Determination of Coenzyme A (CoASH) in the Presence of Different Thiols by Using Flow-Injection with a UV/Vis Spectrophotometric Detector and Potentiometric Determination of CoASH Using an Iodide ISE
Abstract
:1. Introduction
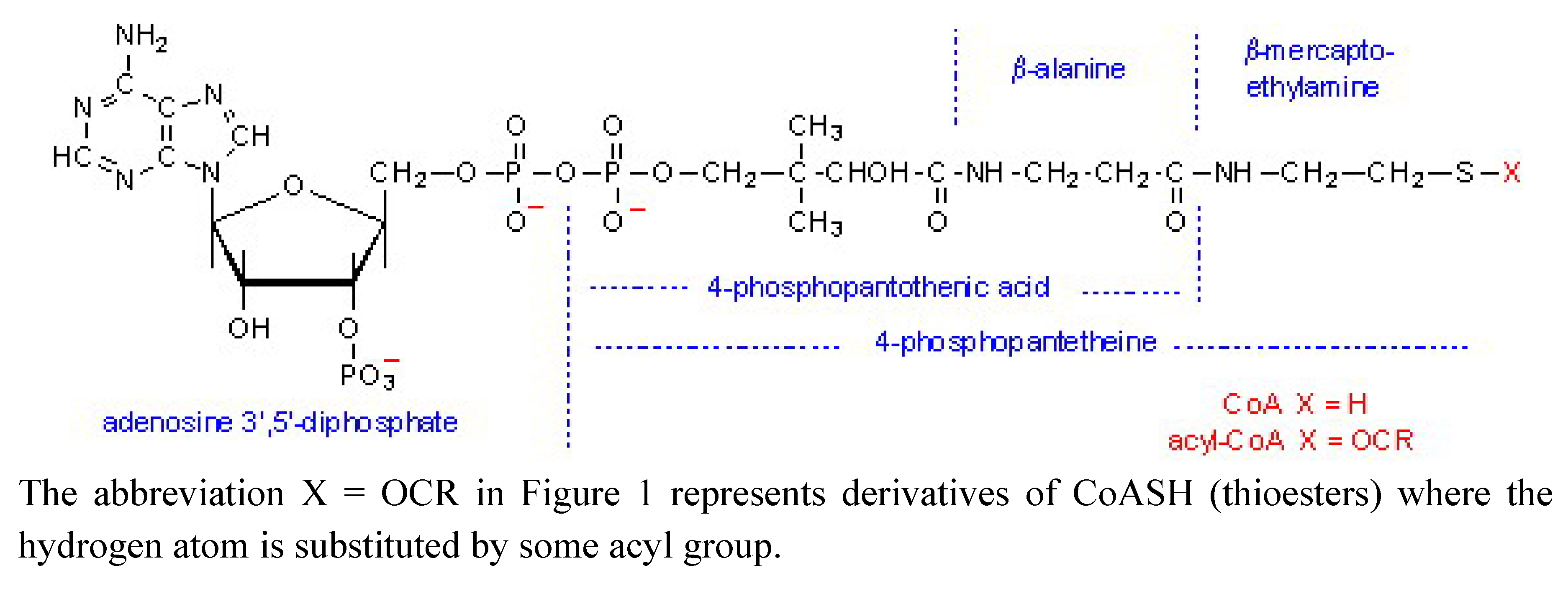
2. Results and Discussion
2.1. Flow-injection determination of CoASH using a potentiometric detector
2.2. Flow-injection system optimization
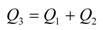
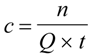
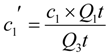
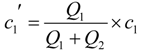
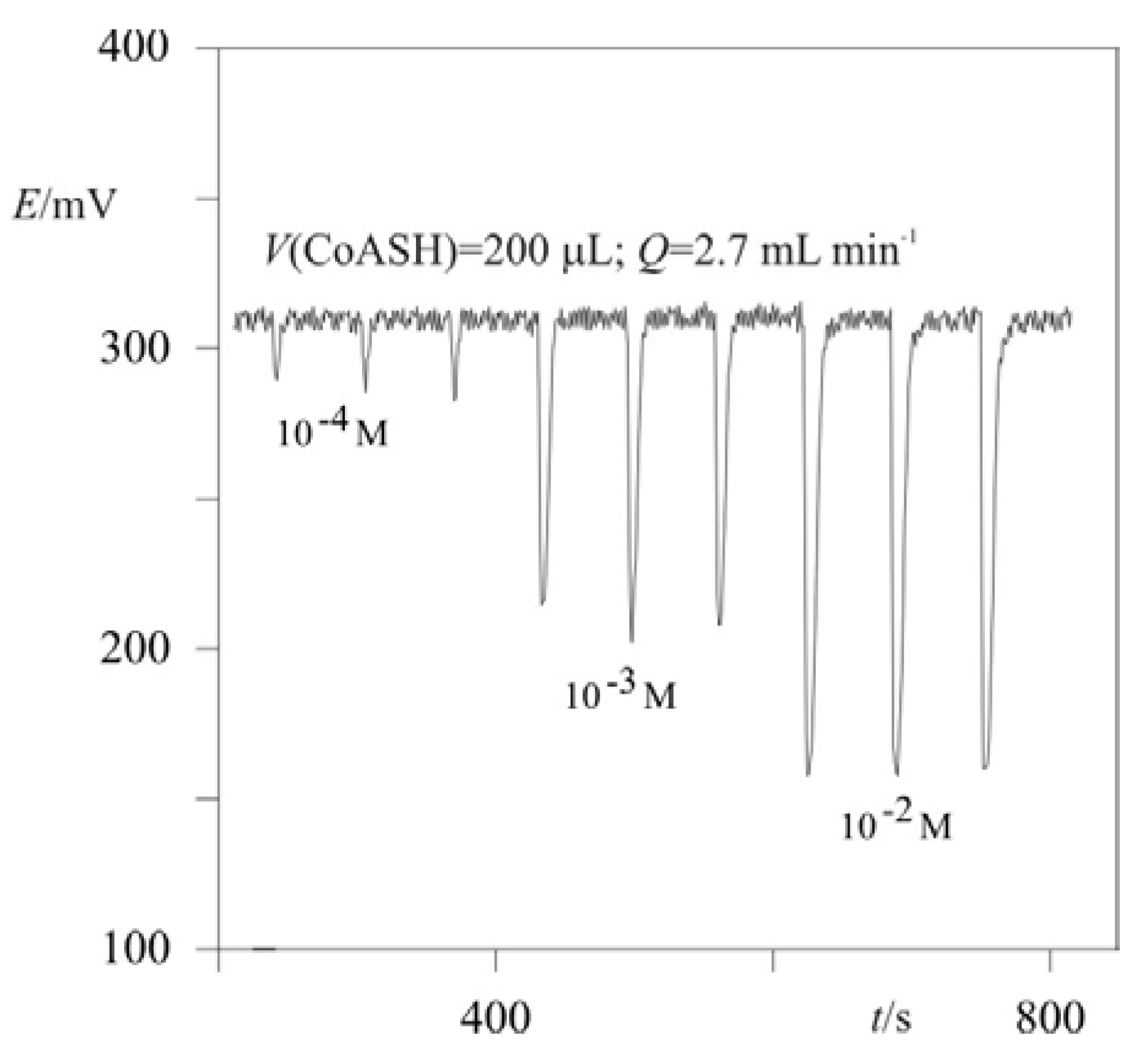
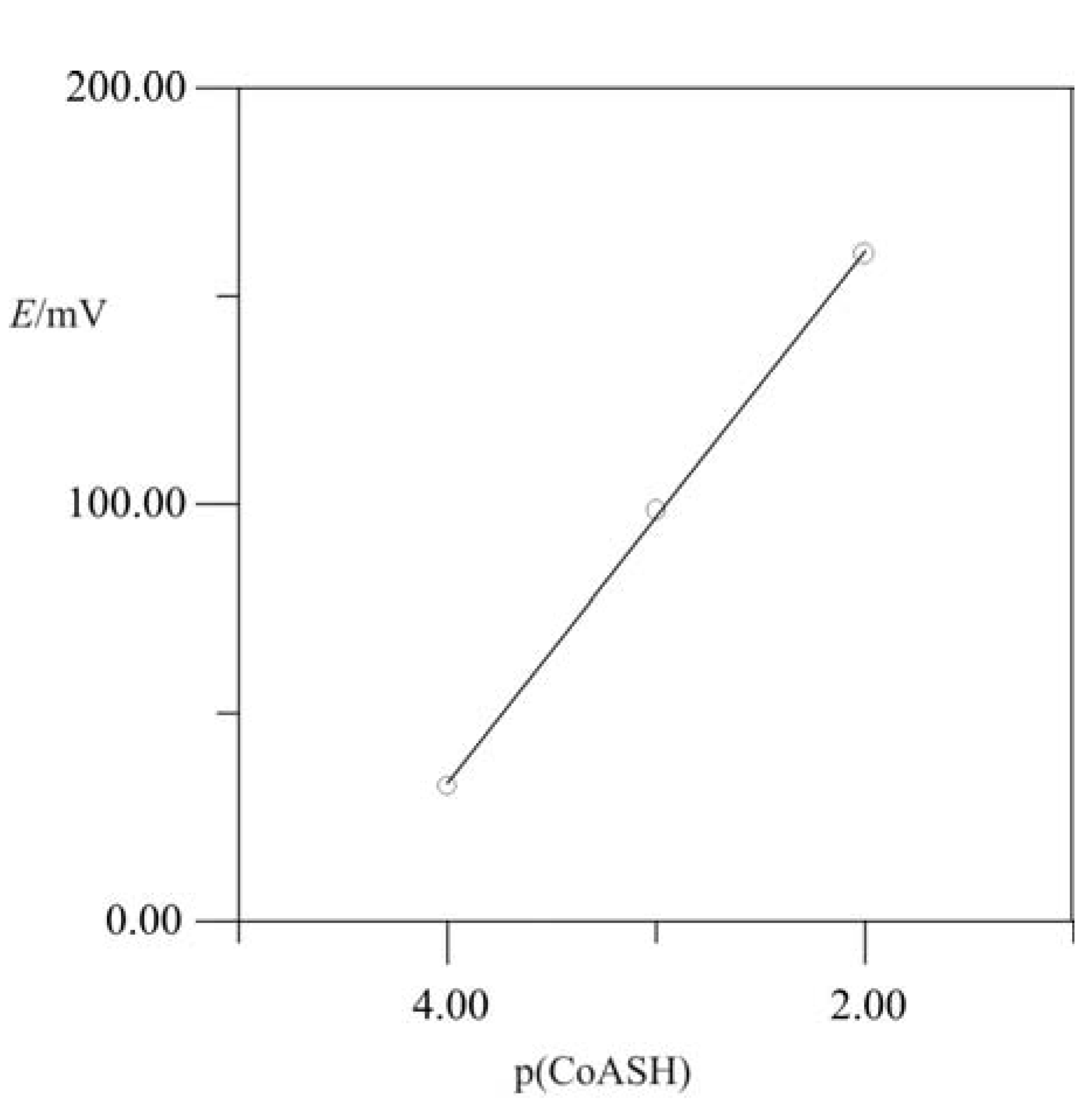
2.3. “Classic” (Batch experiment) potentiometric determination of CoASH
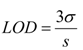
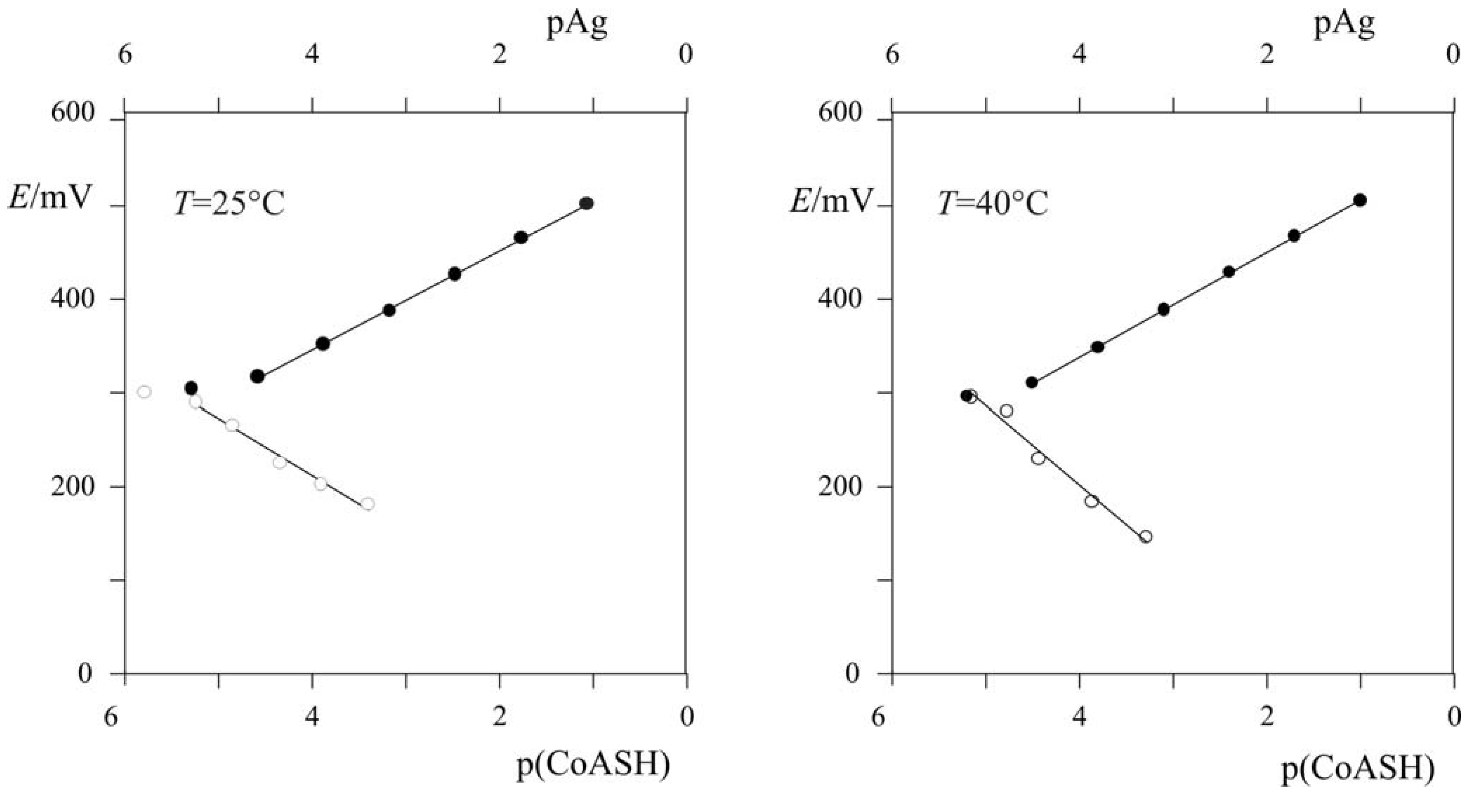
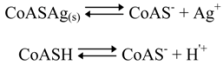
2.3.1. Calculating Ksp(CoAS-Ag)
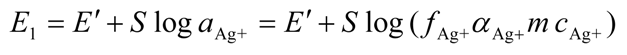
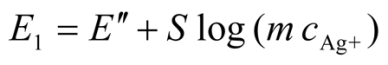
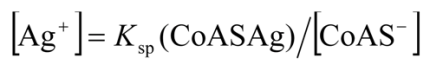
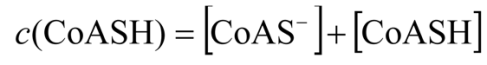
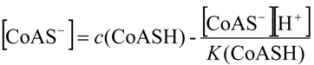
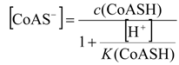
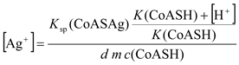
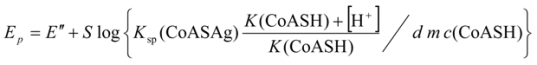
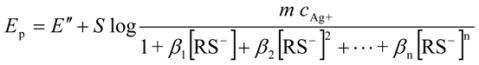
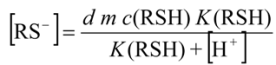
2.3.2. Determination of Solubility Product of CoASAg or Stability Constant of Ag(SAoC)n(1-n)+
 and the solubility product of CoASAg, when pH is fixed and known, can be calculated using equation (17):
and the solubility product of CoASAg, when pH is fixed and known, can be calculated using equation (17):
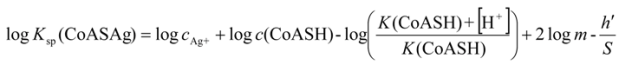
| Used electrode | pKsp | Ksp |
|---|---|---|
| Laboratory home-made electrode with silver pellet | 18.4 | 4.0×10-19 |
| Commercial iodide ISE | 18.7 | 2.0×10-19 |
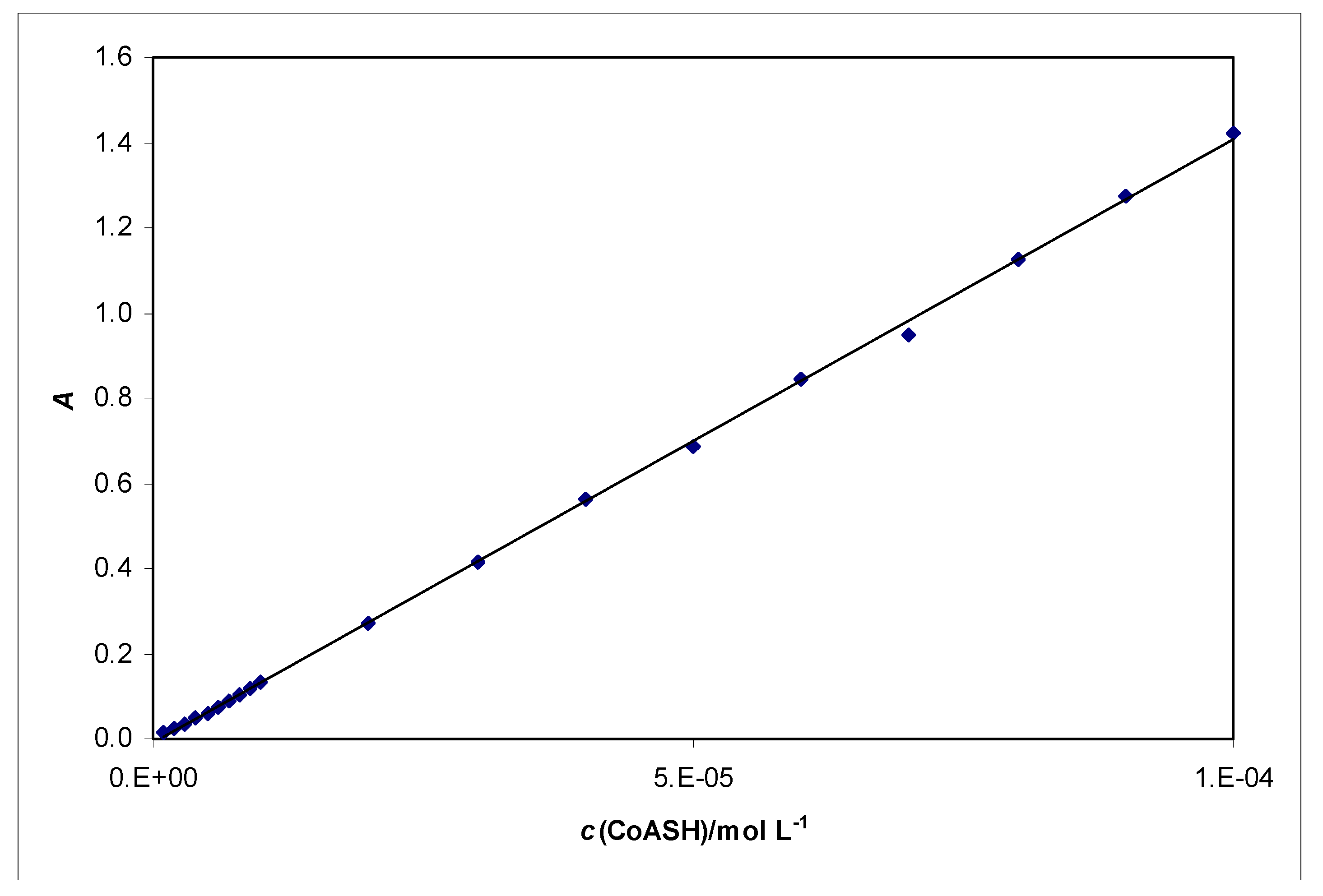
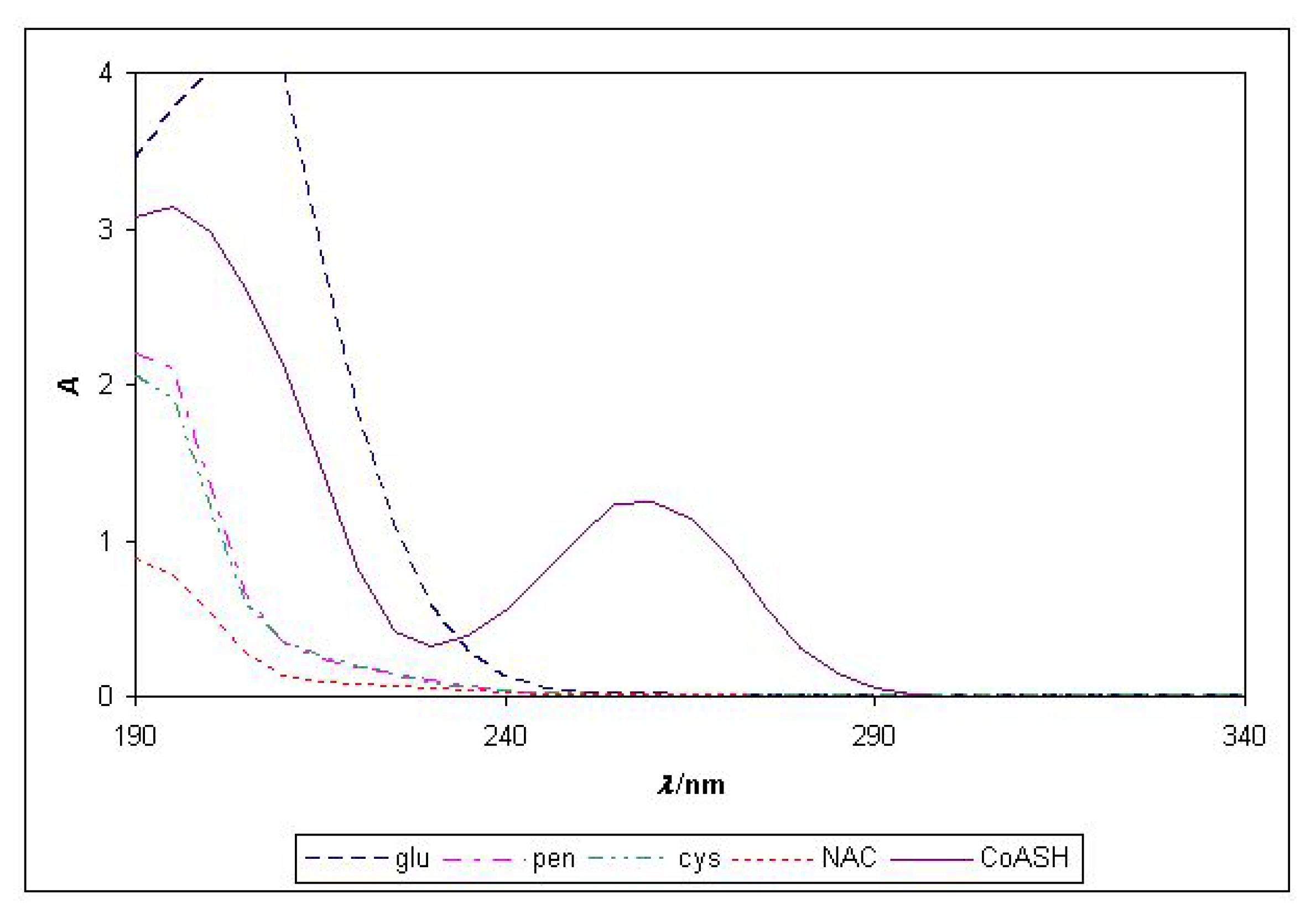
2.4. Spectrophotometric determination of CoASH
| pH | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 | 7.00 | 8.00 | 9.00 | 10.00 | 11.00 | 12.00 | 13.00 |
| A | 1.42 | 1.41 | 1.41 | 1.39 | 1.38 | 1.38 | 1.40 | 1.40 | 1.39 | 1.39 | 1.43 | 1.43 | 1.42 |
| Thiols | A258 | ||
|---|---|---|---|
| c=10-5 M | c=8.0×10-5 M | c=10-4 M | |
| CoASH | 0.143 | 1.142 | 1.429 |
| cys | -0.005 | 0.001 | 0.002 |
| glu | -0.003 | 0.005 | 0.008 |
| NAC | 0.003 | 0.008 | 0.008 |
| pen | -0.006 | -0.002 | -0.001 |
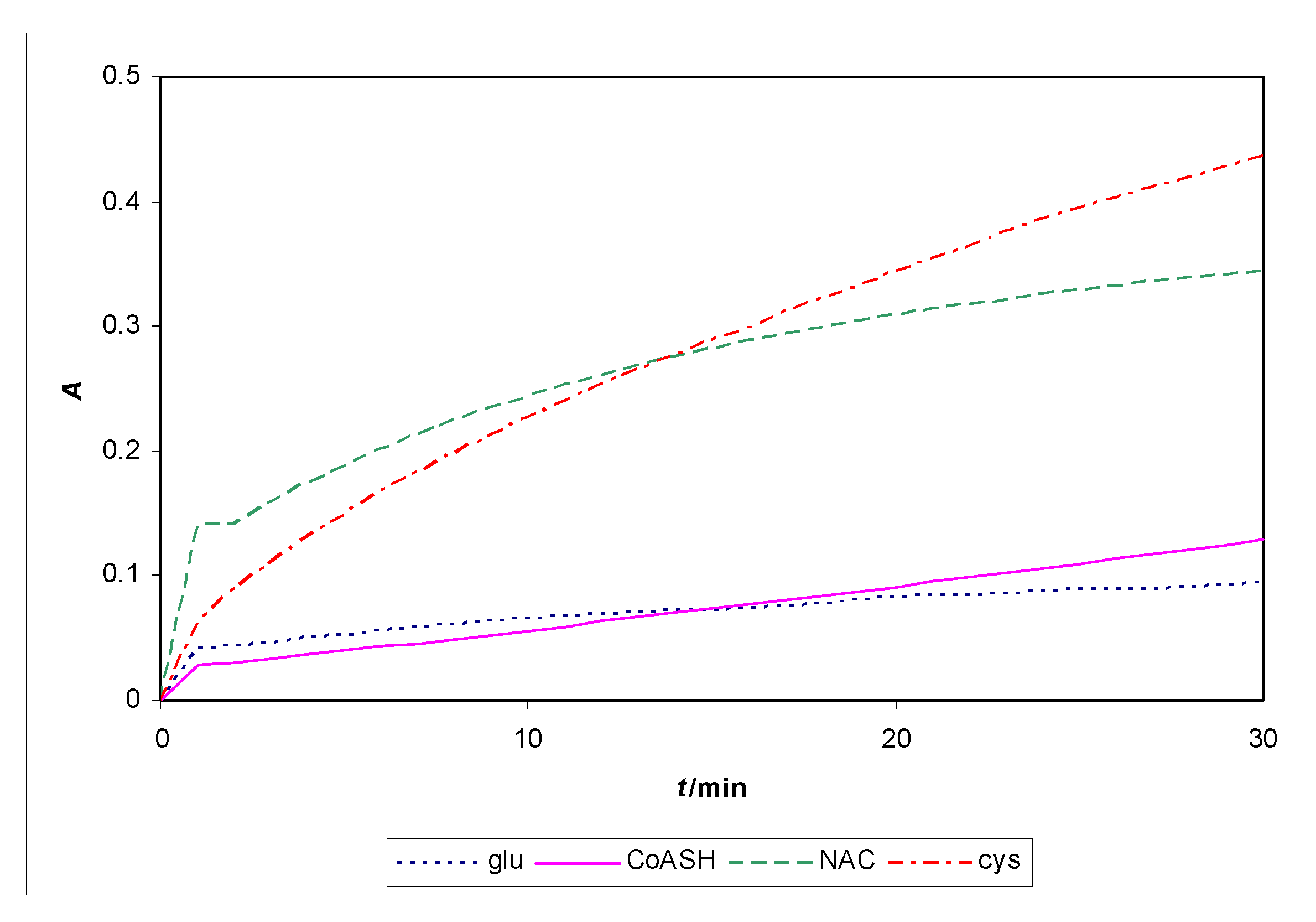
2.5. Kinetic determination of CoASH with a UV/Vis spectrophotometer as detector
3. Experimental
3.1. Chemicals
3.2. Preparation of solutions
3.3. Apparatus
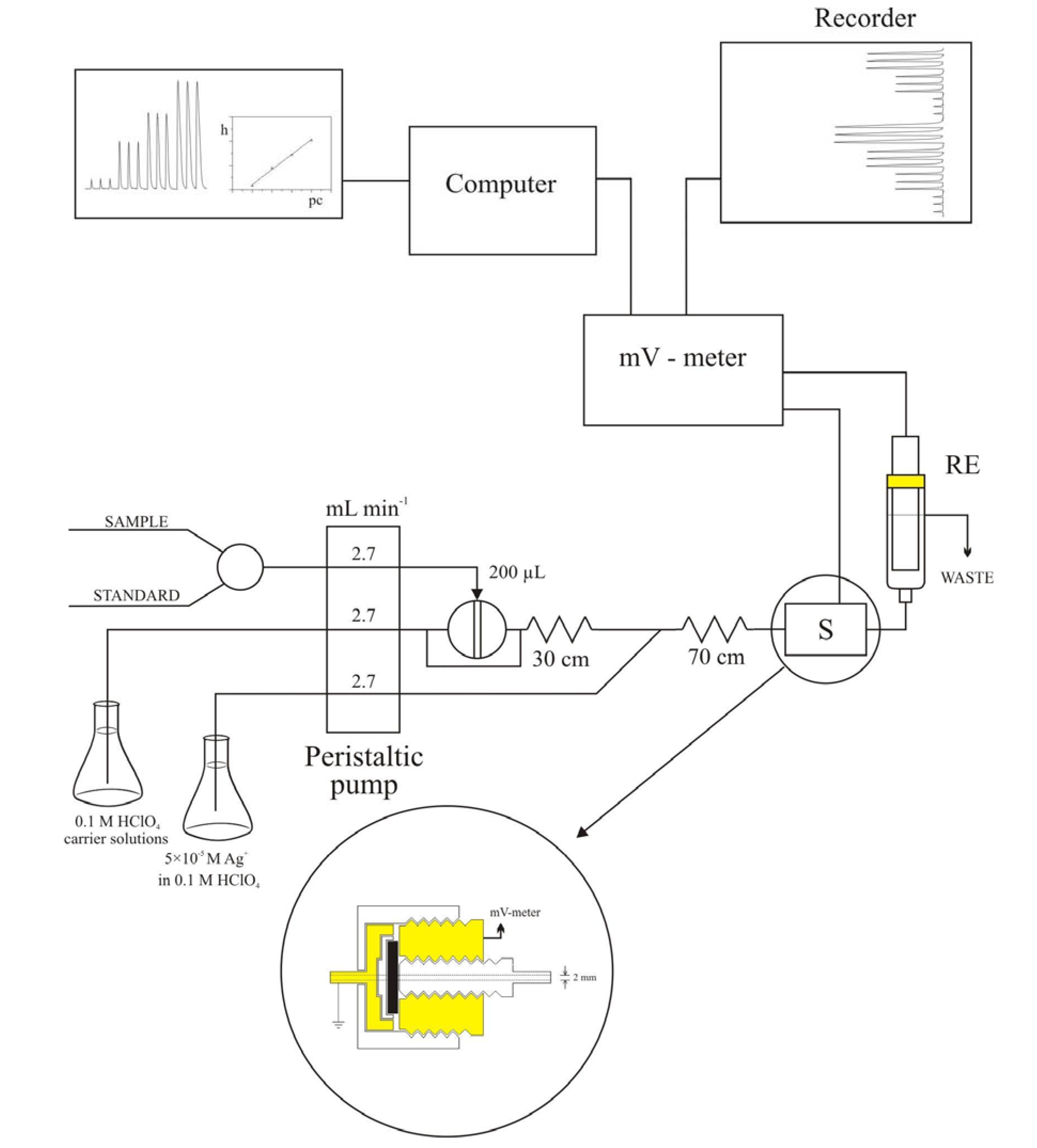
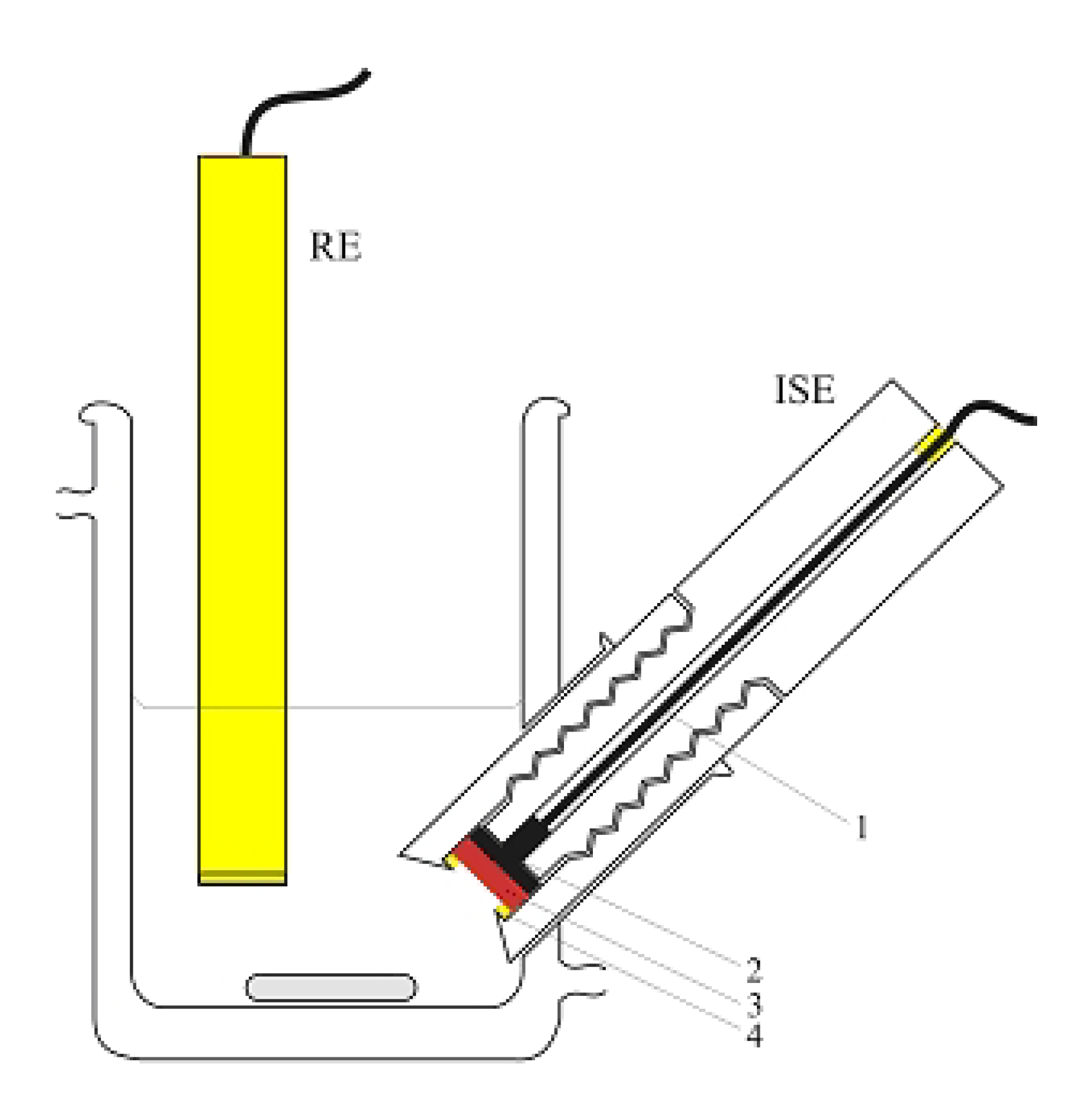
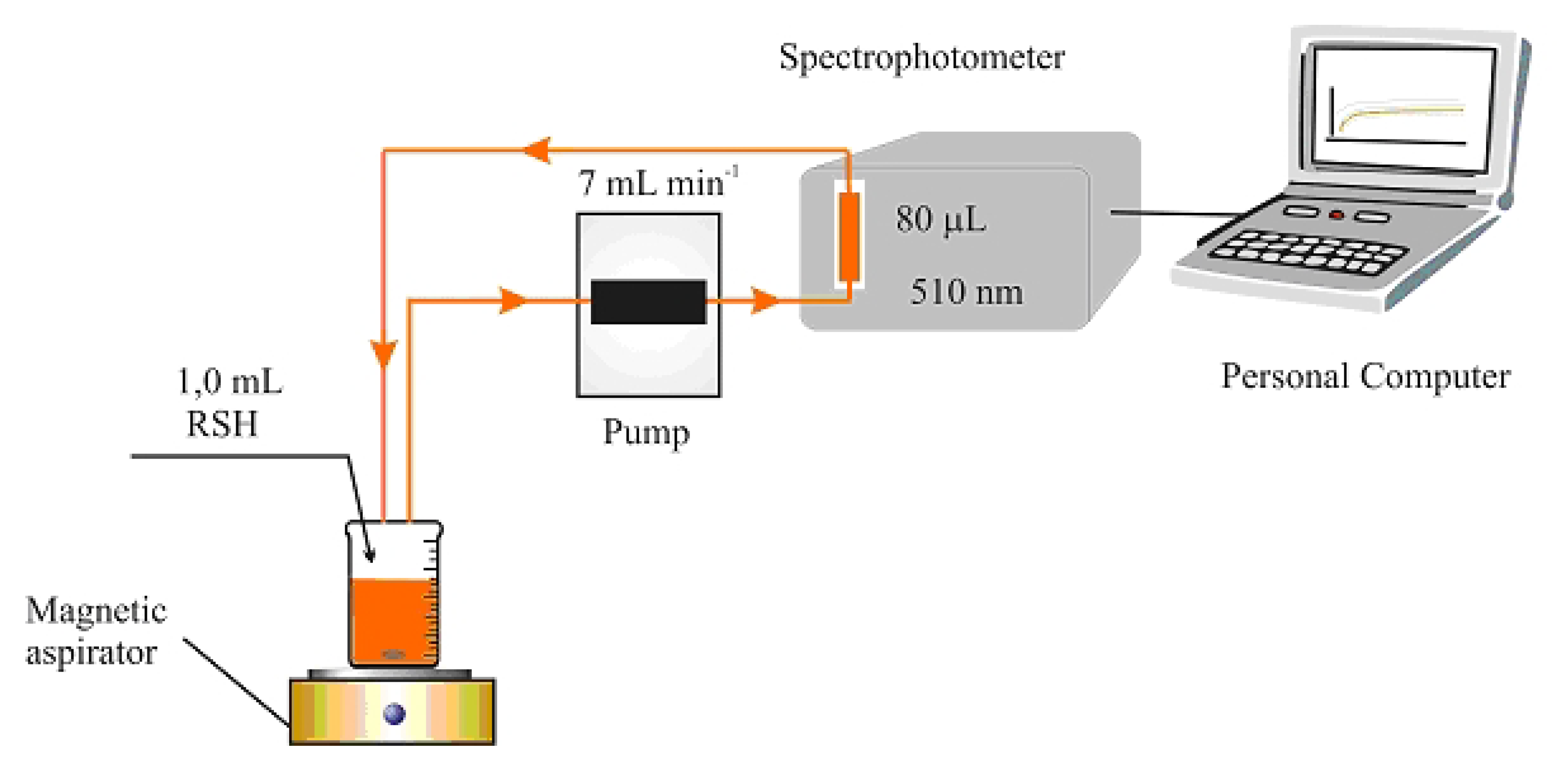
4. Conclusions
- Samples Availability: Not available.
References
- Guanshu, L.; Junnan, C.; Ping, C.; Yinfa, M. Separation and Quantitation of short-chain coenzyme A's in biological samples by capillary electrophoresis. Anal. Chem. 2003, 75, 78–82. [Google Scholar]
- McDougal, D.B., Jr.; Dargar, R.V. A spectrophotometric Cycling assay for reduced coenzyme a and its esters in small amounts of tissue. Anal. Biochem. 1979, 97, 103–115. [Google Scholar]
- Allred, J.B.; Guy, D.G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal. Biochem. 1969, 29, 293–299. [Google Scholar]
- Larson, T.R.; Graham, I.A. A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 2001, 25, 115–125. [Google Scholar]
- Larson, T.R.; Graham, I.A. Application of a new method for the sensitive detection and quantification of acyl-CoA esters in Arabidopsis thaliana seedlings and mature leaves. Biochem. Soc. Trans. 2000, 28, 575–577. [Google Scholar] [CrossRef]
- Han, F.; Huynh, B.H.; Ma, Y.; Lin, B. high-efficiency dna separation by capillary electrophoresis in a polymer solution with ultralow viscosity. Anal. Chem. 1999, 71, 2385–2389. [Google Scholar]
- Han, F.; Huynh, B.H.; Shi, H.; Lin, B.; Ma, Y. pteridine analysis in urine by capillary electrophoresis using laser-induced fluorescence detection. Anal. Chem. 1999, 71, 1265–1269. [Google Scholar] [CrossRef]
- Shi, H.; Ma, Y.; Humphrey, J.H.; Craft, N.E. Determination of vitamin A in dried human blood spots by high-performance capillary electrophoresis with laser-excited fluorescence detection. J. Chromatogr. B Biomed. Appl. 1995, 665, 89–96. [Google Scholar] [CrossRef]
- Xu, J.; Che, P.; Shi, H.; Ma, Y. Quantitative determination of serum iron in human blood by high-performance capillary electrophoresis. J. Chromatogr., B: Biomed. Appl. 1995, 669, 45–51. [Google Scholar]
- Guanshu, L.; Junnan, C.; Ping, C.; Yinfa, M. Separation and Quantitation of short-chain coenzyme A's in biological samples by capillary electrophoresis. Anal. Chem. 2003, 75, 78–82. [Google Scholar] [CrossRef]
- Li, J.; Ge, X.; Jiang, C. Spectrofluorimetric determination of trace amounts of coenzyme A using terbium ion-ciprofloxacin complex probe in the presence of periodic acid. Anal. Bioanal. Chem. 2007, 387, 2083–2089. [Google Scholar] [CrossRef]
- Pande, V.P.; Caramacion; Nylda, M. simple radioisotopic assay of acetylcarnitine and acetyl-CoA at picomolar levels. Anal. Biochem. 1981, 112, 30–38. [Google Scholar]
- Rabier, D.; Briand, P.; Petit, F.; Kamoun, P.; Cathelineau, L. Radioisotopic assay of picomolar amounts of coenzyme A. Anal. Biochem. 1983, 134, 325–329. [Google Scholar]
- Martinović, A.; Radić, Nj. Kinetic Potentiometric Determination of Penicillamine and N-Acetyl-L-Cysteine based on Reaction with Iodate. Acta Chim. Slov. 2009, 56, 503–506. [Google Scholar]
- Fornazari, T.A.L.; Suarez, W.T.; Vieira, H.J.; Fatibello-Fiho, O. Flow Injection Spectrophotometric System for N-Acetyl-L-Cysteine Determination in Pharmaceuticals. Acta Chim. Slov. 2005, 52, 164–167. [Google Scholar]
- Yamato, S.; Shimada, K. Flow-injection spectrophotometric determination of acetyl-coenzyme A with immobilized phosphotransacetylase. Anal. Chim. Acta 1990, 232, 281–286. [Google Scholar] [CrossRef]
- Komljenović, J.; Radić, Nj. Potentiometric determination of thiourea by using ion-selective electrode with AgI-based membrane hydrophobised with PTFE. Acta Pharm. Jugosl. 1991, 41, 41–46. [Google Scholar]
- Radić, N.; Komljenović, J. Potentiometric determination of mercury(II) and Thiourea in strong acid solution using an ion-selective electrode with AgI-based membrane hydrophobised by PTFE. Fresenius J. Anal. Chem. 1991, 341, 592–596. [Google Scholar]
- Komljenović, J.; Radić, Nj. Potentiometric determination of N-acetylcysteine based on its reduction action on Fe(III). Acta Pharm. 1993, 43, 99–106. [Google Scholar]
- Bralić, M.; Radić, N. Direct Potentiometric measurements of cysteine by using iodide-selective electrode. Croat. Chem. Acta 1994, 67, 543–551. [Google Scholar]
- Komljenović, J.; Radić, N. Design and properties of flow-through electrode with AgI-based membrane hydrophobized by PTFE; application to flow-injection determination of thiourea. Sensor. Actuator. B Chem. 1995, 24, 312–316. [Google Scholar] [CrossRef]
- Komljenović, J.; Radić, Nj. Potentiometric flow-injection determination of cysteine in the presence of cystine using an tubular electrode with AgI-based membrane as detector. Analusis 1996, 24, 63–65. [Google Scholar]
- Komljenović, J.; Radić, N. Use of a multi-purpose solid-state ion-selective electrode body and agi-based membrane hydrophobised by PTFE for determination of I− and Hg2+. Analyst 1986, 111, 887–889. [Google Scholar] [CrossRef]
- Morf, W.E.; Kohr, G.; Simon, W. Theoretical treatment of the selectivity and detection limit of silver compound membrane electrodes. Anal. Chem. 1974, 46, 1538–1543. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Giljanović, J.; Prkić, A. Determination of Coenzyme A (CoASH) in the Presence of Different Thiols by Using Flow-Injection with a UV/Vis Spectrophotometric Detector and Potentiometric Determination of CoASH Using an Iodide ISE. Molecules 2010, 15, 100-113. https://doi.org/10.3390/molecules15010100
Giljanović J, Prkić A. Determination of Coenzyme A (CoASH) in the Presence of Different Thiols by Using Flow-Injection with a UV/Vis Spectrophotometric Detector and Potentiometric Determination of CoASH Using an Iodide ISE. Molecules. 2010; 15(1):100-113. https://doi.org/10.3390/molecules15010100
Chicago/Turabian StyleGiljanović, Josipa, and Ante Prkić. 2010. "Determination of Coenzyme A (CoASH) in the Presence of Different Thiols by Using Flow-Injection with a UV/Vis Spectrophotometric Detector and Potentiometric Determination of CoASH Using an Iodide ISE" Molecules 15, no. 1: 100-113. https://doi.org/10.3390/molecules15010100
APA StyleGiljanović, J., & Prkić, A. (2010). Determination of Coenzyme A (CoASH) in the Presence of Different Thiols by Using Flow-Injection with a UV/Vis Spectrophotometric Detector and Potentiometric Determination of CoASH Using an Iodide ISE. Molecules, 15(1), 100-113. https://doi.org/10.3390/molecules15010100




