B.H. Improved liquid chromatographic method for determination of carotenoids in Taiwanese mango (Mangifera indica L.). J. Chromatogr. A 2004, 1054, 261–268. [Google Scholar]
- Rodriguez-Amaya, D.B.; Kimura, M. Harvest Plus Technical Monograph Series 2. In HarvestPlus Handbook for Carotenoid Analysis; International Food Policy Research Institute and International Center for Tropical Agriculture: Washington, DC, USA, 2004. [Google Scholar]
- Jimenez-Escrig, A.; Jimenez-Jimenez, I.; Sanchez-Moreno, C.; Saura-Calixt, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agr. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J. Agric. Food Chem. 2002, 40, 431–434. [Google Scholar]
- El-Demerdash, F.M.; Yousef, M.I.; Kedwany, F.S.; Baghdadi, H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and b-carotene. Food Chem. Toxicol. 2004, 42, 1563–1571. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; International Life Sciences Institute (ILSI) Press: Washington, DC, USA, 2001. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Filipe, P.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Santos, A.; Patterson, L.K.; Silva, J. N.; Cavaleiro, J.A.S.; Freitas, J.P.; Mazière, J.-C.; Santus, R.; Morlière, P. The alkyl chain length of 3-alkyl-3’,4’,5,7-tetrahydroxyflavones modulates effective inhibition of oxidative damage in biological systems: Illustration with LDL, red blood cells and human skin keratinocytes. Biochem. Pharmacol. 2009, 77, 957–964. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Trombino, S.; Lauriola, L.; Ranelletti, F.O.; Calviello, G. Dual role of β-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: Dependence on pO2. Carcinogenesis 2006, 27, 2383–2391. [Google Scholar] [CrossRef]
- Khoo, H.E.; Ismail, A.; Mohd-Esa, N.; Idris, S. Carotenoid content of underutilized fruits. Plant Foods Human Nutri. 2008, 63, 170–175. [Google Scholar] [CrossRef]
- Princen, H.M.; van Poppel, G.; Vogelezang, C.; Buytenhek, R.; Kok, F.J. Supplementation with vitamin E but not beta-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro. Effect of cigarette smoking. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 554–562. [Google Scholar]
- Lau, B.H.S. Suppression of LDL Oxidation by Garlic. J. Nutr. 2001, 131, 985S–988S. [Google Scholar]
- Heinecke, J.W. Lipoprotein oxidation in cardiovascular disease: Chief culprit or innocent bystander? J. Exp. Med. 2006, 203, 813–816. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zheng, T. The in vitro antioxidant activity of lotus germ oil from supercritical fluid carbon dioxide extraction. Food Chem. 2009, 115, 939–944. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Prasada Rao, U.J.S. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Sapitnitskaya, M.; Maul, P.; McCollum, G.T.; Guy, C.L.; Weiss, B.; Samach, A.; Porat, R. Postharvest heat and conditioning treatments activate different molecular responses and reduce chilling injuries in grapefruit. J. Exp. Botany 2006, 57, 2943–2953. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of Daylily (Hemerocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2001, 48, 5962–5968. [Google Scholar]
- Holcombe, D. The fitness for purpose of analytical methods. In EURACHEM Guide; EURACHEM Working Group: Prague, Czech Republic, 1998. [Google Scholar]
- AOAC. Method Validation. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals, AOAC International, Maryland, USA; 2002. Available online: http://www.aoac.org/vmeth/page1.htm/, accessed on 10 September 2009.
- Lai, L.S.; Chou, S.T.; Chao, W.W. Studies on the antioxidative activities of hsian-tsao (Mesona procumbens Hemsl) leaf gum. J. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [CrossRef]
- Chu, Y.F.; Liu, R.H. Cranberries inhibit LDL oxidation and induce LDL receptor expression in hepatocytes. Life Sci. 2005, 77, 1892–1901. [Google Scholar] [CrossRef]
- Graham, J.M.; Higgins, J.A.; Gillott, T.; Taylor, T.; Wilkinson, J.; Ford, T.; Billington, D. A novel method for the rapid separation of plasma lipoproteins using self-generating gradients of iodixanol. Atherosclerosis 1996, 124, 125–135. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Tsoukatos, D.C.; Arborati, M.; Liapikos, T.; Clay, K.L.; Murphy, R.C.; Chapman, M.J.; Ninio, E. Copper-Catalyzed Oxidation Mediates PAF Formation in Human LDL Subspecies. Arter. Thromb. Vasc. Biol. 1997, 17, 3505–3512. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymology 1978, 52, 302–310. [Google Scholar]
- Rodríguez, J.; Di Pierro, D.; Gioia, M.; Monaco, S.; Delgado, R.; Coletta, M.; Marini, S. Effects of a natural extract from Mangifera indica L, and its active compound, mangiferin, on energy state and lipid peroxidation of red blood cells. Biochim. Biophy. Acta 2006, 1760, 1333–1342. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).