Synthesis and Anti-Human Immunodeficiency Virus Type 1 Activity of (E)-N-Phenylstyryl-N-alkylacetamide Derivatives
Abstract
:1. Introduction

2. Results and Discussion
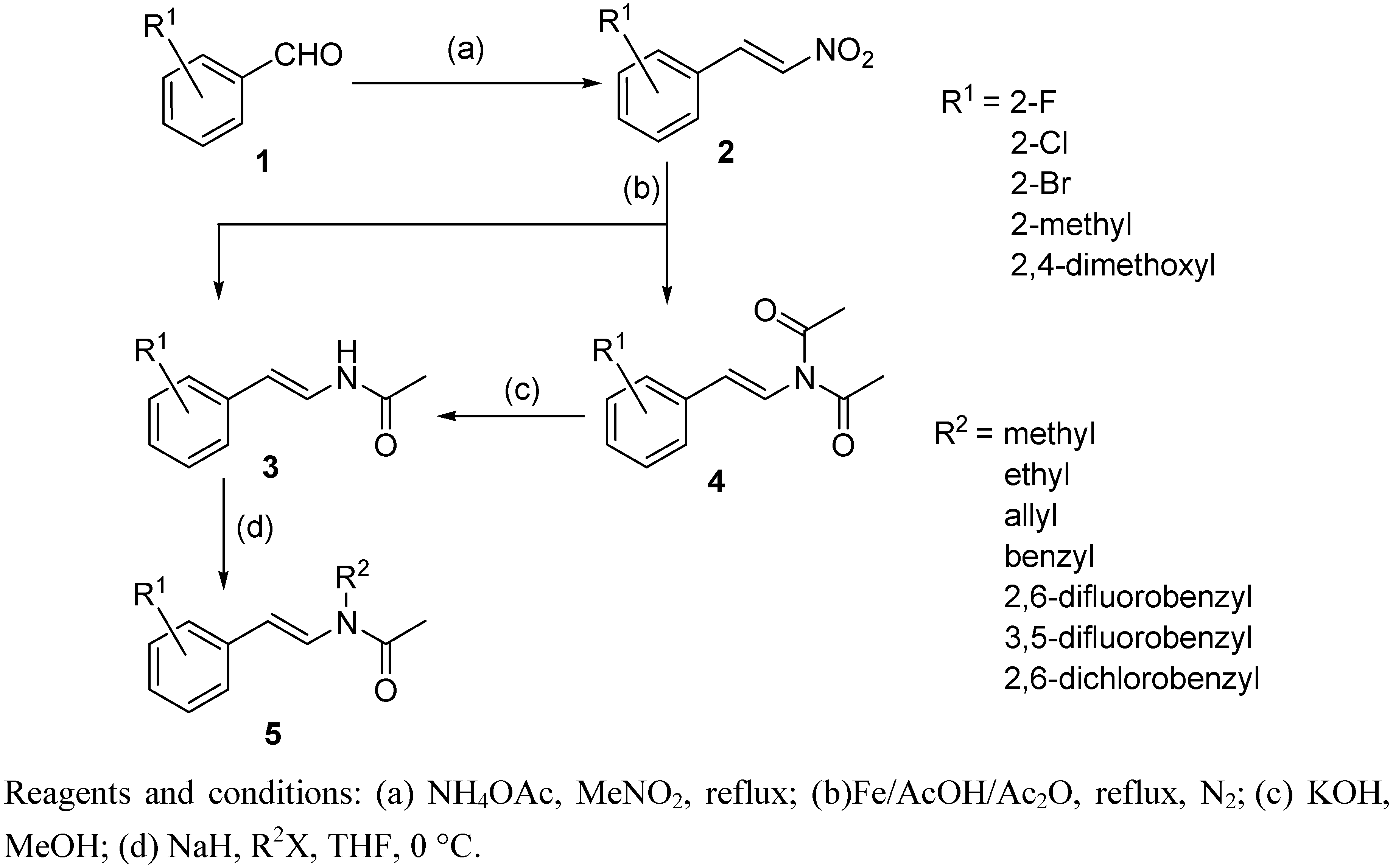
2.1. Chemistry
| Entry | R1 | R2 | RT inhibition (%)b | IC50c (μM) | EC50d (μM) | CC50e (μM) | SIf (CC50/EC50) |
|---|---|---|---|---|---|---|---|
| 5a | 3-Br | methyl | 45.42 | ND | 128 ± 20 | 409 ± 106 | 3.2 |
| 5b | 4-Br | methyl | 16.59 | ND | ND | ND | ND |
| 5c | 2-Br | methyl | 85.27 | 339 ± 107 | 60 ±10 | >789 | >13.2 |
| 5d | 2-Br | ethyl | 46.37 | ND | 48 ± 8 | 358 ± 88 | 7.5 |
| 5e | 2-Br | allyl | 47.76 | ND | 75 ± 13 | 337 ± 154 | 4.5 |
| 5f | 2-Br | acetyl | 60.55 | 320 ± 22 | 8 ± 1 | 85 ± 17 | 10.6 |
| 5g | 2-Br | benzyl | 47.56 | ND | 9 ± 2 | 51 ± 13 | 5.7 |
| 5h | 2-Br | 2,6-difluoro-benzyl | 57.33 | 295 ± 9 | 29 ± 4 | 172 ± 73 | 5.9 |
| 5i | 2-Br | 3,5-difluoro-benzyl | 88.89 | 97 ± 13 | 4 ± 0.5 | 116 ± 13 | 29 |
| 5j | 2-Br | 2,6-dichloro-benzyl | 30.34 | ND | 49 ± 10 | 190 ± 36 | 3.9 |
| 5k | 2-Cl | methyl | 75.22 | 434 ± 44 | 96 ± 20 | 441 ± 18 | 4.6 |
| 5l | 2-Cl | ethyl | 59.10 | 521 ± 11 | 91 ± 15 | 424 ± 22 | 4.7 |
| 5m | 2-Cl | allyl | 29.95 | ND | 118 ± 28 | 536 ± 41 | 4.5 |
| 5n | 2-F | methyl | 61.69 | 477 ± 107 | 130 ± 33 | 664 ± 79 | 5.5 |
| 5o | 2-F | allyl | 38.51 | ND | 261 ± 40 | 538 ± 138 | 2.0 |
| 5p | 2-methyl | methyl | 79.80 | 487 ± 27 | 116 ± 11 | 609 ± 177 | 5.2 |
| 5q | 2-methyl | allyl | 40.30 | ND | 273 ± 43 | >930 | >3.4 |
| 5r | 2,4-dimethoxy | methyl | 69.45 | 373 ± 40 | 32 ± 7 | 256 ± 35 | 8.0 |
| 5s | 2,4-dimethoxy | ethyl | 34.73 | ND | 19 ± 3 | 112 ± 10 | 5.9 |
| 5t | 2,4-dimethoxy | allyl | 36.62 | ND | 55 ± 4 | 290 ± 79 | 5.3 |
| AZTg | NDi | ND | 1.08×10-2 | >509 | >47130 | ||
| PFAh | 96.46 | 5 | ND | ND | ND |
2.2. Biological activity
3. Experimental
3.1. General
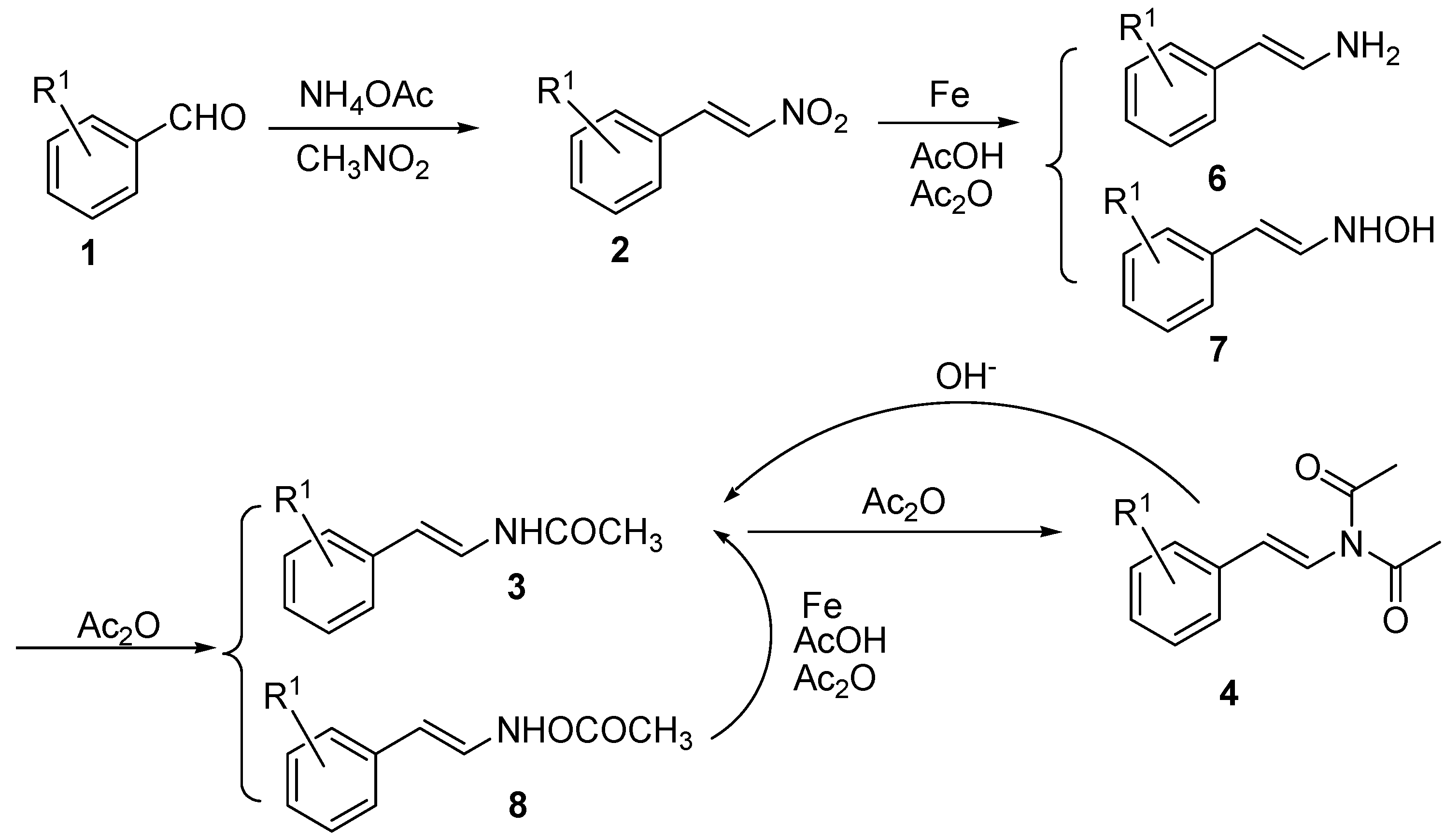
3.2. Preparation of (E)-β-phenylnitroolefins 2
3.3. Preparation of (E)-N-phenylstyrylacetamides 3
3.4. General procedure for the preparation of (E)-N-phenylstyryl-N-alkylacetamides 5: Preparation of (E)-N-(3-bromostyryl)-N-methylacetamide (5a)
3.5. Biological activity assays
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 5a–5t are available from the authors.
References and Notes
- Schinazi, R.F.; Hernandez-Santiago, B.I.; Hurwitz, S.J. Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses. Antivir. Res. 2006, 71, 322–334. [Google Scholar] [CrossRef]
- Didierjean, J.; Isel, C.; Querré, F.; Mouscadet, J.F.; Aubertin, A.M.; Valnot, J.Y.; Piettre, S.R.; Marquet, R. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, RNase H, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother. 2005, 49, 4884–4894. [Google Scholar] [CrossRef]
- Yeni, P. Update on HAART in HIV. J. Hepatol. 2006, 44, S100–S103. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zeng, C.C.; Jiao, Z.G.; Hu, L.M.; Zhong, R.G. Design, synthesis and anti-HIV integrase evaluation of 4-oxo-4H-quinolizine-3-carboxylic acid derivatives. Molecules 2009, 14, 868–883. [Google Scholar]
- Jones, L.H.; Allan, G.; Barba, O.; Burt, C.; Corbau, R.; Dupont, T.; Knochel, T.; Irving, S.; Middleton, D.S.; Mowbray, C.E.; Perros, M.; Ringrose, H.; Swain, N.A.; Webster, R. Westby, M. Phillips. Novel indazole non-nucleoside reverse transcriptase inhibitors using molecular hybridization based on crystallographic overlay. J. Med. Chem. 2009, 52, 1219–1223. [Google Scholar]
- Wu, J.; Liu, X.; Cheng, X.; Cao, Y.; Wang, D.; Li, Z.; Xu, W.; Pannecouque, C.; Witvrouw, M.; Clercq, E.D. Synthesis of novel derivatives of 4-amino-3-(2-furyl)-5-mercapto-1,2,4-triazole as potential HIV-1 NNRTIs. Molecules 2007, 12, 2003–2016. [Google Scholar] [CrossRef]
- Barreca, M.L.; Rao, A.; Luca, L.D.; Iraci, N.; Monforte, A.M.; Maga, G.; Clercq, E.D.; Pannecouque, C.; Balzarini, J.; Chimirri, A. Discovery of novel benzimidazolones as potent non-nucleoside reverse transcriptase inhibitors active against wild-type and mutant HIV-1 strains. Bioorg. Med. Chem. Lett. 2007, 17, 1956–1960. [Google Scholar]
- Gagnon, A.; Landry, S.; Coulombe, R.; Jakalian, A.; Guse, I.; Thavonekham, B.; Bonnear, P.R.; Yoakim, C.; Simoneau, B. Investigation on the role of the tetrazole in the binding of thiotetrazolylacetanilides with HIV-1 wild type and K103N/Y181C double mutant reverse transcriptases. Bioorg. Med. Chem. Lett. 2009, 19, 1199–1205. [Google Scholar] [CrossRef]
- Billamboz, M.; Bailly, F.; Barreca, M.L.; Luca, L.D.; Mouscadet, J.; Calmels, C.; Andréola, M.; Witvrouw, M.; Christ, F.; Debyser, Z.; Cotelle, P. Design, synthesis, and biological evaluation of a series of 2-hydroxyisoquinoline-1,3(2H,4H)-diones as dual inhibitors of human immunodeficiency virus type 1 integrase and the reverse transcriptase RNase H domain. J. Med. Chem. 2008, 51, 7717–7730. [Google Scholar] [CrossRef]
- Burke, J.T.R.; Fesen, M.R.; Mzaumder, A.; Wang, J.; Carothers, A.M.; Grunberger, D.; Driscoll, J.; Kohn, K.; Pormier, Y. Hydroxylated aromatic inhibitors of HIV-1 integrase. J. Med. Chem. 1995, 38, 4171–4178. [Google Scholar]
- Cheng, P.; Jiang, Z.Y.; Wang, R.R.; Zhang, X.M.; Wang, Q.; Zheng, Y.T.; Zhou, J.; Chen, J.J. Synthesis and biological evaluation of N-acetyl-beta-aryl-1,2-didehydroethylamines as new HIV-1 RT inhibitors in vitro. Bioorg. Med. Chem. Lett. 2007, 17, 4476–4480. [Google Scholar]
- Cheng, P.; Huang, N.; Jiang, Z.Y.; Zhang, Q.; Zheng, Y.T.; Chen, J.J.; Zhang, X.M.; Ma, Y.B. 1-Aryltetrahydroisoquinolines as active anti-HIV agents in vitro. Bioorg. Med. Chem. Lett. 2008, 18, 2475–2478. [Google Scholar] [CrossRef]
- Yan, M.H.; Cheng, P.; Jiang, Z.Y.; Ma, Y.B.; Zhang, X.M.; Zhang, F.X.; Yang, L.M.; Zheng, Y.T.; Chen, J.J. Periglaucines A–D, anti-HBV and HIV-1 alkaloids from Pericampylus glaucus. J. Nat. Prod. 2008, 71, 760–763. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.Y.; Pu, S.P.; Zheng, Y.T. Zinc coupling potentiates anti-HIV-1 activity of baicalin. Biochem. Biophys. Res.Commun. 2004, 324, 605–610. [Google Scholar] [CrossRef]
- Jiang, T.; Kuhen, K.L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Wu, T.Y.; He, Y. Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I. Bioorg. Med. Chem. Lett. 2006, 16, 2105–2108. [Google Scholar]
- Barreca, M.L.; Rao, A.; Laura, D.L.; Zappalà, M.; Monforte, A.; Maga, G.; Pannecouque, C.; Balzarini, J.; Clercq, E.D.; Chimirri, A.; Monforte, P. Computational strategies in discovering novel non-nucleoside inhibitors of HIV-1 RT. J. Med. Chem. 2005, 48, 3433–3437. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, P.; Chen, J.-J.; Huang, N.; Wang, R.-R.; Zheng, Y.-T.; Liang, Y.-Z. Synthesis and Anti-Human Immunodeficiency Virus Type 1 Activity of (E)-N-Phenylstyryl-N-alkylacetamide Derivatives. Molecules 2009, 14, 3176-3186. https://doi.org/10.3390/molecules14093176
Cheng P, Chen J-J, Huang N, Wang R-R, Zheng Y-T, Liang Y-Z. Synthesis and Anti-Human Immunodeficiency Virus Type 1 Activity of (E)-N-Phenylstyryl-N-alkylacetamide Derivatives. Molecules. 2009; 14(9):3176-3186. https://doi.org/10.3390/molecules14093176
Chicago/Turabian StyleCheng, Pi, Ji-Jun Chen, Ning Huang, Rui-Rui Wang, Yong-Tang Zheng, and Yi-Zeng Liang. 2009. "Synthesis and Anti-Human Immunodeficiency Virus Type 1 Activity of (E)-N-Phenylstyryl-N-alkylacetamide Derivatives" Molecules 14, no. 9: 3176-3186. https://doi.org/10.3390/molecules14093176
APA StyleCheng, P., Chen, J.-J., Huang, N., Wang, R.-R., Zheng, Y.-T., & Liang, Y.-Z. (2009). Synthesis and Anti-Human Immunodeficiency Virus Type 1 Activity of (E)-N-Phenylstyryl-N-alkylacetamide Derivatives. Molecules, 14(9), 3176-3186. https://doi.org/10.3390/molecules14093176




