Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes
Abstract
:Introduction
Results and Discussion
Relationship between the antifungal activity and chemical structure of thiosemicarbazides
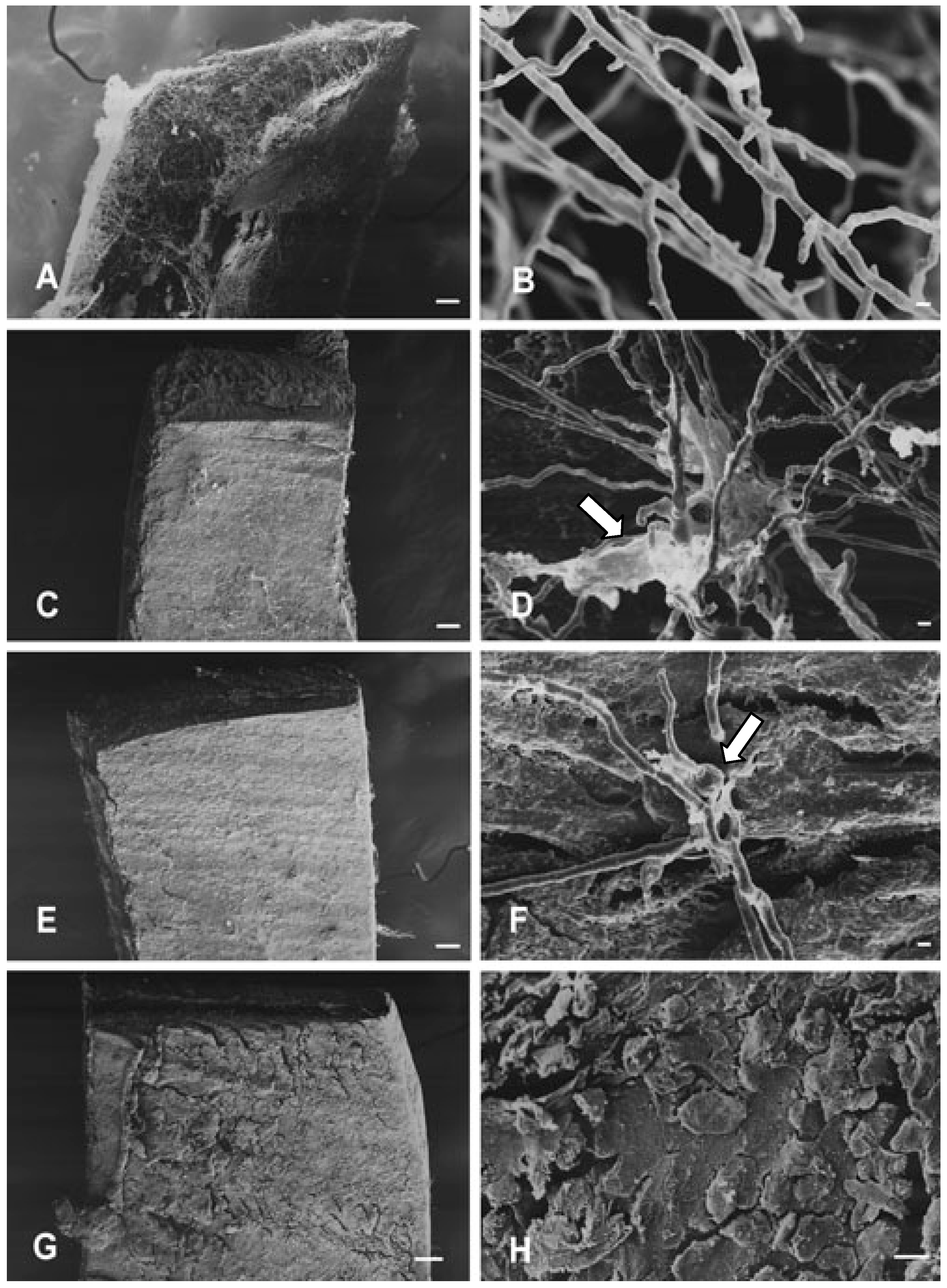
Effects on hyphae structure and inhibitory activity of TIO C on the invasion of nails
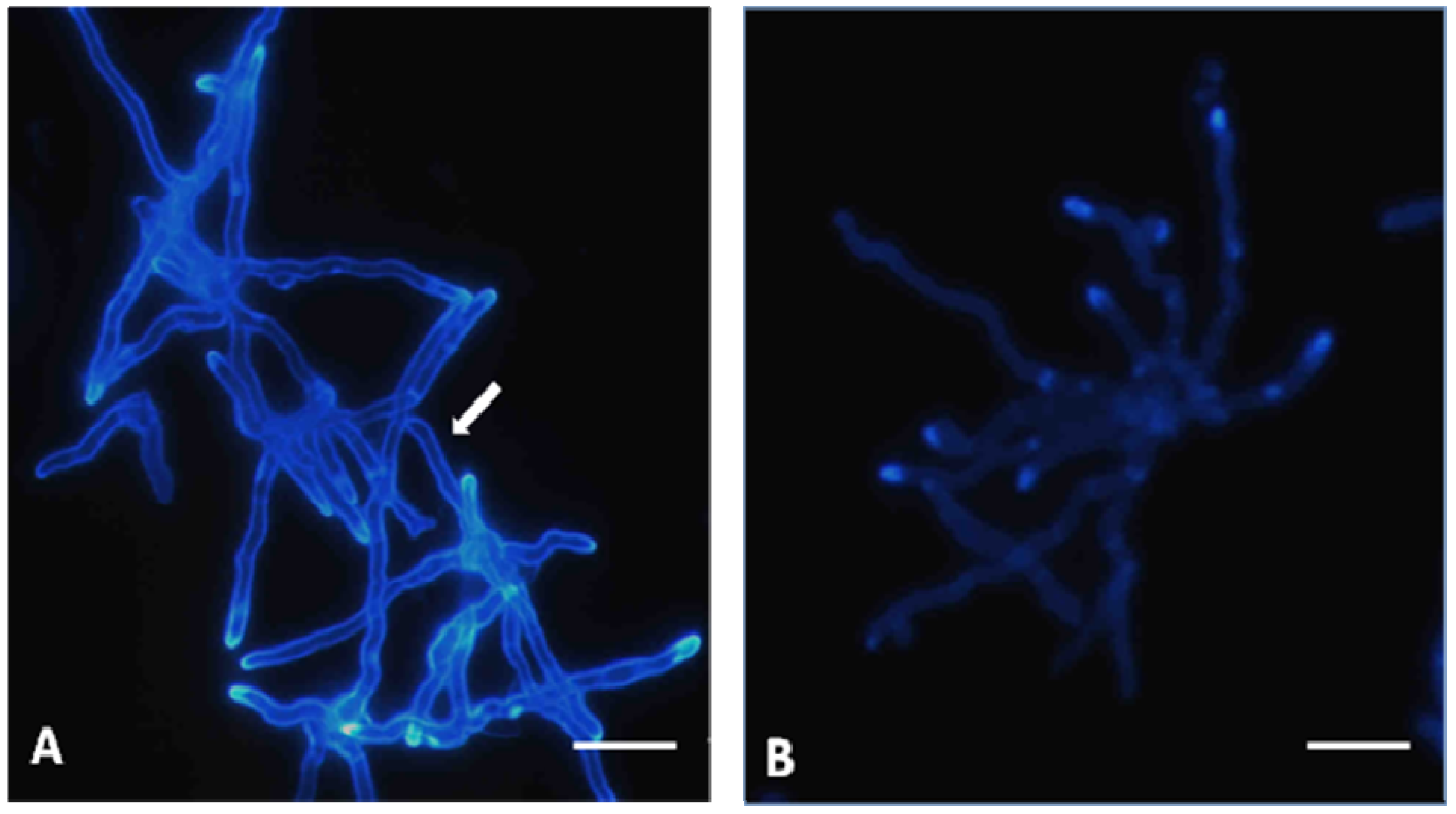
Absence of linkage between ergosterol and TIO C
Effects of TIO C on cell wall structure
Experimental
General
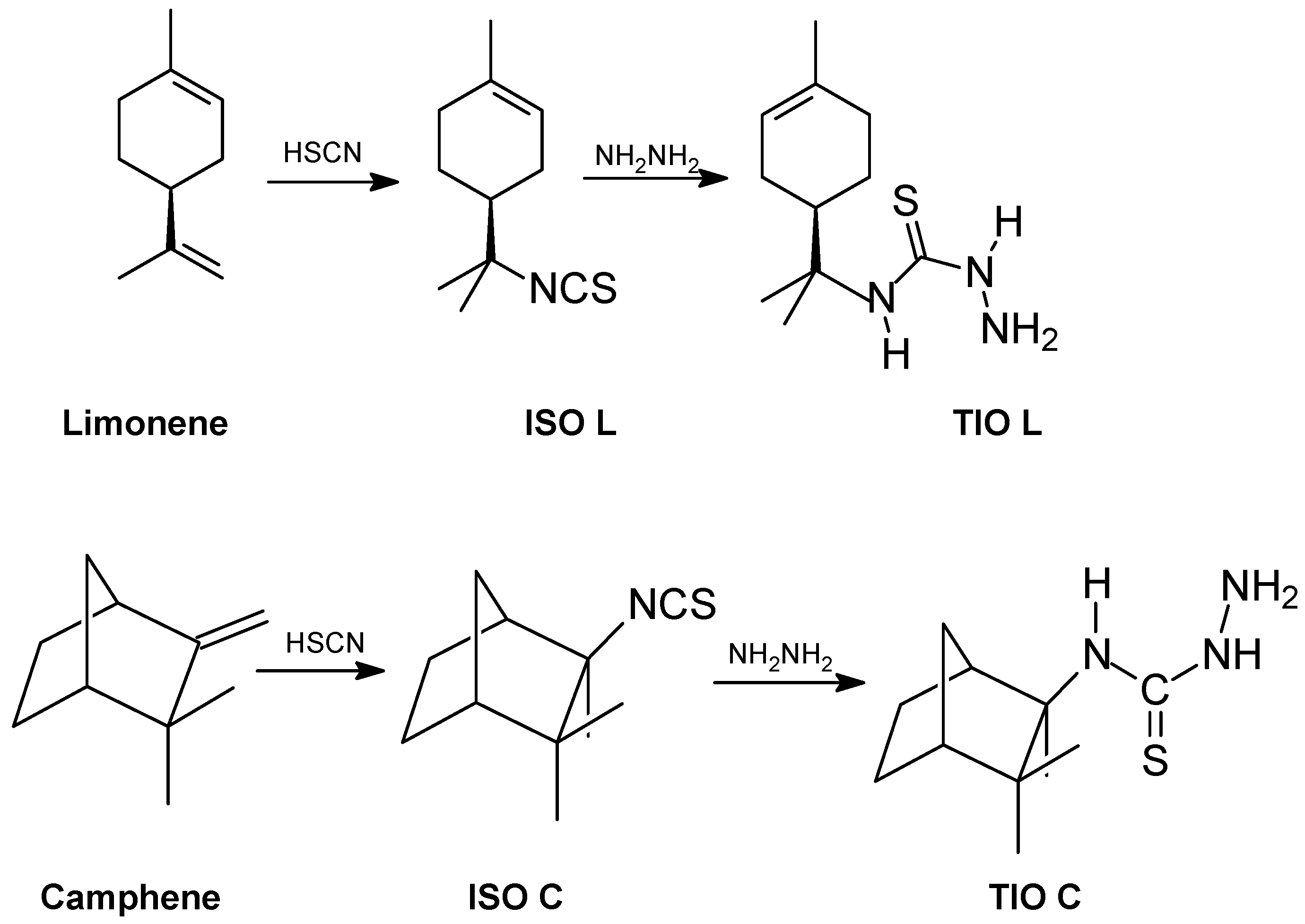
Synthesis of thiosemicarbazides
Strain and culture conditions
Fungal Inhibitory activity
Fungicidal activity
Scanning electron microscopy (SEM)
Nail fragments invasion test
Fungus cell wall integrity analysis
Ergosterol effect assay
Conclusions
Acknowledgements
References and Notes
- Campbell, A.W.; Anyanwu, E.C.; Morad, M. Evaluation of the drug treatment and persistence of onychomycosis. Sci. World J. 2004, 4, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Viktor, A.C.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.S. Onychomycosis in the elderly: drug treatment options. Drugs Aging 2007, 24, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbia.l Res. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Hajjeh, R.A.; Scher, R.; Konnikov, N.; Grupta, A.K.; Summerbell, R.; Sullivan, S.; Daniel, R.; Krusinski, P.; Fleckman, P.; Rich, P.; Odom, R.; Aly, R.; Pariser, D.; Zaiac, M.; Rebell, G.; Lesher, J.; Gerlach, B.; Ponde-de-Leon, G.F.; Ghannoum, A. A large-sacle North American study of fungal isolates from nails: the frequency of onycomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000, 43, 641–648. [Google Scholar]
- Iorizzo, M.; Piraccini, B.M.; Tosti, A. New fungal nail infections. Curr. Opin. Infect. Dis. 2007, 20, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M; Campbell, C.K.; Fraser, M.; Johnson, E.M. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med. Mycol. 2007, 45, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Degreef, H.; Cauwemberg, G. Fungal infections of the skin: infection process and antimycotic therapy. Curr. Drug Targets 2005, 6, 849–862. [Google Scholar] [CrossRef]
- Hainer, B.L. Dermatophyte Infections. Am. Fam. Physician 2003, 76, 101–108. [Google Scholar]
- Seebacher, C. Action mechanisms of modern antifungal agents and resulting problems in the management of onychomycosis. Mycoses 2003, 46, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Mock, M.M.; Baudraz-Rosselet, F.; Panizzon, R.G. Tinea capitis dermatophytes: susceptibility to antifungal drugs tested in vitro and in vivo. Dermatology 1998, 197, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Pintilie, O.; Profire, L.; Sunel, V.R.; Popa, M.; Pui, A. Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D,L-methionine moiety. Molecules 2007, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Athar, F.; Van Zyl, R.L.; Chen, C.T.; Azam, A. Synthesis and biological evaluation of novel 4-substituted 1-{[4-(10,15,20-triphenylporphyrin-5-yl)phenyl]methylidene} Thiosemi-carbazides as new class of potential antiprotozoal agents. Chem. Biodivers. 2008, 5, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Lima, R.S.; Moreira, D.R.; Cardoso, M.V.; Gouveia de Brito, A.C.; Farias dos Santos, L.M.; Hernandes, M.Z.; Kiperstok, A.C.; Lima, R.S.; Soares, M.B. Synthesis, docking, and in vivo activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acyl-thiazolidones against Trypanosoma cruzi. Bioorg. Med. Chem. 2006, 14, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, N.; Veena, A.; Lavarasan, R.; Adiraj, M.; Shanmugapandiyan, P.; Sridhara, S.K. Syntesis and biological activities of 2,6-Diaryl-3-metyl-4-piperidone derivatives. Biol. Pharm. Bull. 2003, 26, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.K.; Singh, L.; Sharma, D.K. Synthesis, spectral, and biological properties of Copper(II) complexes of thiosemicarbazones of Schiff bases derived from 4-aminoantipyrine and aromatic aldehydes. Bioinorg. Chem. Appl. 2006, 2006, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, N.; Karaoglu, S.A.; Demirbas, A.; Sancak, K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4] triazole derivatives. Eur. J. Med. Chem. 2004, 39, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Dogan, N.H.; Rollas, S.; Erdeniz, H. Synthesis, structure elucidation and antimicrobial activity of some 3-hydroxy-2-naphthoic acid hydrazide derivatives. Il Farmaco 1998, 30, 462–467. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: mechanism of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derivates from isatin derivatives and N-[4-(4’-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, S.H.; Li, Z.M.; Su, N.; Zhao, W.Z. Synthesis of novel 2-phenylsulfonyl-hydrazono-3-(2′,3′,4′,6′-tetra-O-acetyl-beta-d-glucopyranosyl) thiazolidine-4-ones from thiosemi-carbazide precursors. Carbohydr. Res. 2006, 341, 2867–2870. [Google Scholar] [CrossRef] [PubMed]
- Salgin-Göksen, U.; Gökhan-Kelekçi, N.; Göktaş, O.; Köysal, Y.; Kiliç, E.; Işik, S.; Aktay, G.; Ozalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kong, C.H.; Zhang, C.X. Chemical composition and antimicrobial activity of the essential oil from Ambrosia trifida L. Molecules 2006, 11, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Letchamo, W.; Ward, W.; Heard, B.; Heard, D. Essential oil of Valeriana officinalis L. cultivars and their antimicrobial activity as influenced by harvesting time under commercial organic cultivation. J. Agric. Food Chem. 2004, 52, 3915–3919. [Google Scholar] [CrossRef] [PubMed]
- Magwa, M.L.; Gundidza, M.; Gwerua, N.; Humphrey, G. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, E.; Cardia, M.C.; Bonsignore, L.; Plumitallo, A.; Pellerano, M.L.; De Logu, A. Synthesis and anti-microbial activity of isothiosemicarbazones and cyclic analogues. Il Farmaco 2002, 57, 809–817. [Google Scholar] [CrossRef]
- Maccioni, E.; Cardia, M.C.; Distinto, S.; Bonsignore, L.; De Logu, A. An investigation of biological effect of structural modifications investigation of isothiosemicarbazones and their cyclic analogues. Il Farmaco 2003, 58, 951–959. [Google Scholar] [CrossRef]
- Peng, C.; Dong, C.; Hou, Qiaomin, H.; Xu, C.; Zhao, J. The hydrophobic surface of PaAMP from pokeweed seeds is essential to its interaction with fungal membrane lipid and the antifungal activity. FEBS 2005, 579, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.W. Amphotericin B: an introduction. J. Antimicrob. Chemother. 1991, 28, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sortino, M.; Delgado, P; Juarez, S.; Quiroga, J.; Aboni, R.; Insuasty, B.; Nogueras, M.; Rodero, L.; Garibotto, F.M.; Enriz, R.D.; Zacchino, A.A. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg. Med. Chem. 2007, 15, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Almagro, V.; Marsaioli, A.J. A direct route to terpene isothiocyanates. Tetrahedron Lett. 2003, 34, 6717–6720. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard M38-A; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Rashid, A.; Scott, E.M.; Richardson, M.D. Inhibitory effect of terbinafine on the invasion of nails by T. mentagrophytes. J. Am. Acad. Dermatol. 1995, 33, 718–723. [Google Scholar] [CrossRef]
Sample Availability: The samples are available from the authors. |
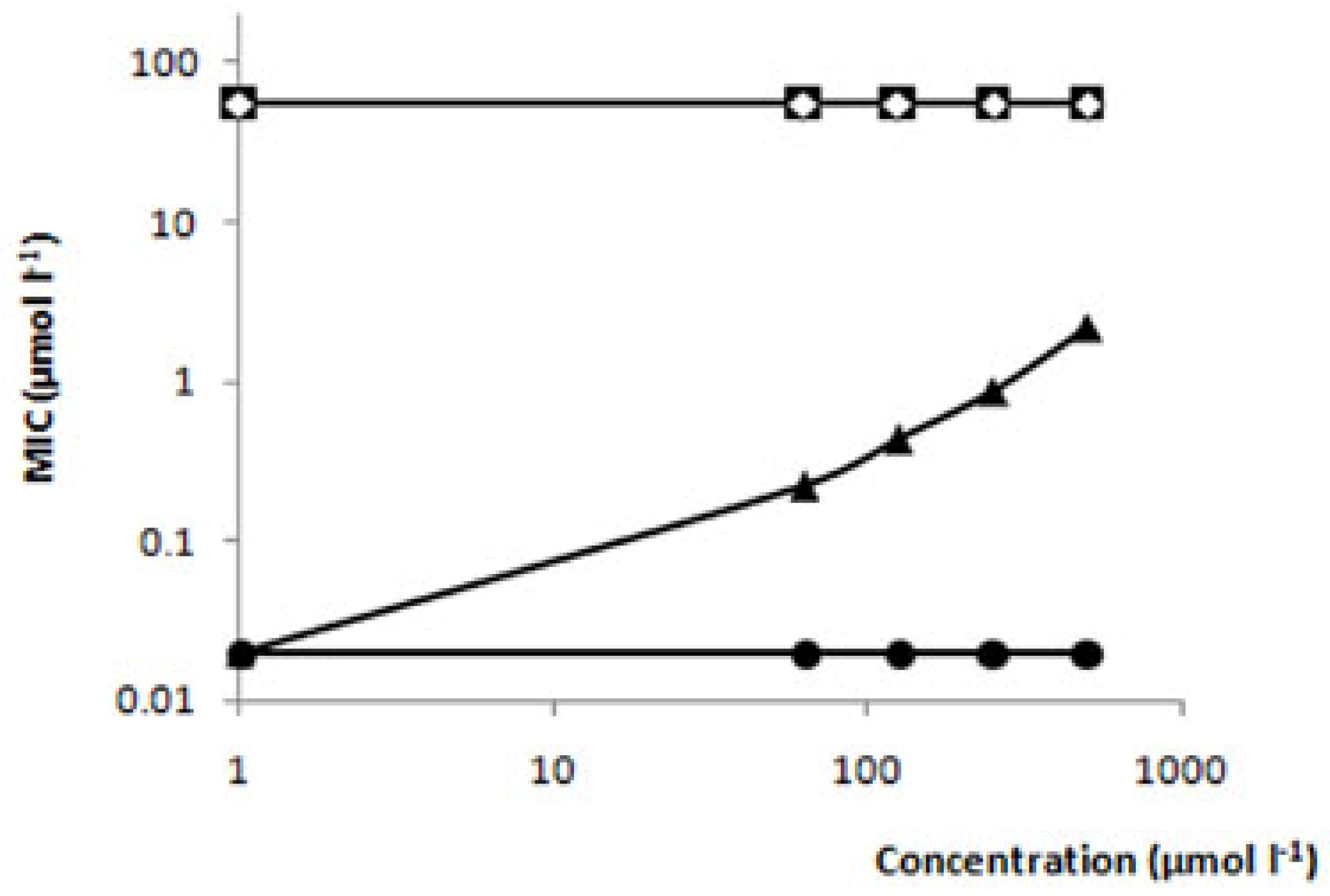
| Compound | Structure | MIC (μmol L-1) | MFC (μmol L-1) |
|---|---|---|---|
| Limonene | 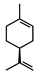 | >735 | >735 |
| Camphene | 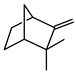 | >735 | >735 |
| TIO | 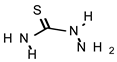 | 548 | >735 |
| TIO L | 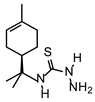 | 110 | 220 |
| TIO C | 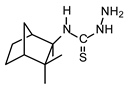 | 55 | 110 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamaguchi, M.U.; Barbosa da Silva, A.P.; Ueda-Nakamura, T.; Dias Filho, B.P.; Conceição da Silva, C.; Nakamura, C.V. Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796-1807. https://doi.org/10.3390/molecules14051796
Yamaguchi MU, Barbosa da Silva AP, Ueda-Nakamura T, Dias Filho BP, Conceição da Silva C, Nakamura CV. Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes. Molecules. 2009; 14(5):1796-1807. https://doi.org/10.3390/molecules14051796
Chicago/Turabian StyleYamaguchi, Mirian Ueda, Ana Paula Barbosa da Silva, Tânia Ueda-Nakamura, Benedito Prado Dias Filho, Cleuza Conceição da Silva, and Celso Vataru Nakamura. 2009. "Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes" Molecules 14, no. 5: 1796-1807. https://doi.org/10.3390/molecules14051796
APA StyleYamaguchi, M. U., Barbosa da Silva, A. P., Ueda-Nakamura, T., Dias Filho, B. P., Conceição da Silva, C., & Nakamura, C. V. (2009). Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes. Molecules, 14(5), 1796-1807. https://doi.org/10.3390/molecules14051796




