Elucidating the Structure-Activity Relationships of the Vasorelaxation and Antioxidation Properties of Thionicotinic Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
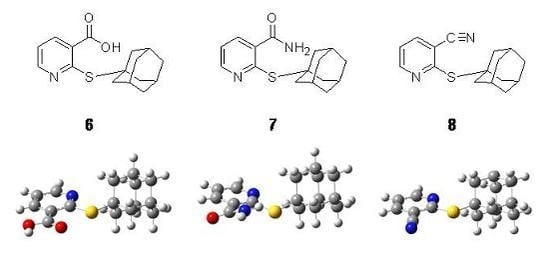
2.1. Tested compounds
2.2. Vasorelaxant activity
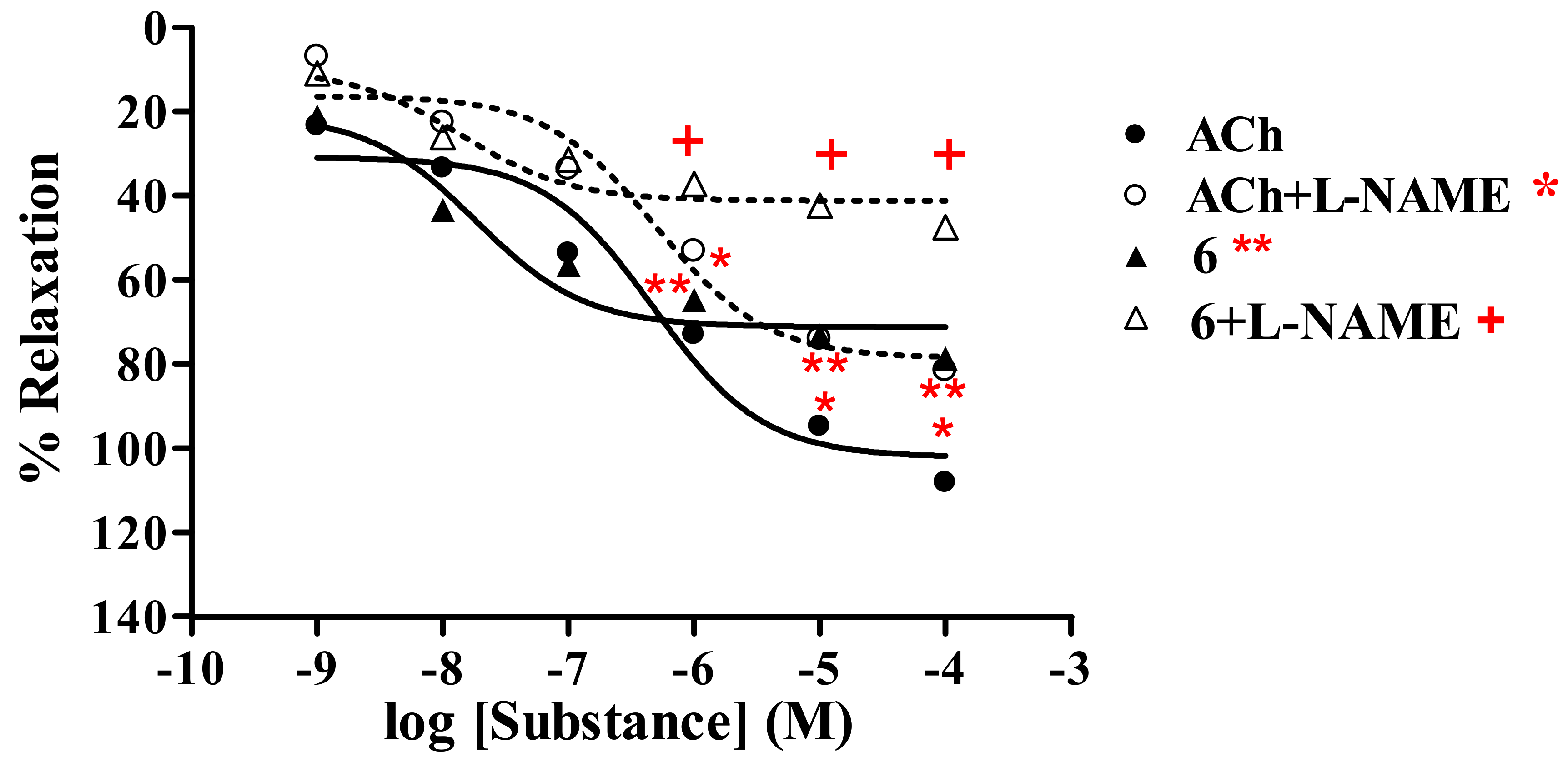
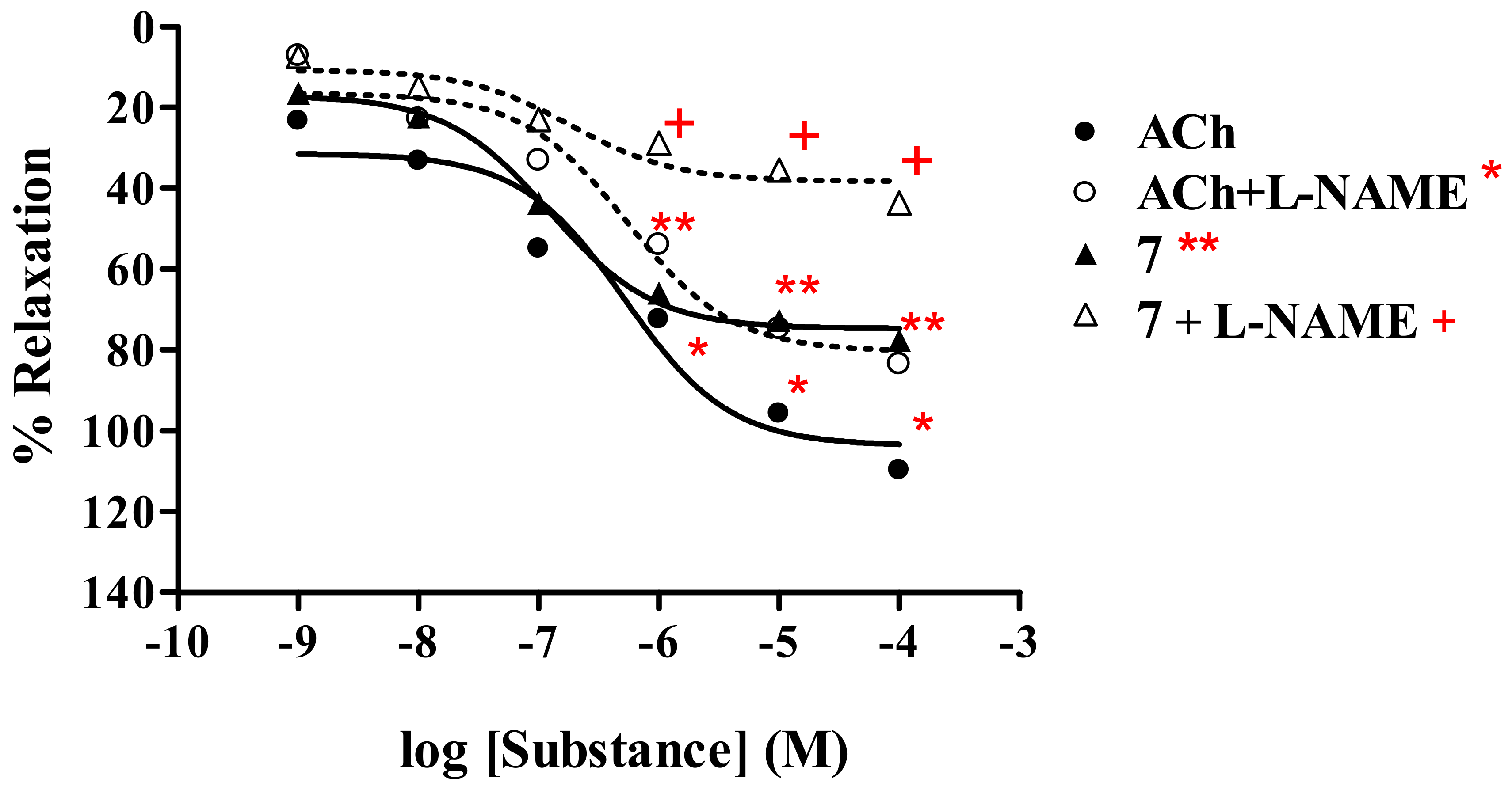
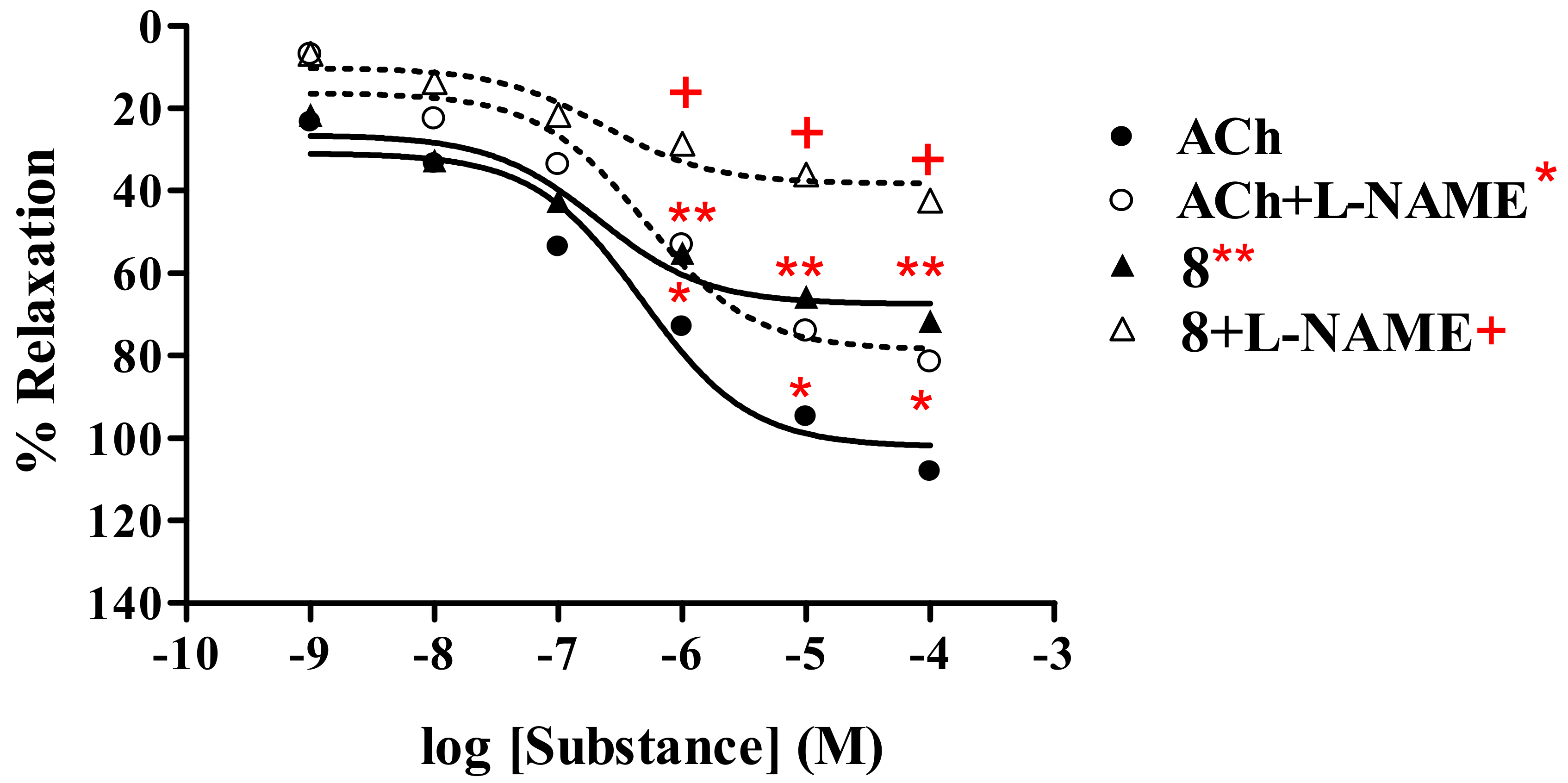
2.2.1. Effect of thionicotinic acid derivatives 6-8 on the vascular function of rat thoracic aorta in the presence and absence of L-NAME.
Thionicotinic acid 6
Thionicotinamide analog 7
Thionicotinonitrile analog 8
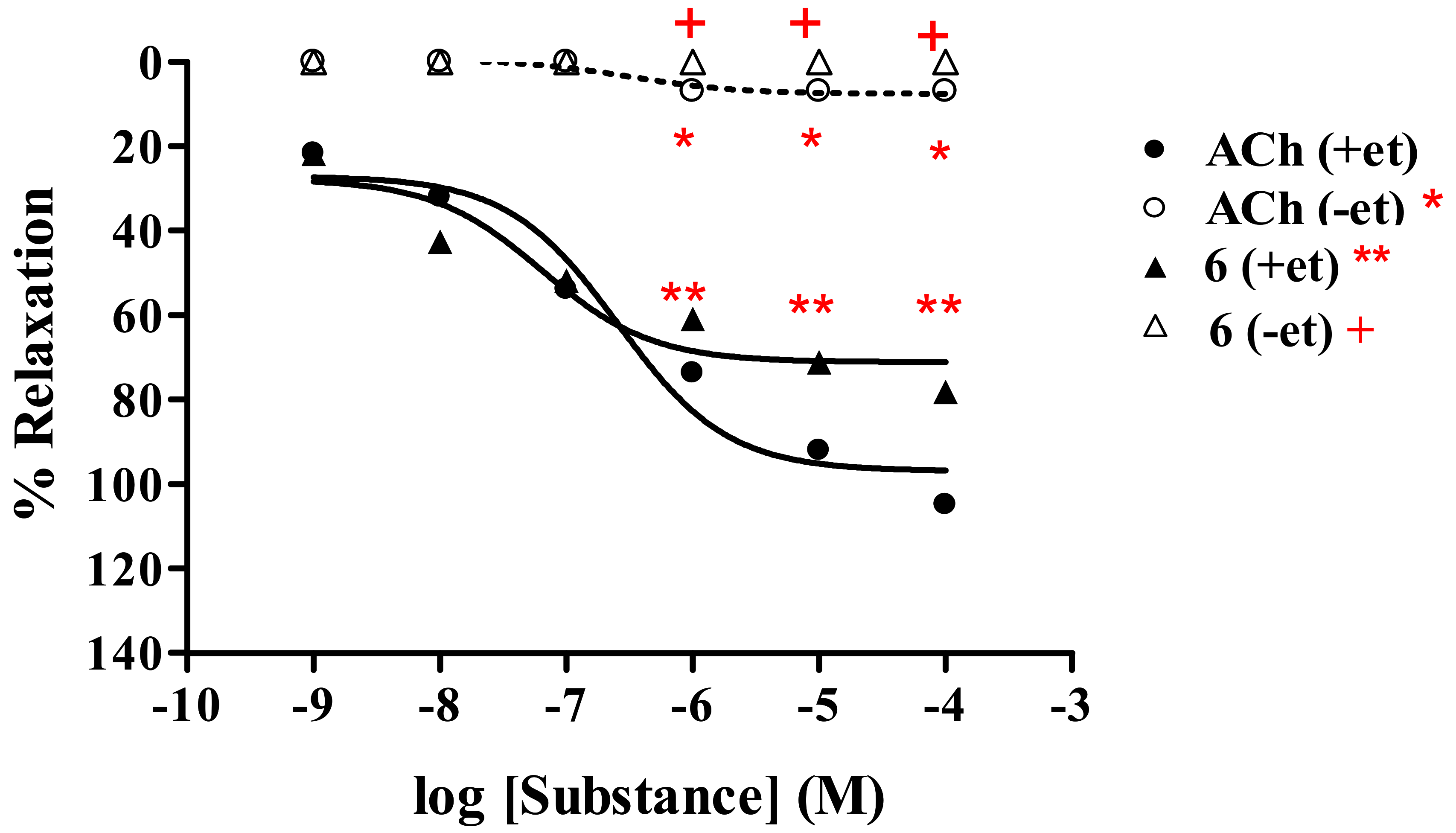
2.2.2. Effect of endothelial cells on vasorelaxant activity of thionicotinic acid derivatives 6-8
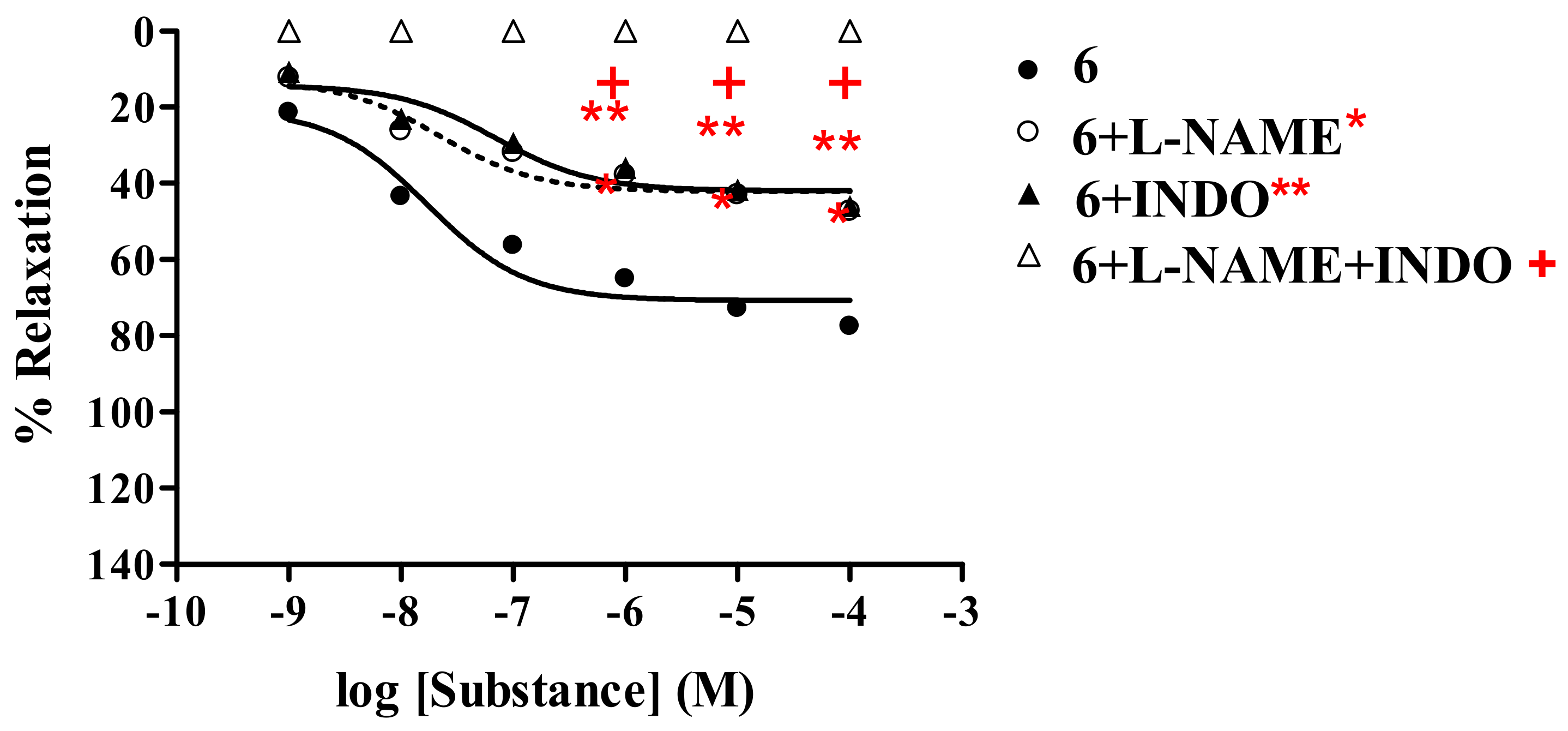
2.2.3. Effect of derivatives 6-8 on the vascular function of rat thoracic aorta in the presence of cyclo-oxygenase inhibitor (INDO)
2.3. Antioxidative activity
2.4. Molecular modeling of vasorelaxant and antioxidative activities
3. Conclusions
4. Experimental
4.1. General
4.2. Tested compounds 6-8
4.3. Vasorelaxant assay
4.3.1. Isometric tension measurements
4.3.2. Statistical analyses
4.4. Antioxidative assay
4.5. Molecular modeling analysis
Acknowledgments
References
- Offermanns, H.; Kleemann, A.; Tanner, H.; Beschke, H.; Friedrich, H. Vitamins, nicotinamide and nicotinic acid (B3). In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Grayson, M., Eckroth, D., Eds.; Wiley: New York, N.Y., USA, 1983; Volume 24, p. 59. [Google Scholar]
- Lai, E.; De Lepeleire, I.; Crumley, T.M.; Liu, F.; Wenning, L.A.; Michiels, N.; Vets, E.; O’Neill, G.; Wagner, J.A.; Gottesdiener, K. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin. Pharmacol. Ther. 2007, 81, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Parsons, W.G.; Roberts, L.J. Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins 1989, 38, 263–274. [Google Scholar] [CrossRef]
- Gille, A.; Bodor, E.T.; Ahmed, K.; Offermanns, S. Nicotinic acid: Pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Nandy, P. Vasodilatory effect of nicotinamide on the fluidity of erythrocyte membrane and liposomes. J. Surf. Sci. Technol. 1989, 5, 13–20. [Google Scholar]
- Ruddock, M.W.; Hirst, D.G. Nicotinamide relaxes vascular smooth muscle by inhibiting myosin light chain kinase-dependent signaling pathways: implications for anticancer efficacy. Oncol. Res. 2004, 14, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Christensen, F.K. Topical agent for application to the skin prior to luminous treatment. US Pat. 2004191278, 2004. [Chem. Abstr. 2004, 141, 282850]. [Google Scholar]
- Walling, D.W.; Vatter, M.L. Cosmetic compositions containing vitamin B3. US Pat. 6455055, 2002. [Chem. Abstr. 2002, 137, 252724]. [Google Scholar]
- Bodor, E.T.; Offermanns, S. Nicotinic acid: an old drug with a promising future. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S68–S75. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.-P.; Schroeder, V. Nicorandil-Review of pharmacological properties and clinical applications. Heart Drug 2005, 5, 220–229. [Google Scholar] [CrossRef]
- Trcka, V.; Vejdelek, Z.J. Vasodilating action of several series of pyridine derivatives. Pharmazie 1956, 11, 242–247. [Google Scholar] [PubMed]
- Zhou, Z.; Walsh, M.; Hramiec, J. Nicotinamide adenine dinucleotide induces relaxation in vitro rat aortic rings by acting on adenosine receptors. Surg. Forum 1999, 50, 479–481. [Google Scholar]
- Finch, N.; Campbell, T.R.; Gemenden, C.W.; Antonaccio, M.J.; Polvaski, H.J. Synthesis and antihypertensive action of 5-thio-2-pyridinecarboxylic acid derivatives. J. Med. Chem. 1987, 21, 1269–1274. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Buer, L. The deoxydative substitution reactions of nicotinamide and nicotinic acid N-oxides by 1-adamantanethiol in acetic anhydride. J. Heterocyclic Chem. 1985, 22, 771–775. [Google Scholar] [CrossRef]
- Quignard, J.F.; Félétou, M.; Thollon, C.; Vilaine, J.P.; Duhault, J.; Vanhoutte, P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; J., M.; Encabo, A.; Alonso, M.J.; Balfagón, G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen. Pharmacol. 1999, 33, 35–41. [Google Scholar] [CrossRef]
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Eklund, B.; Kaijser, L.; Nowak, J.; Wennmalm, A. Prostaglandins contribute to the vasodilation induced by nicotinic acid. Prostaglandins 1979, 17, 821–830. [Google Scholar] [CrossRef]
- Kaijser, L.; Eklund, B.; Olsson, A.G.; Carlson, L.A. Dissociation of the effects of nicotinic acid on vasodilatation and lipolysis by a prostaglandin synthesis inhibitor, indomethacin, in man. Med. Biol. 1979, 57, 114–117. [Google Scholar] [PubMed]
- Zardi, E.M.; Dobrina, A.; Amoroso, A.; Afeltra, A. Prostacyclin in liver disease: a potential therapeutic option. Expert Opin. Biol. Ther. 2007, 7, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Arana, S.; Caceres, A.; di Villa Bianca, R.; Sorrentino, R.; Rastrelli, L. New lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities. J. Nat. Prod. 2004, 67, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Meeker, W.F.; Boujaoude, M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure–activity relationships. J. Cardiovasc. Pharm. 2005, 46, 302–309. [Google Scholar] [CrossRef]
- Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Naenna, T.; Prachayasittikul, V. Quantitative structure-imprinting factor relationship of molecularly imprinted polymers. Biosens. Bioelectron. 2007, 22, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Tansila, N.; Naenna, T.; Prachayasittikul, V. Prediction of GFP spectral properties using artificial neural network. J. Comput. Chem. 2007, 28, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Naenna, T.; Isarankura Na-Ayudhya, C.; Prachayasittikul, V. Quantitative prediction of imprinting factor of molecularly imprinted polymers by artificial neural network. J. Comput. Aid. Mol. Des. 2005, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Piacham, T.; Tantimongcolwat, T.; Naenna, T.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. QSAR model of the quorum-quenching N-acyl-homoserine lactone lactonase activity. J. Biol. Syst. 2008, 16, 279–293. [Google Scholar] [CrossRef]
- Piacham, T.; Isarankura-Na-Ayudhya, C.; Nantasenamat, C.; Yainoy, S.; Ye, L.; Bülow, L.; Prachayasittikul, V. Metalloantibiotic Mn(II)-bacitracin complex mimicking manganese superoxide dismutase. Biochem. Biophys. Res. Commun. 2006, 341, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Thippakorn, C.; Suksrichavalit, T.; Nantasenamat, C.; Tantimongcolwat, T.; Isarankura-Na-Ayudhya, C.; Naenna, T.; Prachayasittikul, V. Modeling the LPS neutralization activity of anti-endotoxins. Molecules 2009, 14, 1869–1888. [Google Scholar] [CrossRef] [PubMed]
- Worachartcheewan, A.; Nantasenamat, C.; Naenna, T.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Modeling the activity of furin inhibitors using artificial neural network. Eur. J. Med. Chem. 2009, 44, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Absolute electronegativity and hardness: applications to organic chemistry. J. Org. Chem. 2002, 54, 1423–1430. [Google Scholar] [CrossRef]
- Zhou, Z.; Parr, R.G. Activation hardness: new index for describing the orientation of electrophilic aromatic substitution. J. Am. Chem. Soc. 2002, 112, 5720–5724. [Google Scholar] [CrossRef]
- Faust, W.L. Explosive Molecular Ionic Crystals. Science 1989, 245, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.L.; Nordblom, G.D.; Mayeda, E.A. Simple, comprehensive correlation of organic oxidation and ionization potentials. J. Org. Chem. 2002, 37, 916–918. [Google Scholar] [CrossRef]
- Politzer, P.; Abu-Awwad, F.; Murray, J.S. Comparison of density functional and Hartree-Fock average local ionization energies on molecular surfaces. Int. J. Quantum Chem. 1998, 69, 607–613. [Google Scholar] [CrossRef]
- Zhan, C.-G.; Nichols, J.A.; Dixon, D.A. Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: Molecular properties from density functional theory orbital energies. J. Phys. Chem. A 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Migliavacca, E.; Carrupt, P.-A.; Testa, B. Theoretical parameters to characterize antioxidants. Part 1. The case of vitamin E and analogs. Helv. Chim. Acta 1997, 80, 1613–1626. [Google Scholar] [CrossRef]
- Mohajeri, A.; Asemani, S.S. Theoretical investigation on antioxidant activity of vitamins and phenolic acids for designing a novel antioxidant. J. Mol. Struct. 2009, 930, 15–20. [Google Scholar] [CrossRef]
- Chen, W.; Song, J.; Guo, P.; Cao, W.; Bian, J. Exploring a possible way to synthesize novel better antioxidants based on vitamin E: A DFT study. Bioorg. Med. Chem. Lett. 2006, 16, 5874–5877. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Naenna, T.; Prachayasittikul, V. Prediction of bond dissociation enthalpy of antioxidant phenols by support vector machine. J. Mol. Graph. Model. 2008, 27, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Suksrichavalit, T.; Prachayasittikul, S.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Suksrichavalit, T.; Prachayasittikul, S.; Piacham, T.; Isarankura-Na-Ayudhya, C.; Nantasenamat, C.; Prachayasittikul, V. Copper complexes of nicotinic-aromatic carboxylic acids as superoxide dismutase mimetics. Molecules 2008, 13, 3040–3056. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Wongsawatkul, O.; Sobey, C.G. Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries. Clin. Exp. Pharmacol. Physiol. 2000, 27, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and Antioxidant Activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724–2744. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Suksrichavalit, T.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of 1-adamantylthio derivatives of 3-substituted pyridines. EXCLI. J. 2008, 7, 63–70. [Google Scholar]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R., II; Keith, T.; Millam, J.; Eppinnett, K.; Hovell, W.L.; Gilliland, R. GaussView, Version 3.09; Semichem, Inc.: Shawnee Mission, KS, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03; Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
Sample Availability: Contact the authors. |
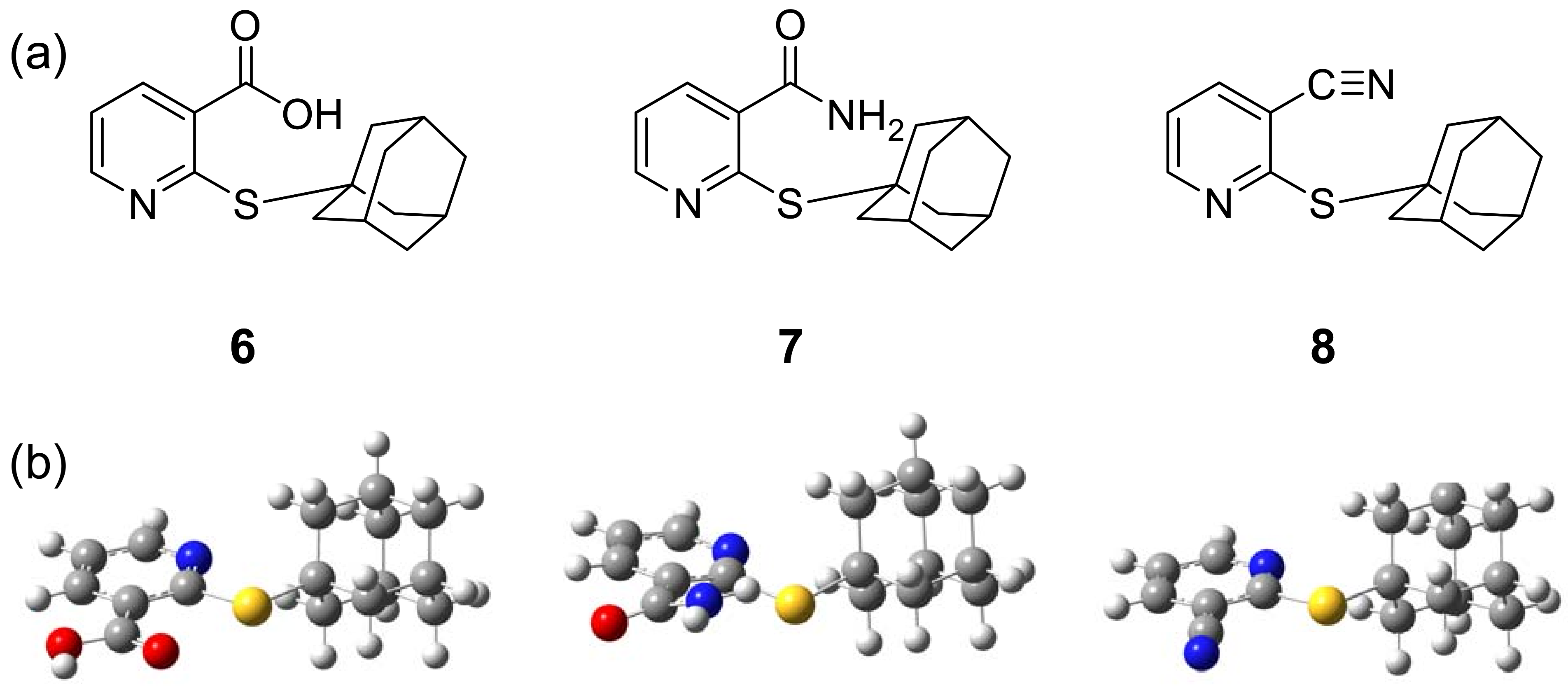
| Compound | Vasorelaxant activity | ||||
|---|---|---|---|---|---|
| Without L-NAME | With L-NAME (1mM) | ||||
| Rmax (%) | ED50 (M) | Rmax (%) | ED50 (M) | ||
| 6a | 78.67 ± 0.48 | 2.13 × 10-8 | 47.60 ± 0.83 | 2.50 × 10-8 | |
| ACha | 108.17 ± 1.22 | 4.72 × 10-7 | 81.59 ± 0.63 | 4.92 × 10-7 | |
| 7b | 77.69 ± 0.47 | 1.25 × 10-7 | 43.72 ± 0.70 | 2.66 × 10-7 | |
| AChb | 109.86 ± 0.65 | 5.29 × 10-7 | 83.54 ± 0.91 | 5.49 × 10-7 | |
| 8a | 71.64 ± 0.55 | 2.44 × 10-7 | 42.36 ± 0.98 | 3.05 × 10-7 | |
| ACha | 108.17 ± 1.22 | 4.72 × 10-7 | 81.59 ± 0.27 | 4.92 × 10-7 | |
| Compound | Vasorelaxant activity | ||||
|---|---|---|---|---|---|
| +et a | −et b | ||||
| Rmax (%) | ED50 (M) | Rmax (%) | ED50 (M) | ||
| 6c | 78.18 ± 0.79 | 6.64 × 10-8 | 0 | − | |
| AChc | 104.89 ± 1.33 | 2.66 × 10-7 | 7.11 ± 0.35 | 3.13 × 10-7 | |
| 7d | 75.19 ± 0.59 | 1.05 × 10-7 | 0 | − | |
| AChd | 103.45 ± 1.12 | 3.35 × 10-7 | 7.47 ± 0.15 | 3.13 × 10-7 | |
| 8d | 71.92 ± 0.52 | 3.82 × 10-7 | 0 | − | |
| AChd | 103.45 ± 1.12 | 3.35 × 10-7 | 7.47 ± 0.15 | 3.13 × 10-7 | |
| Compound | Vasorelaxant activity | |||||||
|---|---|---|---|---|---|---|---|---|
| −Inhibitora | +L-NAME (1mM) | +INDO (1mM) | +L-NAME (1mM) +INDO (1mM) | |||||
| Rmax (%) | ED50 (M) | Rmax (%) | ED50 (M) | Rmax (%) | ED50 (M) | Rmax (%) | ED50 (M) | |
| AChb | 121.7 ± 1.44 | 9.99 × 10-7 | 81.34 ± 0.77 | 5.44 × 10-7 | 68.78 ± 0.92 | 4.58 × 10-7 | 0 | − |
| 6b | 77.67±0.66 | 1.78 × 10-8 | 47.44 ± 0.44 | 3.55 × 10-8 | 46.05 ± 0.26 | 7.17 × 10-8 | 0 | − |
| 7b | 76.93±0.56 | 1.23 × 10-7 | 43.22 ± 0.66 | 3.88 × 10-7 | 36.49 ± 0.60 | 6.34 × 10-7 | 0 | − |
| 8b | 71.47±0.42 | 2.05 × 10-7 | 42.10 ± 0.65 | 3.43 × 10-7 | 37.63 ± 0.62 | 6.14 × 10-7 | 0 | − |
| SNPc | 120.81±1.18 | 3.16 × 10-7 | 116.70 ± 1.30 | 3.17 × 10-7 | 112.93 ± 0.61 | 3.16 × 10-7 | 104.98±1.41 | 3.17×10-7 |
| Compound | % DPPH radical scavenging activitya | % NBT inhibitionb |
|---|---|---|
| 6 | 33.20 | 15.40 |
| 7 | 0.57 | 15.45 |
| 8 | 0.30 | 17.31 |
| Compound | Dipole moment (Debye) | IP (eV) | HOMO-LUMO gap (eV) | ED50 (M) |
|---|---|---|---|---|
| 6 | 2.4098 | 0.2184 | 0.1605 | 2.13×10-8 |
| 7 | 3.6920 | 0.2265 | 0.1837 | 1.25×10-7 |
| 8 | 4.8092 | 0.2285 | 0.1669 | 2.44×10-7 |
| ACh | 13.1100 | 0.3976 | 0.2800 | 4.72×10-7 |
| Correlation with ED50 | 0.9594 | 0.9038 | 0.8879 |
| Compound | Dipole moment (Debye) | IP (eV) | HOMO-LUMO gap (eV) | DPPH (%) | NBT (%) |
|---|---|---|---|---|---|
| 6 | 2.4098 | 0.2184 | 0.1605 | 33.20 | 15.40 |
| 7 | 3.6920 | 0.2265 | 0.1837 | 0.57 | 15.45 |
| 8 | 4.8092 | 0.2285 | 0.1669 | 0.30 | 17.31 |
| Correlation with DPPH | -0.8885 | -0.9826 | -0.7086 | ||
| Correlation with NBT | 0.8575 | 0.6748 | -0.2276 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prachayasittikul, S.; Wongsawatkul, O.; Worachartcheewan, A.; Nantasenamat, C.; Ruchirawat, S.; Prachayasittikul, V. Elucidating the Structure-Activity Relationships of the Vasorelaxation and Antioxidation Properties of Thionicotinic Acid Derivatives. Molecules 2010, 15, 198-214. https://doi.org/10.3390/molecules15010198
Prachayasittikul S, Wongsawatkul O, Worachartcheewan A, Nantasenamat C, Ruchirawat S, Prachayasittikul V. Elucidating the Structure-Activity Relationships of the Vasorelaxation and Antioxidation Properties of Thionicotinic Acid Derivatives. Molecules. 2010; 15(1):198-214. https://doi.org/10.3390/molecules15010198
Chicago/Turabian StylePrachayasittikul, Supaluk, Orapin Wongsawatkul, Apilak Worachartcheewan, Chanin Nantasenamat, Somsak Ruchirawat, and Virapong Prachayasittikul. 2010. "Elucidating the Structure-Activity Relationships of the Vasorelaxation and Antioxidation Properties of Thionicotinic Acid Derivatives" Molecules 15, no. 1: 198-214. https://doi.org/10.3390/molecules15010198
APA StylePrachayasittikul, S., Wongsawatkul, O., Worachartcheewan, A., Nantasenamat, C., Ruchirawat, S., & Prachayasittikul, V. (2010). Elucidating the Structure-Activity Relationships of the Vasorelaxation and Antioxidation Properties of Thionicotinic Acid Derivatives. Molecules, 15(1), 198-214. https://doi.org/10.3390/molecules15010198