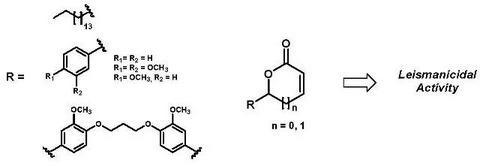
Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity
Abstract
:1. Introduction
2. Results and Discussion
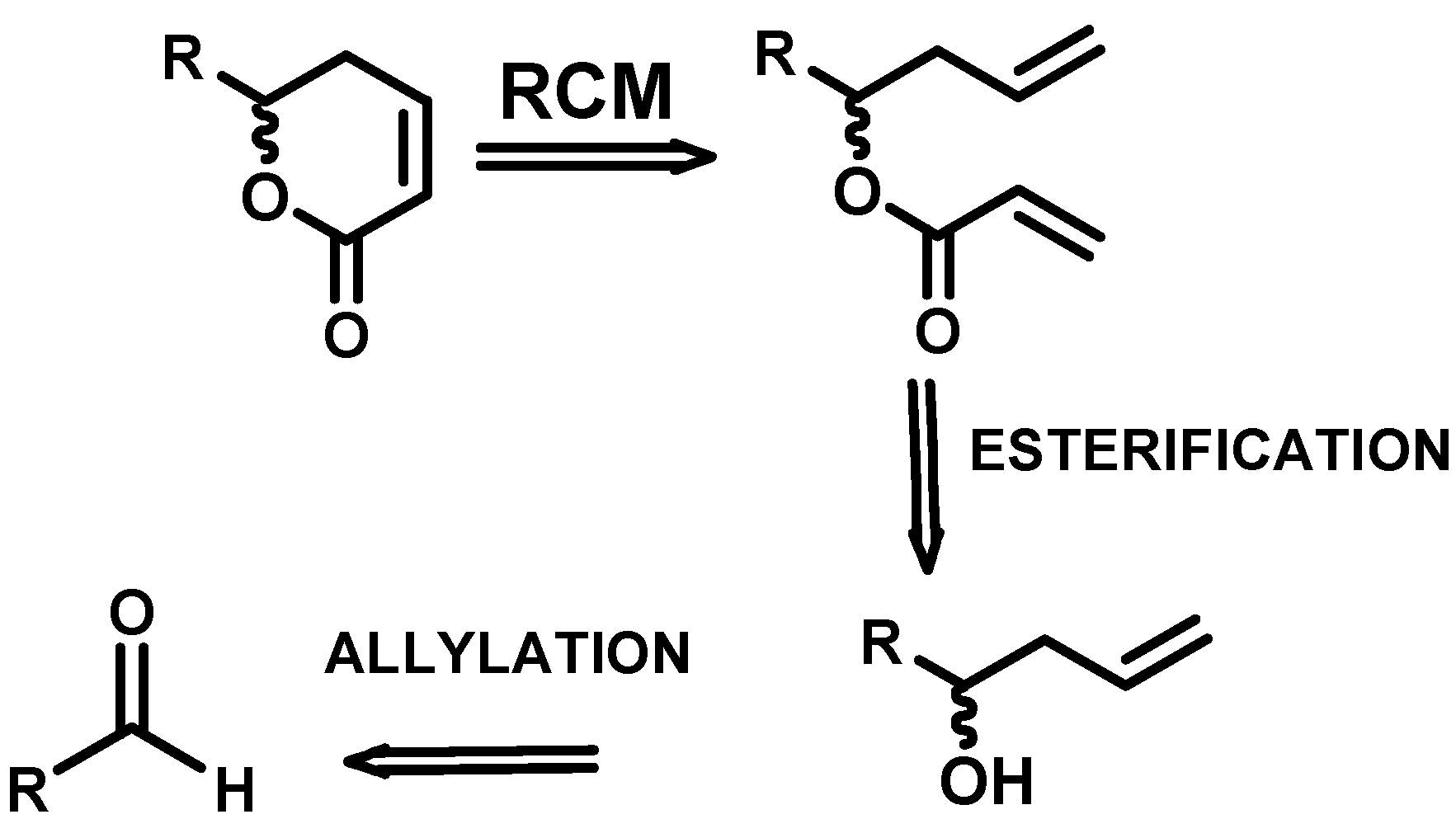
2.1. Synthetic plan
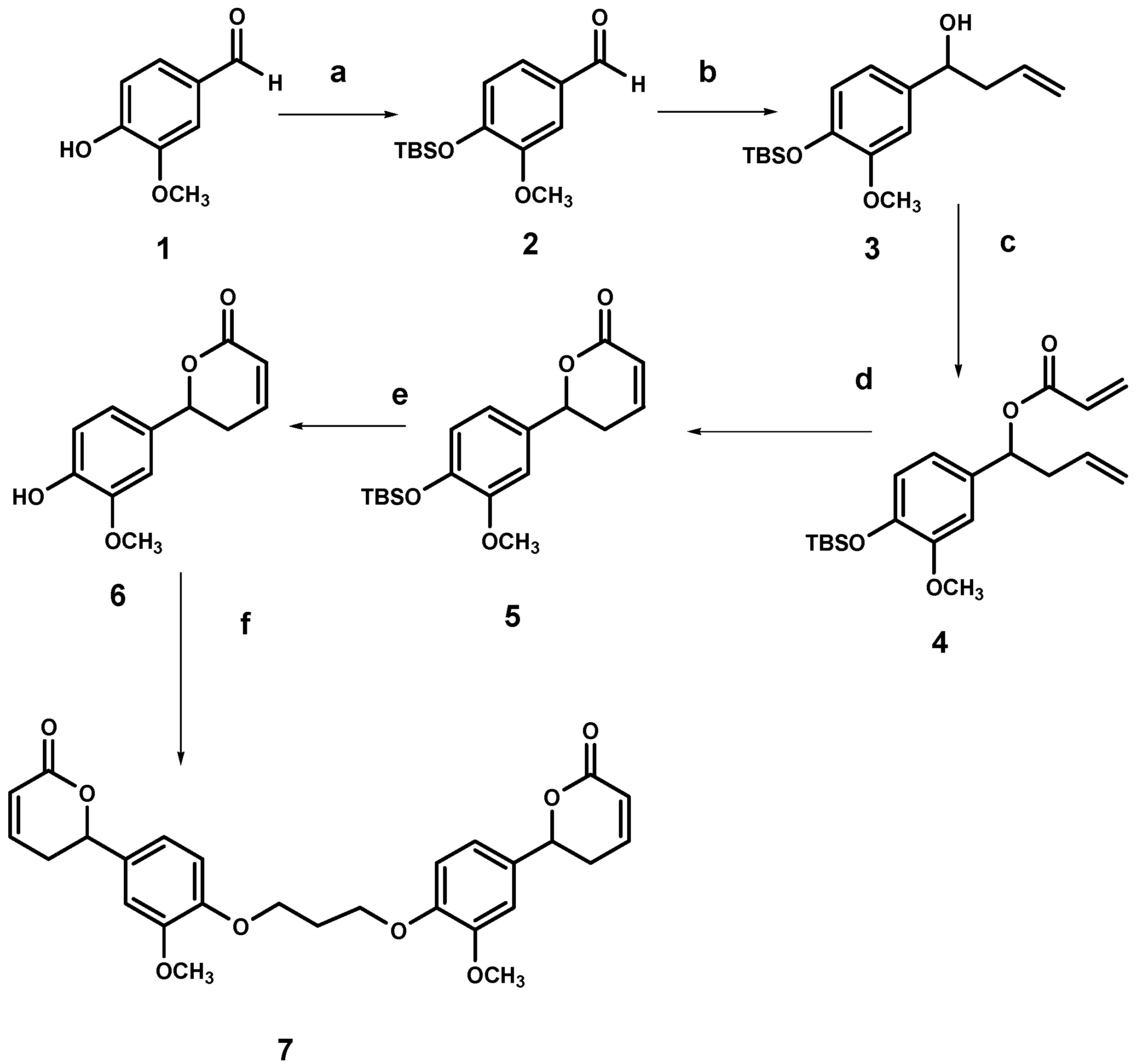
2.2. Synthesis of the dimer of 6-(4-hydroxy-3-methoxyphenyl)-5,6-dihydro-pyran-2-one (7)
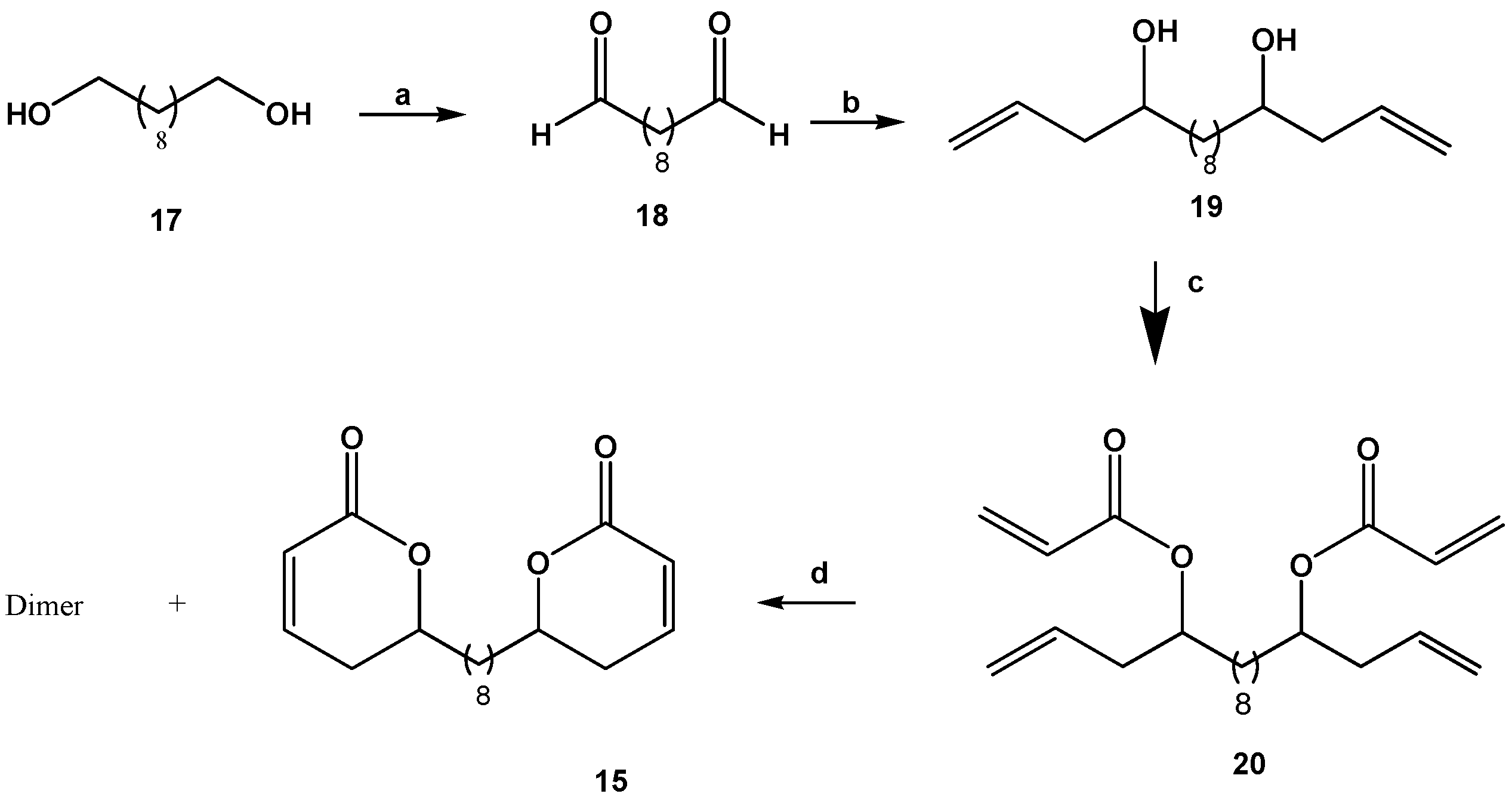
2.3. Synthesis of Dilactone 15
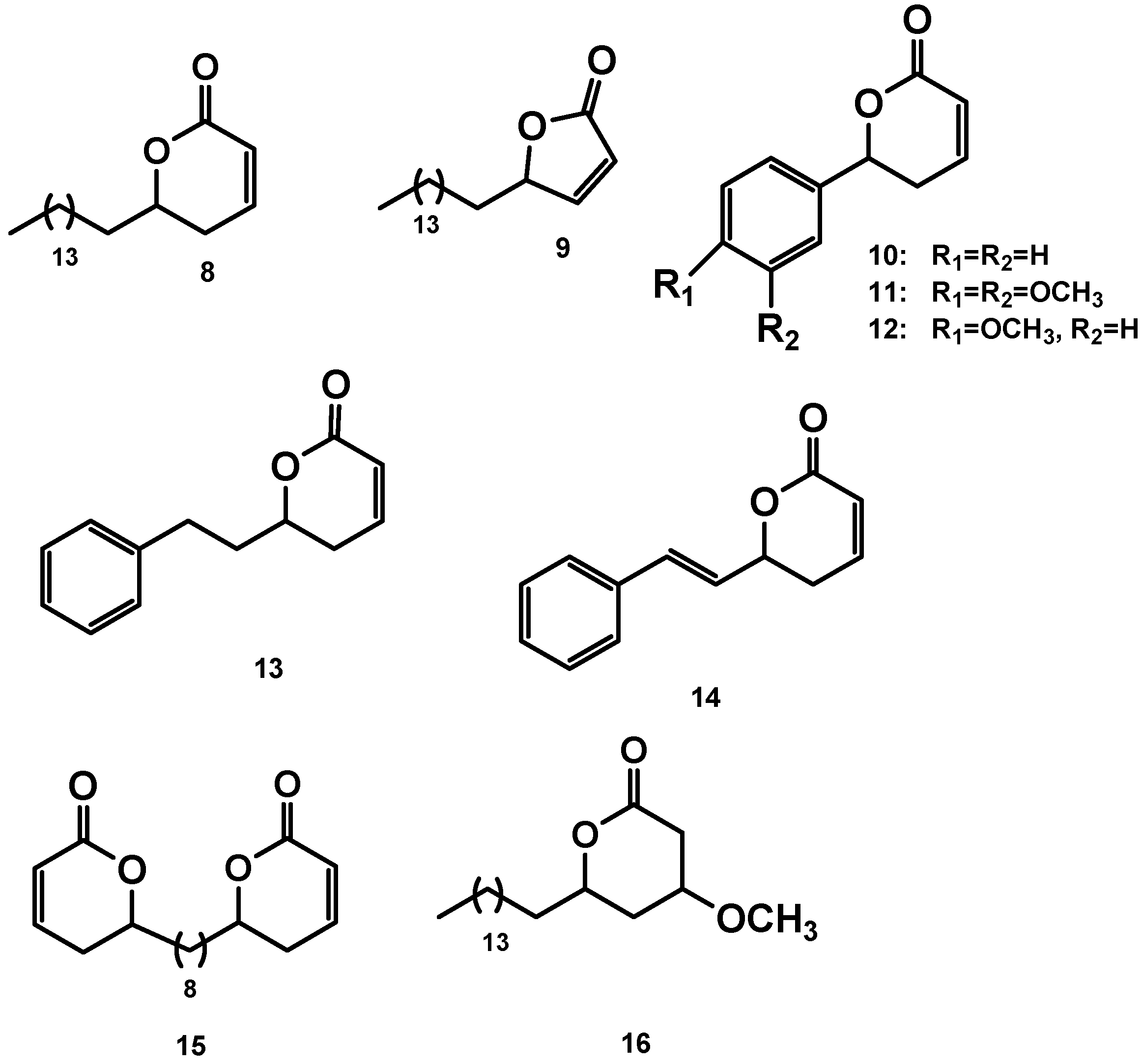
2.4. Synthesis of compounds 9, 15 and the Michael adduct 16
2.5. Leishmanicidal Activity Studies
| Compound | Cytotoxicity | Leishmanicidal Activity | |
|---|---|---|---|
| LC50 (μg/mL) | EC50 (μg/mL) | SI* | |
| 6 | 27.6 ± 5.9 | 37.9 ± 1.4 | 0.7 |
| 7 | 54.1 ± 3.1 | 22.2 ± 3.2 | 2.4 |
| 8 | 3.5 ± 0.4 | 0.8 ± 0.2 | 4.4 |
| 9 | 33.9 ± 1.4 | 2.8 ± 0.8 | 12.1 |
| 10 | 1.4 ± 0.1 | 4.5 ± 0.3 | 0.3 |
| 11 | 0.4 ± 0.03 | 1.6 ± 0.2 | 0.3 |
| 12 | 1.0 ± 0.04 | 8.6 ± 0.3 | 0.1 |
| 13 | 2.5 ± 0.3 | 7.5 ± 2.1 | 0.3 |
| 14 | 2.2 ± 0.3 | 1.9 ± 0.1 | 1.2 |
| 15 | 1.0 ± 0.01 | 1.4 ± 0.3 | 0.7 |
| 16 | 51.2 ± 6.0 | 47.2 ±10.3 | 1.1 |
 | |||
| 3.7** | 0.20** | 18.5** | |
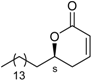 | |||
| 4.0** | 0.22** | 18.1** | |
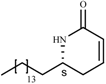 | |||
| 45.1** | 3.42** | 13.2** | |
| Glucantime | 416.4 | 6.7 | 59.6 |
3. Experimental
3.1. General
3.2. General synthetic procedures
3.3. Bioassays
4. Conclusions
Acknowledgements
References and Notes
- Ouellette, M. Biochemical and molecular mechanisms of drug resistance in parasites. Trop. Med. Int. Health 2001, 6, 874–882. [Google Scholar] [CrossRef]
- Available online: www.who.int/tdr/diseases/default.htm.
- Available online: www.who.int/infectious-disease-report/2009/ch4.htm.
- Hoffmann, H.M.R.; Rabe, J. Synthesis and Biological Activity of α -Methylene-γ-butyrolactones. Angew. Chem. Int. Ed. Engl. 1985, 24, 94–110. [Google Scholar] [CrossRef]
- Negishi, E.; Kotora, M. Regio- and stereoselective synthesis of gamma-alkylidene butenolides and related compounds. Tetrahedron 1997, 53, 6707–6738. [Google Scholar] [CrossRef]
- Collins, I. Saturated and unsaturated lactones. J. Chem. Soc. Perkin Trans. I 1999, 1377–1396. [Google Scholar] [CrossRef]
- Carter, N.B.; Nadany, A.E.; Sweeney, J.B. Recent developments in the synthesis of furan-2(5H)-ones. J. Chem. Soc. Perkin Trans. I 2002, 34, 2324–2342. [Google Scholar] [CrossRef]
- Saeed, M.; Thomas, H.; Schick, M.; Abbas, M.; Voelter, W. Total synthesis and antileishmanial activity of R-(-)-argentilactone. Tetrahedron Lett. 2001, 42, 7401–7403. [Google Scholar] [CrossRef]
- Davies-Coleman, M.; Rivett, D. Stereochemical studies on boronolide, an α-pyrone from Tetradenia barberae. Phytochemistry 1987, 26, 3047–3050. [Google Scholar] [CrossRef]
- Cardona, W.; Quinones, W.; Robledo, S.; Velez, I.; Murga, J.; Garcıa-Fortanet, J.; Carda, M.; Cardona, D.; Echeverri, F. Antiparasite and antimycobacterial activity of passifloricin analogues. Tetrahedron 2006, 62, 4086–4092. [Google Scholar] [CrossRef]
- Kirkland, T.A.; Grubbs, R.H. Effects of Olefin Substitution on the Ring-Closing Metathesis of Dienes. J. Org. Chem. 1997, 62, 7310–7318. [Google Scholar] [CrossRef]
- Bassetti, M.; D’Annibale, A. Synthesis of α,β-Unsaturated 4,5-Disubstituted γ-Lactones via Ring-Closing Metathesis Catalyzed by the First-Generation Grubbs' Catalyst. Org. Lett. 2005, 7, 1805–1808. [Google Scholar] [CrossRef]
- Schuster, M.; Blechert, S.A. An Olefin Metathesis/Double Bond Isomerization Sequence Catalyzed by an In Situ Generated Ruthenium Hydride Species. Angew. Chem. Int. Ed. 1997, 36, 2036–2055. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Chang, S. Recent Advances in Olefin Metathesis and Its Application in Organic Synthesis. Tetrahedron 1998, 54, 4413–4450. [Google Scholar] [CrossRef]
- Fürstner, A.; Grela, K. Ring Closing Alkyne Metathesis. Application to the Stereoselective Total Synthesis of Prostaglandin E2-1,15 Lactone. Angew. Chem. Int. Ed. 2000, 39, 1234–1236. [Google Scholar] [CrossRef]
- Armstrong, S.K. Ring closing diene metathesis in organic synthesis. J. Chem. Soc. Perkin Trans. 1998, 1, 371–388. [Google Scholar] [CrossRef]
- Gosh, A.K.; Liu, C.A. Stereoselective Synthesis of (-)-Tetrahydrolipstatin. Chem. Commun. 1999, 1743–1744. [Google Scholar]
- Cossy, J.; Bauer, D.; Bellosta, V. Oxidation of Substituted Spiro[bicyclo[n.1.0]alkane-2,2’-[1,3]dioxolanes]. Formation of substituted lactones. Tetrahedron Lett. 1999, 40, 4187–4188. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Reddy, M.V.R.; Brown, H.C. Asymmetric synthesis of goniothalamin, hexadecanolide, massoia lactone, and parasorbic acid via sequential allylboration-esterification ring-closing metathesis reactions. Tetrahedron Lett. 2000, 41, 583–586. [Google Scholar] [CrossRef]
- Carda, M.; Rodrıguez, S.; Gonzalez, F.; Castillo, E.; Villanueva, A.; Marco, J.A. Stereoselective Synthesis of the Naturally Occurring Lactones (-)-Osmundalactone and (-)-Muricatacine Using Ring-Closing Metathesis. Eur. J. Org. Chem. 2002, 15, 2649–2655. [Google Scholar]
- Cardona, W.; Quinones, W.; Echeverri, F. Leishmanicidal Activity of Passifloricin A and Derivatives. Molecules 2004, 9, 666–672. [Google Scholar] [CrossRef]
- Corey, E.J.; Cho, H.; Rucker, C.; Hua, D. Studies with trialkylsilyltriflates: new syntheses and applications. Tetrahedron Lett. 1981, 22, 3455–3458. [Google Scholar]
- Cossy, J.; Bauer, D.; Bellosta, V. Formal total synthesis of (+)-methynolide. Tetrahedron 2002, 58, 5909–5922. [Google Scholar] [CrossRef]
- Trnka, T.; Grubbs, R. Development of L2X2Ru▬CHR Olefin Metathesis Catalysts: An Organometallic Success Story: Grubbs. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Clark, S.; Whitlock, G. A Short Synthesis of (±)-Decarestrictine L. Tetrahedron Lett. 1994, 35, 6381–6382. [Google Scholar] [CrossRef]
- Mayence, A.; Pietka, A.; Collins, M.S.; Cushion, M.T.; Tekwani, B.L.; Huang, T.L.; Vanden Eynde, J.J. Novel bisbenzimidazoles with antileishmanial effectiveness. Bioorg. Med. Chem. Lett. 2008, 18, 2658–2661. [Google Scholar] [CrossRef]
- Nakashima, K.; Imoto, M.; Miki, T.; Miyake, T.; Fujisaki, N.; Fukunaga, S.; Mizutani, R.; Sono, M.; Tori, M. Ring closing metathesis reaction of dienes with acrylate moiety leading to 5- to 7-membered lactones and cyclization to 14-membered rings. Heterocycles 2002, 56, 85–89. [Google Scholar] [CrossRef]
- Pilli, R.; Fatima, N. Synthetic process for the preparation of (R)-(+)- and (S)-(-)-goniothalamin and derivatives via asymmetric catalytic allylation of aldehydes, esterification, and olefin metathesis reaction. BR 2003003756 A 2005 0419, 2005. [Google Scholar]
- Murga, J.; Garcia-Fortanet, J.; Carda, M.; Marco, J.A. Asymmetric synthesis of passifloricin A: a correction in structure. Tetrahedron Lett. 2003, 44, 7909–7912. [Google Scholar]
- Sereno, D.; Lemestre, L. Use of an enzymatic micromethod to quantify amastigote stage of Leishmania amazonensis in vitro. Parasitol. Res. 1997, 83, 401–403. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 8-16 and NMR and MS spectra are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castaño, M.; Cardona, W.; Quiñones, W.; Robledo, S.; Echeverri, F. Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity. Molecules 2009, 14, 2491-2500. https://doi.org/10.3390/molecules14072491
Castaño M, Cardona W, Quiñones W, Robledo S, Echeverri F. Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity. Molecules. 2009; 14(7):2491-2500. https://doi.org/10.3390/molecules14072491
Chicago/Turabian StyleCastaño, Marcela, Wilson Cardona, Winston Quiñones, Sara Robledo, and Fernando Echeverri. 2009. "Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity" Molecules 14, no. 7: 2491-2500. https://doi.org/10.3390/molecules14072491
APA StyleCastaño, M., Cardona, W., Quiñones, W., Robledo, S., & Echeverri, F. (2009). Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity. Molecules, 14(7), 2491-2500. https://doi.org/10.3390/molecules14072491