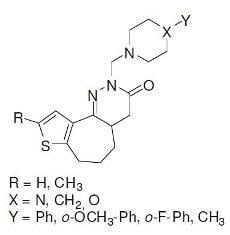
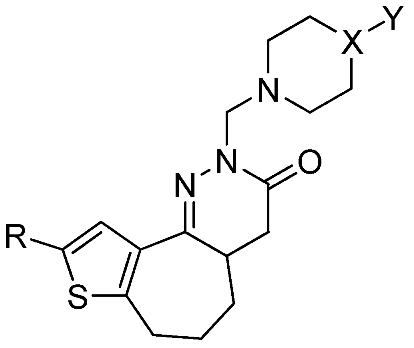
Synthesis and Cytotoxicity of Novel Hexahydrothienocycloheptapyridazinone Derivatives
Abstract
:1. Introduction
| 7 | a | b | c | d | e | f | g | h | i | j | k | l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | H | H | H | H | CH3 | CH3 | CH3 | CH3 | H | CH3 | H | CH3 |
| X | N | N | N | N | N | N | N | N | CH2 | CH2 | O | O |
| Y | Ph | o-OCH3-Ph | o-F-Ph | CH3 | Ph | o-OCH3-Ph | o-F-Ph | CH3 | CH3 | CH3 | - | - |
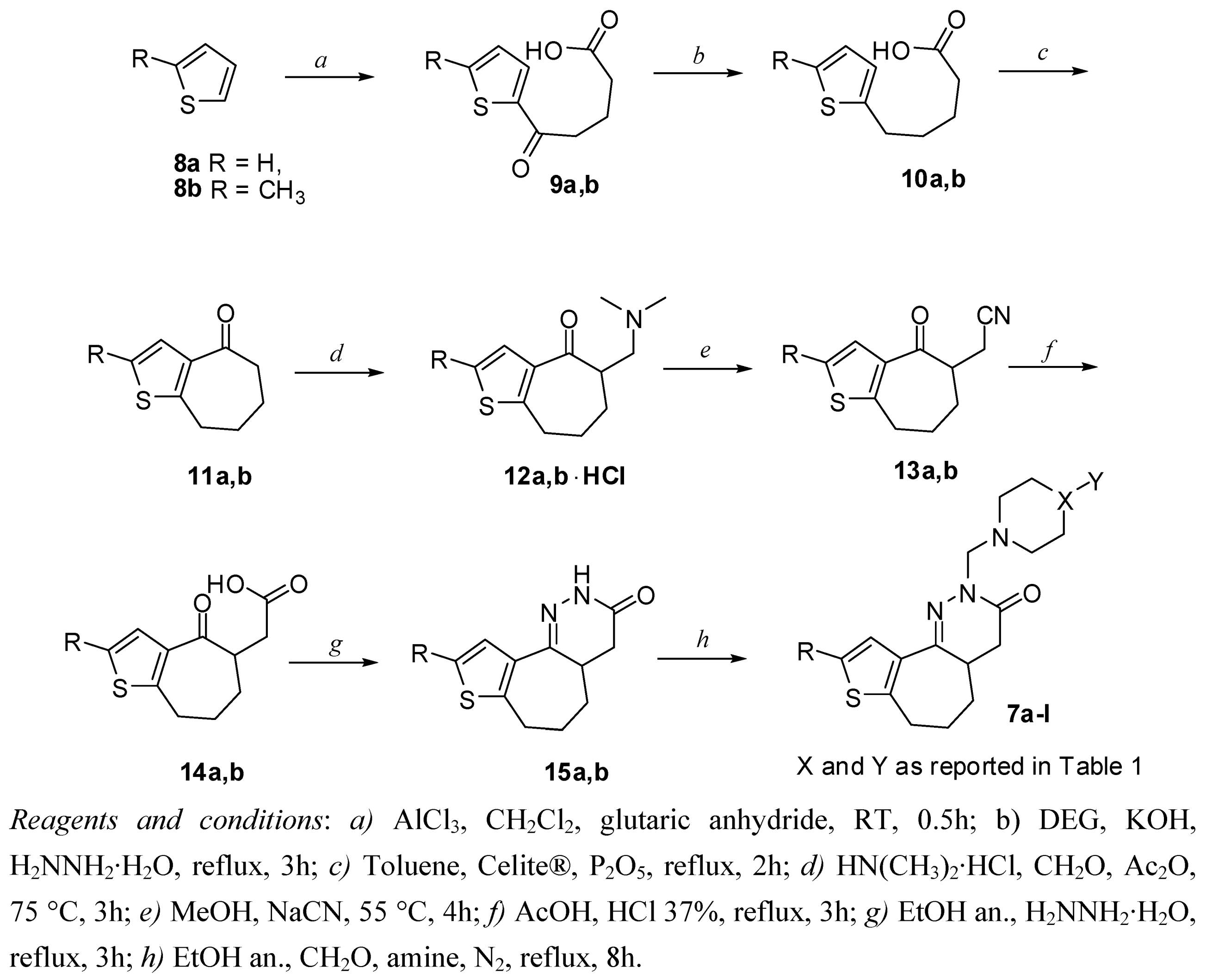
2. Chemistry
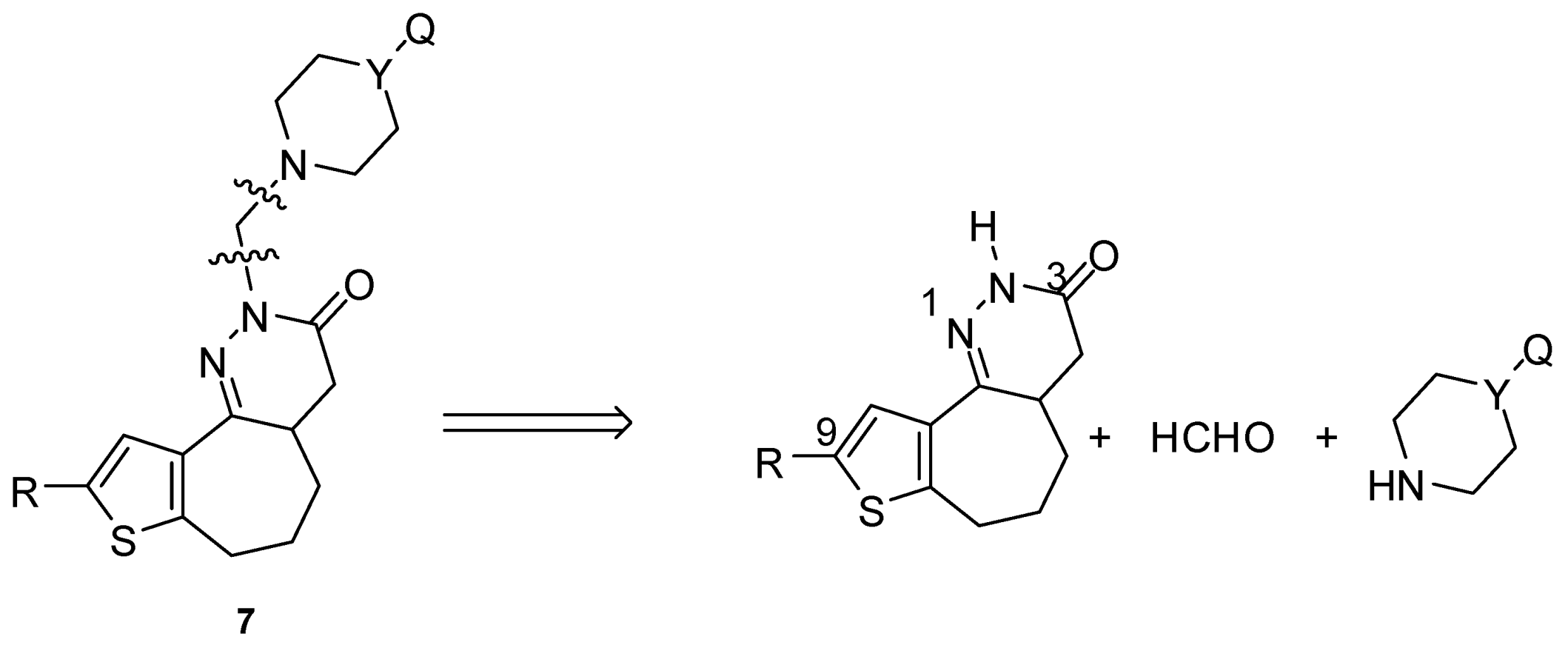
3. Results and Discussion
| Panel/Cell Lines | Compounds | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-small cell lung cancer | 7c | 7d | 7e | 7h | 7i | 7j | 7k | 7l |
| Mean growth % | 97.85 | 98.09 | 103.17 | 101.55 | 101.33 | 101.88 | 96.66 | 102.97 |
| Range of growth % | 81.03 to 113.17 | 72.06 to 120.26 | 78.91 to 108.81 | 92.87 to 109.80 | 80.20 to110.82 | 81.70 to 119.13 | 75.17 to 113.32 | 93.09 to 112.16 |
| Colon cancer | ||||||||
| Mean growth % | 107.29 | 105.03 | 109.01 | 104.88 | 103.17 | 104.91 | 101.79 | 123.28 |
| Range of growth % | 100.56 to 117.52 | 96.19 to 111.60 | 97.01 to 119.39 | 93.70 to 123.28 | 93.76 to 117.4 | 95.50 to 112.76 | 93.53 to 114.38 | 99.22 to 110.16 |
| Breast Cancer | ||||||||
| Mean growth % | 103.25 | 100.59 | 107.91 | 105.20 | 104.68 | 107.49 | 101.71 | 108.39 |
| Range of growth % | 92.60 to 113.98 | 91.34 to 109.30 | 96.71 to 121.53 | 91.23 to 120.16 | 88.76 to122.29 | 91.22 to 124.23 | 94.33 to 110.47 | 87.28 to 129.10 |
| Ovarian Cancer | ||||||||
| Mean growth % | 104.72 | 101.64 | 108.09 | 102.84 | 109.28 | 102.64 | 103.69 | 104.24 |
| Range of growth % | 99.67 to 111.88 | 98.15 to 108.92 | 95.14 to 119.38 | 96.73 to 120.49 | 92.31to 170.96 | 94.48 to 117.11 | 92.28 to 117.79 | 96.32 to 122.92 |
| Leukemia | ||||||||
| Mean growth % | 100.46 | 92.31 | 98.55 | 102.95 | 94.13 | 100.01 | 86.67 | 98.54 |
| Range of growth % | 91.68 to 107.61 | 81.94 to 113.17 | 91.36 to 111.53 | 85.24 to 120.85 | 80.99 to | 90.34 to 112.16 | 76.07 to 94.26 | 75.05 to 112.55 |
| Renal Cancer | ||||||||
| Mean growth % | 101.77 | 101.13 | 103.67 | 95.02 | 103.58 | 100.19 | 104.36 | 101.80 |
| Range of growth % | 93.09 to 110.89 | 95.38 to 108.40 | 94.16 to 114.16 | 77.38 to 114.45 | 94.14 to113.75 | 86.51 to 121.05 | 93.91 to 114.99 | 85.76 to 112.31 |
| Melanoma | ||||||||
| Mean growth % | 106.50 | 102.46 | 106.62 | 101.06 | 102.95 | 107.10 | 101.57 | 104.80 |
| Range of growth % | 100.28 to110.39 | 88.88 to 111.60 | 101.92 to116.24 | 92.73 to 112.29 | 87.54 to119.80 | 92.35 to 124.26 | 91.52 to 114.53 | 93.82 to 112.20 |
| Prostate Cancer | ||||||||
| Mean growth % | 102.25 | 100.46 | 114.38 | 109.08 | 101.76 | 99.92 | 103.85 | 109.31 |
| Range of growth % | 98.48 to 106.02 | 93.16 to 107.76 | 108.08 to 120.68 | 104.25 to 113.91 | 99.78 to 103.76 | 96.26 to 103.58 | 102.00 to105.70 | 102.40 to 116.22 |
| CNS Cancer | ||||||||
| Mean growth | 99.49 | 100.85 | 100.78 | 82.03 | 111.34 | 98.38 | 107.23 | 97.20 |
| Range of growth % | 91.08 to 108.91 | 77.31 to 131.16 | 89.96 to 110.76 | 72.14 to 115.77 | 80.17 to 194.64 | 85.30 to 105.97 | 81.54 to 164.17 | 82.07 to 106.60 |
| Compd | Panel/Cell Lines | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-small cell lung cancer | Leukemia | Renal cancer | CNS cancer | |||||
| EKVX* | HOP-92 | SR | RPMI-8226 | CAKI-1 | UO-31 | SNB-75* | ||
| 7d | 27.94 | 20.09 | 22.69 | |||||
| 7e | 21.09 | |||||||
| 7h | 22.62 | 22.23 | 27.86 | |||||
| 7k | 23.93 | 24.83 | ||||||
| 7l | 24.95 | |||||||
4. Conclusions
5. Experimental
5.1. General
5.2. General procedure for the synthesis of 5-oxopentanoic acids 9a,b
5.3. General procedure for the synthesis of pentanoic acids 10a,b
5.4. General procedure for the synthesis of ketones 11a,b
5.5. General procedure for the synthesis of Mannich bases 12a,b
5.6. General procedure for the synthesis of nitriles 13a,b
5.7. General procedure for the synthesis of acids 14a,b
5.8. General procedure for the synthesis of pyridazinones 15a,b
5.9. General procedure for the synthesis of 2-N-substituted-2,4,4a,5,6,7-hexahydro-3H-thieno[2’,3’:6,7]cyclohepta[1,2-c]pyridazin-3-one derivatives 7a-l
Acknowledgements
- Sample Availability: Samples of the compounds 7g and 7l are available from the authors.
References
- Fidler, I.J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Chabner, B.A.; Amrein, P.C.; Druker, B.J.; Michaelson, M.D.; Mitsiades, C.S.; Goss, P.E.; Ryan, D.P.; Ramachandra, S.; Richardson, P.G.; Supko, J.G.; Wilson, W.H. Antineoplastic Agents. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 11th; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; Mc Graw-Hill Professional: New York, NY, USA, 2005; pp. 1315–1403. [Google Scholar]
- Gilchrest, B.A.; Eller, M.S. Cancer therapeutics: Smart and smarter. Drugs Future 2009, 34, 205–216. [Google Scholar] [CrossRef]
- Tišler, M.; Stanovnik, B. Pyridazines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry, The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds, 1st; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Bristol, UK, 1984; Volume 3, pp. 1–56. [Google Scholar]
- Pinna, G.A.; Murineddu, G.; Murruzzu, C.; Zuco, V.; Zunino, F.; Cappelletti, G.; Artali, R.; Cignarella, G.; Solano, L.; Villa, S. Synthesis, modelling, and antimitotic properties of tricyclic systems characterised by a 2-(5-Phenyl-1H-pyrrol-3-yl)-1,3,4-oxadiazole moiety. Chem. Med. Chem. 2009, 4, 998–1009. [Google Scholar]
- Cignarella, G.; Barlocco, D.; Pinna, G.A.; Loriga, M.; Curzu, M.M.; Tofanetti, O.; Germini, M.; Cazzulani, P.; Cavalletti, E. Synthesis and biological evaluation of substituted benzo[h]cinnolinones and 3H-benzo[6,7]cyclohepta[1,2-c]pyridazinones: higher homologues of the antihypertensive and antithrombotic 5H-indeno[1,2-c]pyridazinones. J. Med. Chem. 1989, 32, 2277–2282. [Google Scholar] [CrossRef]
- Cignarella, G.; Barlocco, D.; Curzu, M.M.; Pinna, G.A.; Cazzulani, P.; Cassin, M.; Lumachi, B. Synthesis and pharmacological evaluation of 4,4a-dihydro-5H-[1]benzopyrano[4,3-c]pyridazin-3(2H)-ones bioisosters of antihypertensive and antithrombotic benzo[h]cinnolinones. Eur. J. Med. Chem. 1990, 25, 749–756. [Google Scholar] [CrossRef]
- Pinna, G.A.; Curzu, M.M.; Fraghì, P.; Gavini, E. Synthesis and pharmacological evaluation of 5,6-dihydrobenzo[f]cinnolin-2(3H)ones analogues of antihypertensive and antiaggregating benzo[h]cinnolinones. Farmaco 1996, 51, 653–658. [Google Scholar]
- Pinna, G.A.; Salis, E.; Berta, D.; Gavini, E. Synthesis and pharmacological evaluation of 4a-methyl-4,4a,5,6-tetrahydrothieno[2,3-h]cinnolin-3(2H)-ones. Farmaco 1997, 52, 29–33. [Google Scholar]
- Tanaka, H.; Kirihara, S.; Yasumatsu, H.; Yakushiji, T.; Nakao, T. Synthesis and evaluation of novel 2-aryl-2,5,6,7-tetrahydro-3H-thieno [2′,3′:6,7]cyclohepta[1,2-c]pyridazin-3-ones and 2-aryl-5, 6-dihydrothieno[2,3-h]cinnolin-3(2H)-ones as anxiolytics. Eur. J. Med. Chem. 1997, 32, 607–615. [Google Scholar] [CrossRef]
- Pinna, G.A.; Curzu, M.M.; Murineddu, G.; Chelucci, G.; Cignarella, G.; Menta, E.; Krell, H.W.; Rastelli, G.; Ferrari, A.M. Preparation of thieno[3,2-h]cinnolinones as matrix metalloproteinase inhibitors. Arch. Pharm. Pharm. Med. Chem. 2000, 333, 37–47. [Google Scholar] [CrossRef]
- Costantino, L.; Rastelli, G.; Vescovini, K.; Cignarella, G.; Vianello, P.; Corso, A.D.; Cappiello, M.; Mura, U.; Barlocco, D. Synthesis, activity, and molecular modeling of a new series of tricyclic pyridazinones as selective aldose reductase inhibitors. J. Med. Chem. 1996, 39, 4396–4405. [Google Scholar] [CrossRef]
- Pau, A.; Asproni, B.; Boatto, G.; Grella, G.E.; De Caprariis, P.; Costantino, L.; Pinna, G.A. Synthesis and aldose reductase inhibitory activities of novel thienocinnolinone derivatives. Eur. J. Pharm. Sci. 2004, 21, 545–552. [Google Scholar] [CrossRef]
- Pirisi, M.A.; Murineddu, G.; Mussinu, J.M.; Pinna, G.A. Synthesis and cytotoxicity evaluation of thiophene analogues of 1-methyl-2, 3-bis(hydroxymethyl)benzo[g]indole bis[N-(2-propyl)carbamate]. Farmaco 2002, 57, 331–335. [Google Scholar] [CrossRef]
- Grella, G.E.; Cabras, M.C.; Murineddu, G.; Pau, A.; Pinna, G.A. Synthesis and cytotoxicity of substituted 2-benzylnaphth[2,3-d]imidazoles. Eur. J. Pharm. Sci. 2003, 20, 267–272. [Google Scholar] [CrossRef]
- Murineddu, G.; Cignarella, G.; Chelucci, G.; Loriga, G.; Pinna, G.A. Synthesis and cytotoxic activities of pyrrole[2,3-d]pyridazin-4-one derivatives. Chem. Pharm. Bull. 2002, 50, 754–759. [Google Scholar] [CrossRef]
- Silverman, R.J. The Organic Chemistry of Drug Design and Drug Action, 2nd ed; Elsevier Academic Press: Burlington, MA, USA, 2004; pp. 13–14. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.D.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Boyd, M.R. In Cancer Drug Discovery and Development; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 2, pp. 23–43. [Google Scholar]
- Block, H.J.; Beale, J.M. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical chemistry, 11t ed; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2004; pp. 390–394. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pau, A.; Murineddu, G.; Asproni, B.; Murruzzu, C.; Grella, G.E.; Pinna, G.A.; Curzu, M.M.; Marchesi, I.; Bagella, L. Synthesis and Cytotoxicity of Novel Hexahydrothienocycloheptapyridazinone Derivatives. Molecules 2009, 14, 3494-3508. https://doi.org/10.3390/molecules14093494
Pau A, Murineddu G, Asproni B, Murruzzu C, Grella GE, Pinna GA, Curzu MM, Marchesi I, Bagella L. Synthesis and Cytotoxicity of Novel Hexahydrothienocycloheptapyridazinone Derivatives. Molecules. 2009; 14(9):3494-3508. https://doi.org/10.3390/molecules14093494
Chicago/Turabian StylePau, Amedeo, Gabriele Murineddu, Battistina Asproni, Caterina Murruzzu, Giuseppe E. Grella, Gérard A. Pinna, Maria M. Curzu, Irene Marchesi, and Luigi Bagella. 2009. "Synthesis and Cytotoxicity of Novel Hexahydrothienocycloheptapyridazinone Derivatives" Molecules 14, no. 9: 3494-3508. https://doi.org/10.3390/molecules14093494
APA StylePau, A., Murineddu, G., Asproni, B., Murruzzu, C., Grella, G. E., Pinna, G. A., Curzu, M. M., Marchesi, I., & Bagella, L. (2009). Synthesis and Cytotoxicity of Novel Hexahydrothienocycloheptapyridazinone Derivatives. Molecules, 14(9), 3494-3508. https://doi.org/10.3390/molecules14093494