Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets
Abstract
:1. Introduction
2. Results and Discussion
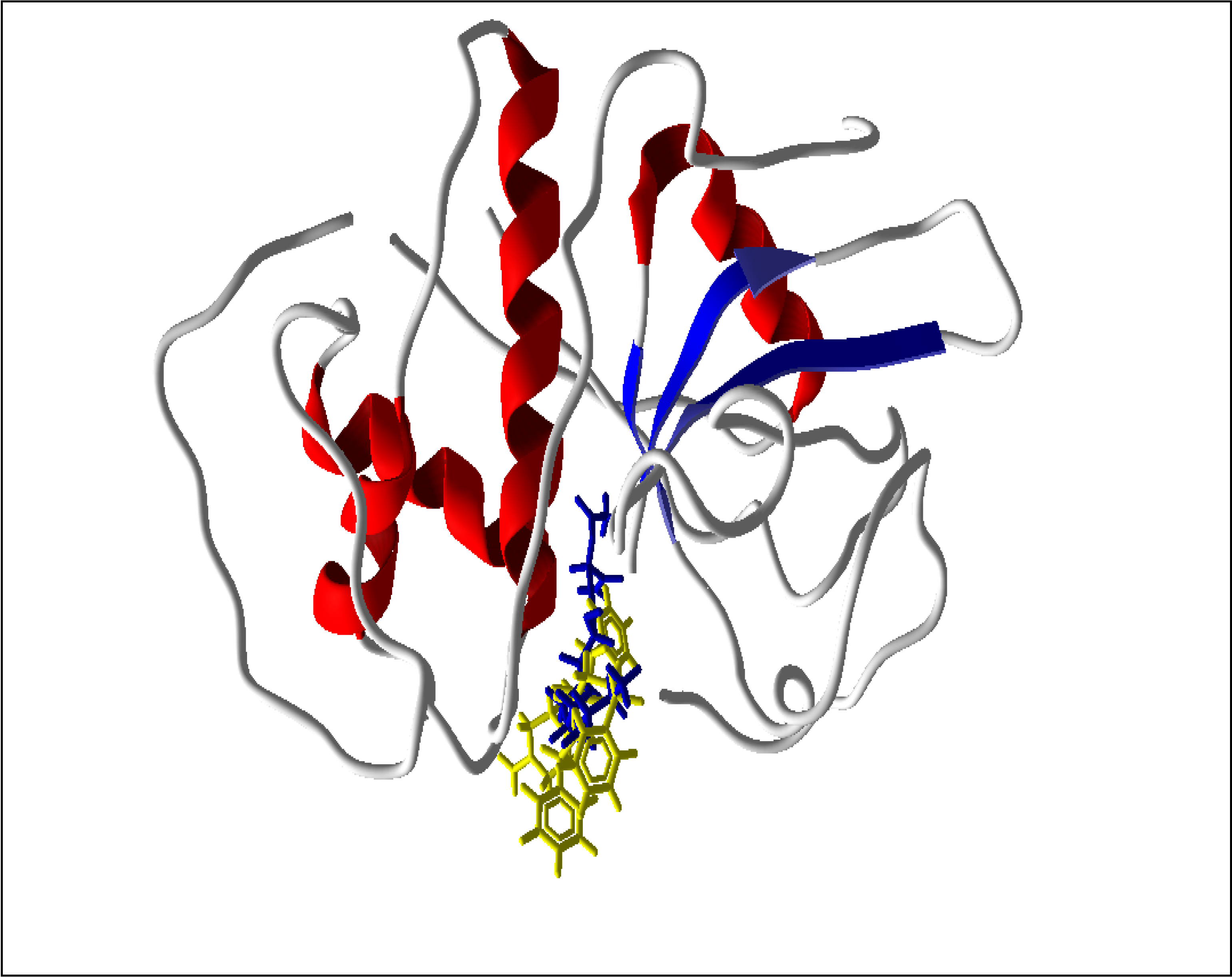
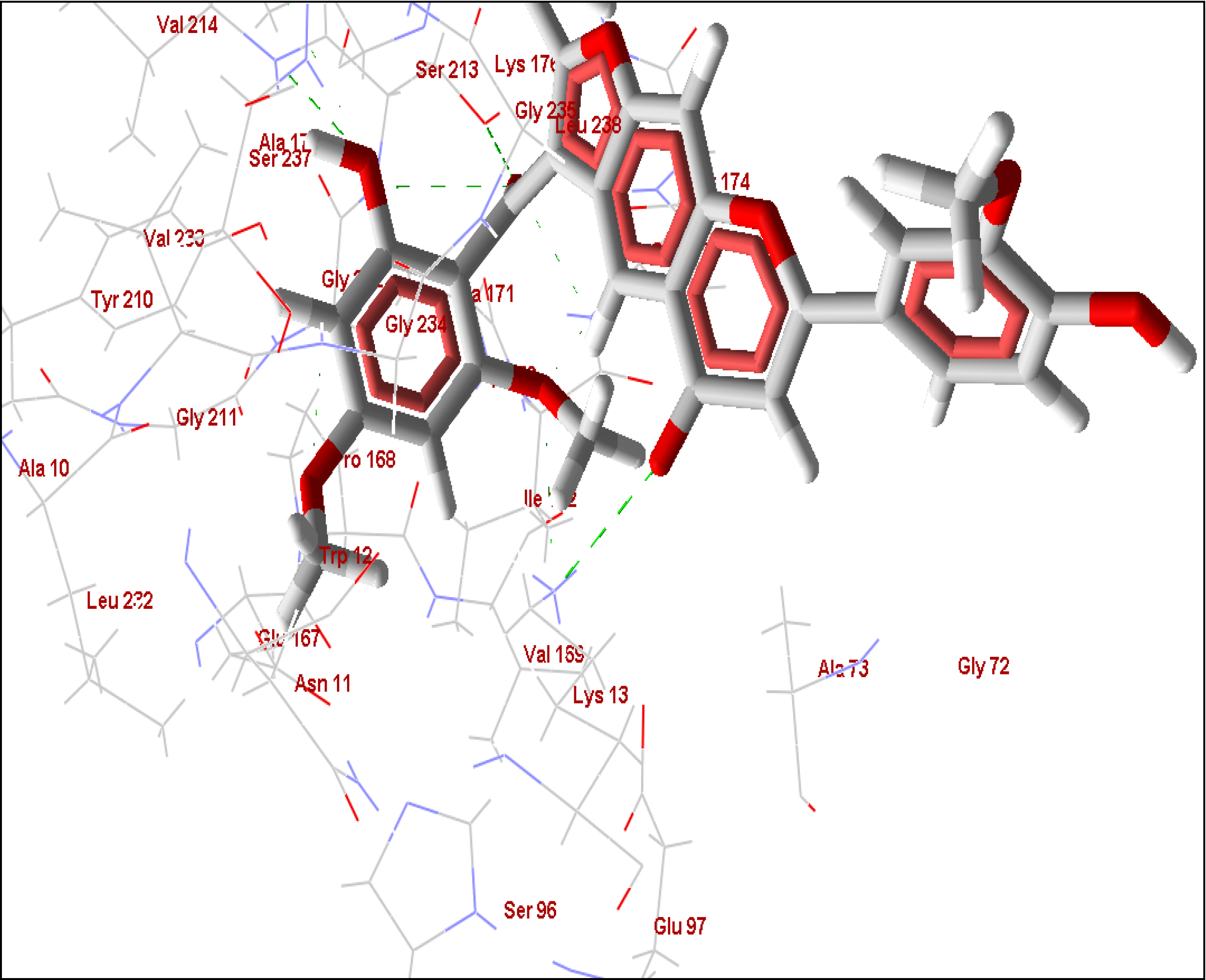
2.1. Farnesyl Diphosphate Synthase
| Compounds | Rerank Pose Scorea | ||||
|---|---|---|---|---|---|
| Rhodesain | Triosephosphate Isomerase (TIM) | Farnesyl diphosphate synthase (FDS) | Trypanothione reductase (TR) | IC50 for T. brucei (µM) | |
| Iridiods | |||||
| 6-O-methylcatalpol | -83.03 | -86.80 | -110.00 | -104.04 | 86 [10] |
| 6-O-β-d-xylopyranosylaucubin | -83.91 | -77.47 | -121.65 | -83.30 | 79 [10] |
| Ajugol | -21.62 | -87.10 | -109.50 | -102.89 | 91 [10] |
| Ajugoside | -78.81 | -99.60 | -113.83 | -113.83 | 144 [10] |
| Aucubin | -80.26 | -98.53 | -101.28 | -119.70 | 148 [10] |
| Catalpol | -66.34 | -75.50 | -75.14 | -81.19 | 151 [10] |
| Ningpogenin | -101.68 | -79.62 | -124.27 | -122.97 | 172 [10] |
| Scrolepidoside | -68.48 | -68.83 | -101.37 | -89.86 | 49 [10] |
| Isoquinone Alkaloids | |||||
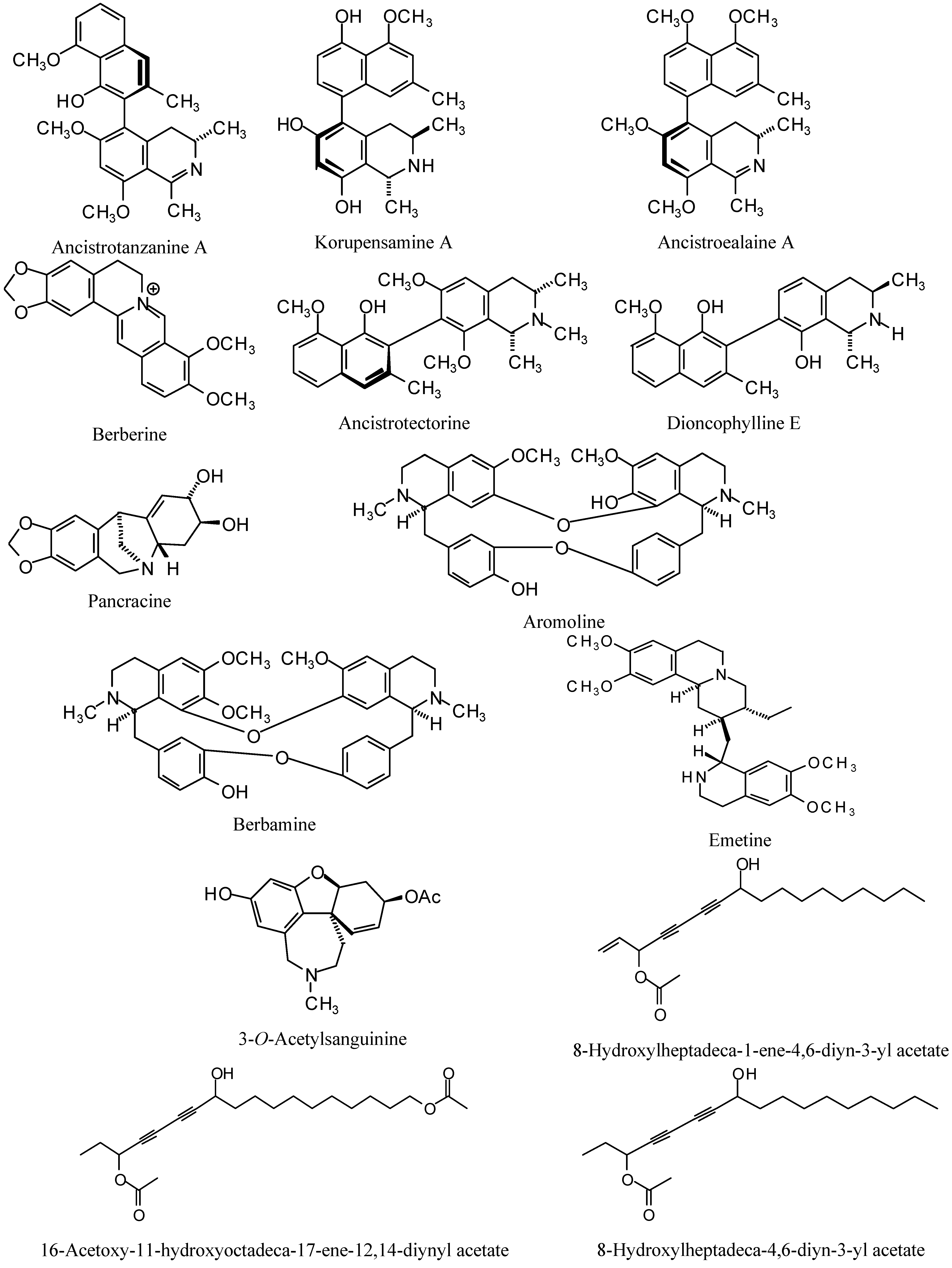
| Ancistroealaine A | -70.34 | -68.84 | -57.11 | -74.17 | 8.25 [11] |
| Ancistrogriffine A | -67.72 | -79.84 | -77.90 | -88.33 | 5.53 [12] |
| Ancistrogriffine C | -64.00 | -86.71 | -88.96 | -78.06 | 7.85 [12] |
| Ancistrogriffithine A | -85.35 | -95.68 | 137.47 | -53.62 | 1.15 [12] |
| Ancistrolikokine D | -79.61 | -81.58 | -94.94 | -68.74 | 6.93 [13] |
| Ancistrotanzanine A | -76.87 | -90.86 | -43.39 | -53.78 | 1.7 [14] |
| Ancistrotanzanine B | -71.15 | -89.98 | -83.70 | -73.43 | 1.7 [16] |
| Ancistrotanzanine C | -76.33 | -84.97 | -35.30 | -6.62 | 3.2 [15] |
| Ancistrotectonine | -68.12 | -84.82 | -93.37 | 14.88 | 10.2 [15] |
| Aromoline | -29.94 | -76.77 | 160.86 | -51.33 | 1.48 [16] |
| Berbamine | 20.95 | -36.57 | 478.31 | -24.28 | 2.6 [17] |
| Berberine | -82.07 | -86.90 | -92.33 | -107.69 | 0.53 [17] |
| Dioncophylline E | -74.74 | -93.87 | -71.17 | -88.05 | 2.1 [18] |
| Emetine | -69.79 | -98.46 | -111.37 | -103.12 | 0.039 [17] |
| Korupensamine A | -69.39 | -89.57 | -76.20 | -4.14 | 4.93 [19] |
| Nangustine | -29.03 | -83.45 | -88.71 | -91.92 | 33 [20] |
| Pancracine | -64.51 | -87.31 | -86.07 | -89.97 | 2.4 [20] |
| Miscellaneous Alkaloid | |||||
| 3-O-Acetylsanguinine | -66.14 | -77.68 | -84.68 | -85.43 | 3.5 [21] |
| Miscellaneous Compounds | |||||
| 8-Hydroxyheptadeca-1-ene-4,6-diyn-3-yl acetate | -93.68 | -109.03 | -118.13 | -132.24 | 0.46 [22] |
| 8-Hydroxylheptadeca-4,6-diyn-3-yl acetate | -105.81 | -100.58 | -103.24 | -127.20 | 18 [22] |
| 16-Acetoxy-11-hydroxyoctadeca-17-ene-12,14-diynyl acetate | -88.38 | -88.12 | -94.64 | -117.40 | 1.1 [22] |
| Aculeatin D | -58.63 | -92.09 | -122.28 | -129.51 | 0.48 [23] |
| Phenolics | |||||
| 1,7-bis(4-hydrophenyl)-heptane-3,5-dione | -42.99 | -49.29 | -76.29 | -91.90 | 7.4-8.3 [24] |
| 1,7-bis(4-hydrophenyl)-heptene-3,5-dione | -49.35 | -53.42 | -72.18 | -97.44 | 8.4 [24] |
| Ambigol A | -83.14 | -90.16 | -96.41 | -106.15 | 33 [25]b |
| Ambigol C | -85.42 | -95.93 | -101.70 | -111.03 | 11 [25]b |
| Angoroside C | -63.53 | -93.95 | -152.16 | -98.74 | 75 [10] |
| Chaetoxanthone A | -51.70 | -52.59 | -52.79 | -43.16 | 12.69 [26] |
| Chaetoxanthone B | -47.56 | -57.78 | -48.26 | -29.30 | 26.26 [26] |
| Cissampeloflavone | -105.24 | -114.60 | -141.43 | -135.28 | 1.99 [27] |
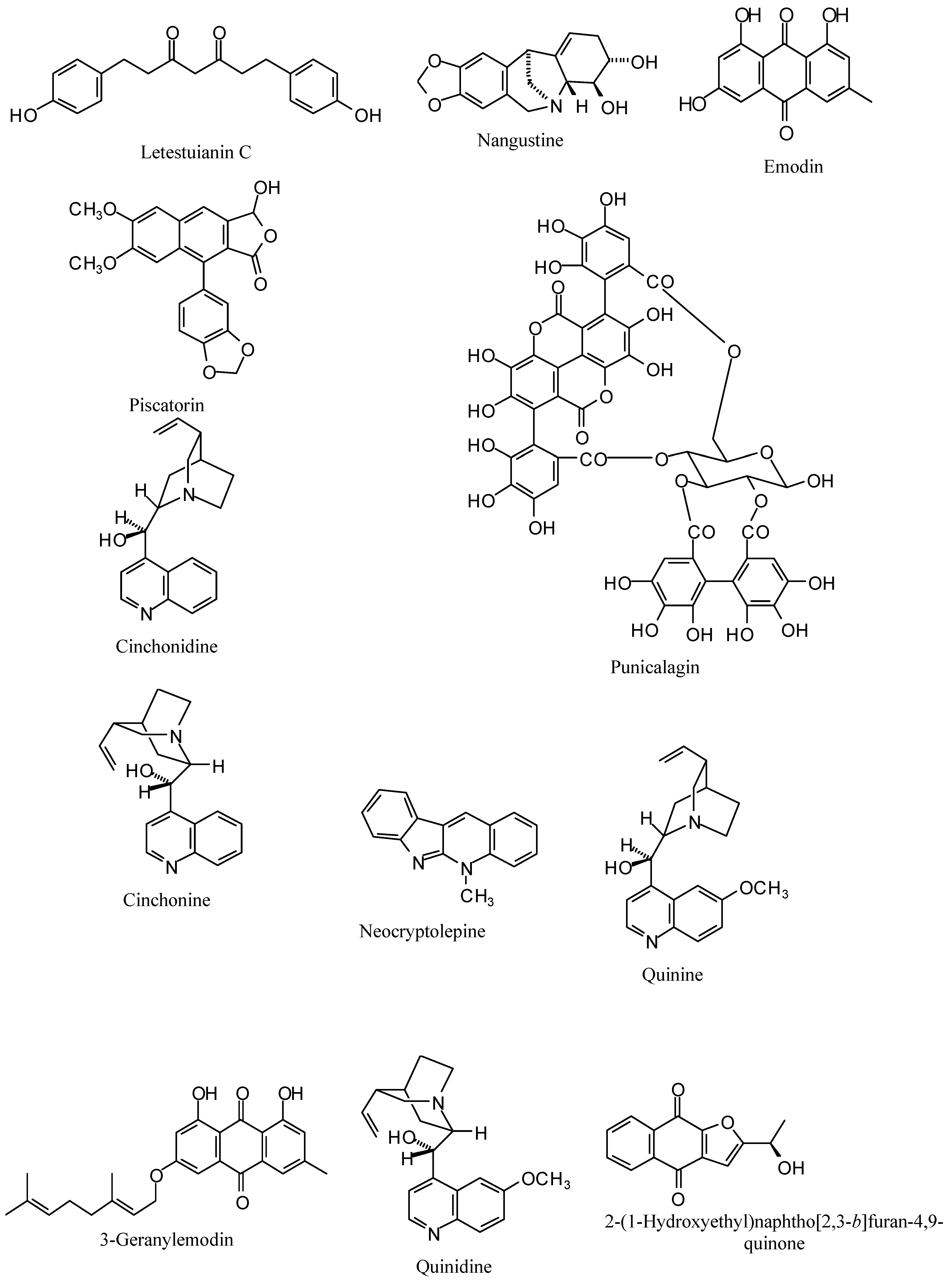
| Letestuianin C | -73.82 | -69.42 | -89.89 | -112.69 | 7.36 [24] |
| Piscatorin | -89.25 | -110.52 | -118.69 | -123.04 | 6.10 [28] |
| Punicalagin | -31.50 | 10.55 | 389.14 | 189.60 | 1.75 [29] |
| Vismione D | -97.84 | -102.02 | -121.43 | -127.88 | 22 [30] |
| Quinoline Alkaloids | |||||
| Cinchonidine | -40.27 | -77.57 | -93.47 | -92.77 | 7.1 [17] |
| Cinchonine | -47.11 | -80.25 | -93.21 | -114.67 | 1.2 [17] |
| Cryptolepine | -73.02 | -82.82 | -72.71 | -90.88 | 0.6 [31] |
| Neocryptolepine | -73.12 | -87.48 | -78.55 | -89.50 | 2.23 [31] |
| Quinidine | -61.61 | -84.40 | -84.17 | -98.87 | 0.77 [17] |
| Quinine | -60.77 | -84.20 | -85.38 | -114.96 | 4.9 [17] |
| Quinones | |||||
| 2-(1-hydroxylethyl)naphtho[2,3-b]furan-4,9-quinone | -75.43 | -90.81 | -74.83 | -99.66 | 0.05 [32] |
| 3-Geranylemodin | -93.20 | -97.03 | -129.13 | -138.76 | 35.4 [30] |
| 4°-O-demethylknipholone-4°-O-β-D-glucopyranoside | -78.06 | -92.32 | -129.29 | -115.47 | 1.2 [33] |
| Emodin | -79.37 | -93.13 | -82.46 | -83.17 | 67 [30] |
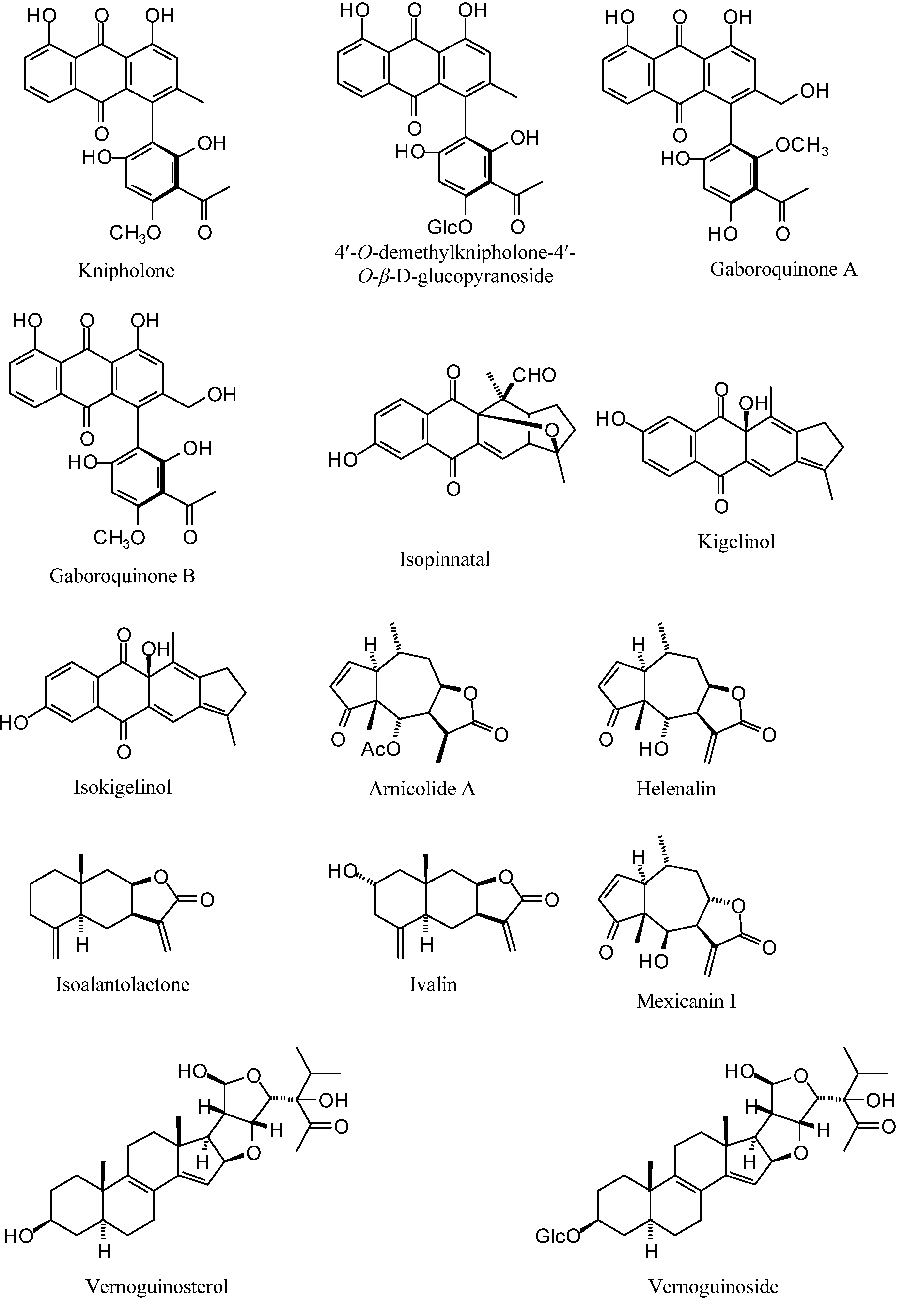
| Gabroquinone A | -90.99 | -96.68 | -113.22 | -70.45 | 11.3 [33] |
| Gabroquinone B | -91.59 | -95.34 | -114.86 | -86.95 | 101 [33] |
| Isokigelinol | -64.21 | -59.06 | -68.31 | -66.08 | 11.11 [32] |
| Isopinnatal | -55.03 | -55.51 | -77.04 | -93.08 | 0.73 [32] |
| Kigelinol | -59.03 | -74.05 | -64.27 | -70.88 | 21.28 [32] |
| Knipholone | -77.99 | -100.24 | -83.91 | -63.32 | 21.4 [33] |
| Terpenoids | |||||
| Arnicolide A | -73.97 | -73.66 | -82.65 | -56.31 | 1.42 [34] |
| Helenalin | -77.08 | -71.00 | -84.45 | -63.25 | 0.051 [34] |
| Isoalantolactone | -61.91 | -69.31 | -62.40 | -78.73 | 23.4 [34] |
| Ivalin | -56.83 | -72.21 | -66.62 | -78.14 | 7.8 [34] |
| Mexicanin 1 | -56.40 | -62.47 | -74.63 | -76.12 | 0.318 [34] |
| Vernoguinoside | -86.81 | -69.78 | -108.12 | -95.31 | 6 [35] |
| Vernoguinosterol | -60.89 | -78.61 | -107.89 | -50.99 | 8 [35] |
| Compounds | Class of Compound | Rerank Pose Score | H-bonding (kJ/mol) | ETotal (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| T. brucei | Human | T. brucei | Human | T. brucei | Human | ||
| Angoroside C | phenolic | -152.16 | 80.14 | -43.56 | -33.83 | -207.79 | -56.03 |
| Cissampeloflavone | phenolic | -141.43 | 54.45 | -8.01 | -15.44 | -192.64 | -88.88 |
| 4°-O-demethylknipholone- | quinone | -129.29 | 148.82 | -41.50 | -8.81 | -159.31 | -44.02 |
| 4°-O-β-D-glucopyranoside | |||||||
| 3-Geranylemodin | quinone | -129.13 | -69.92 | -13.43 | -14.75 | -147.73 | -121.73 |
| Ningpogenin | iridoid | -124.27 | -79.16 | -7.14 | -10.41 | -164.20 | -94.61 |
| BPH-210 a | --- | 114.55 | --- | -8.65 | --- | -138.81 | --- |
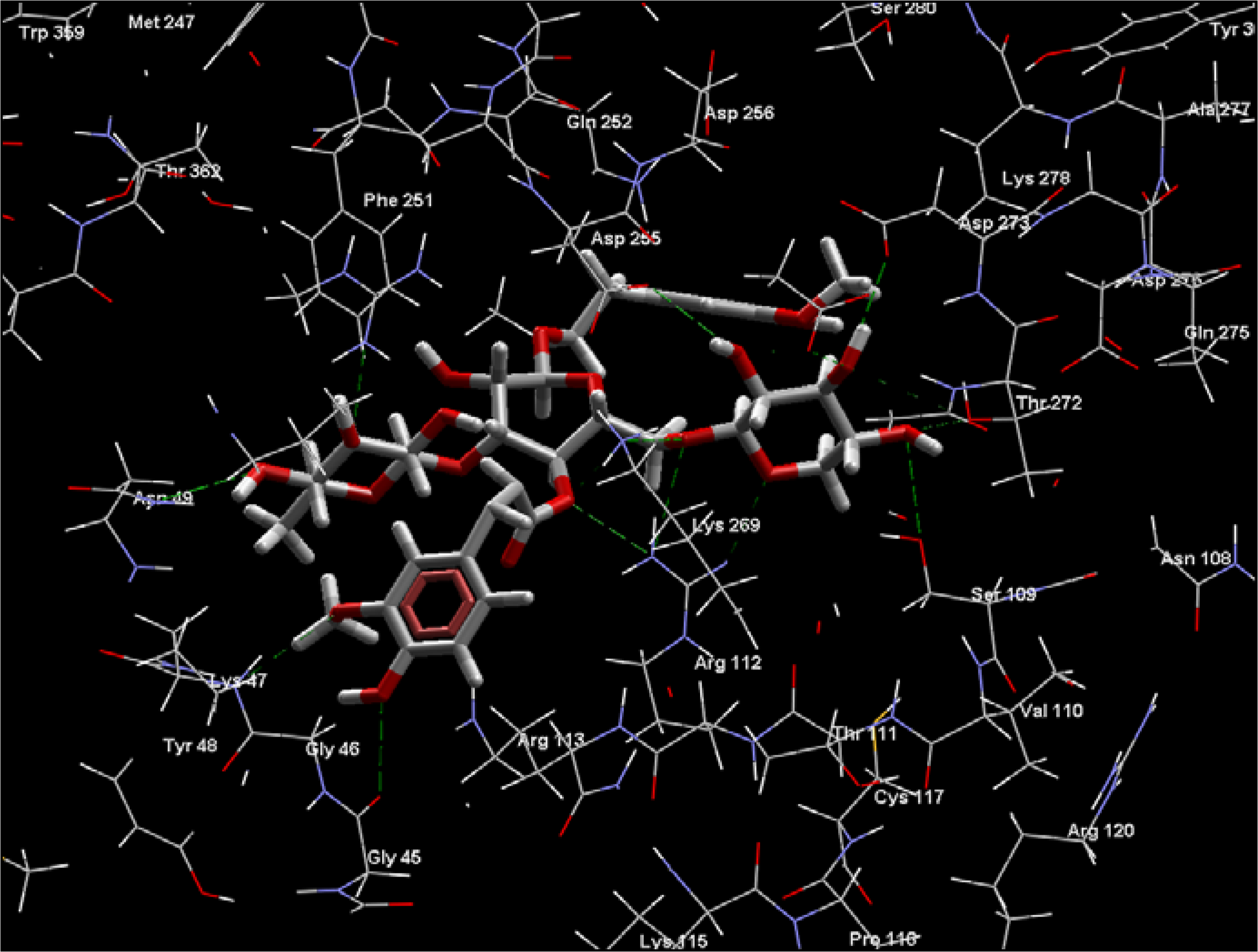
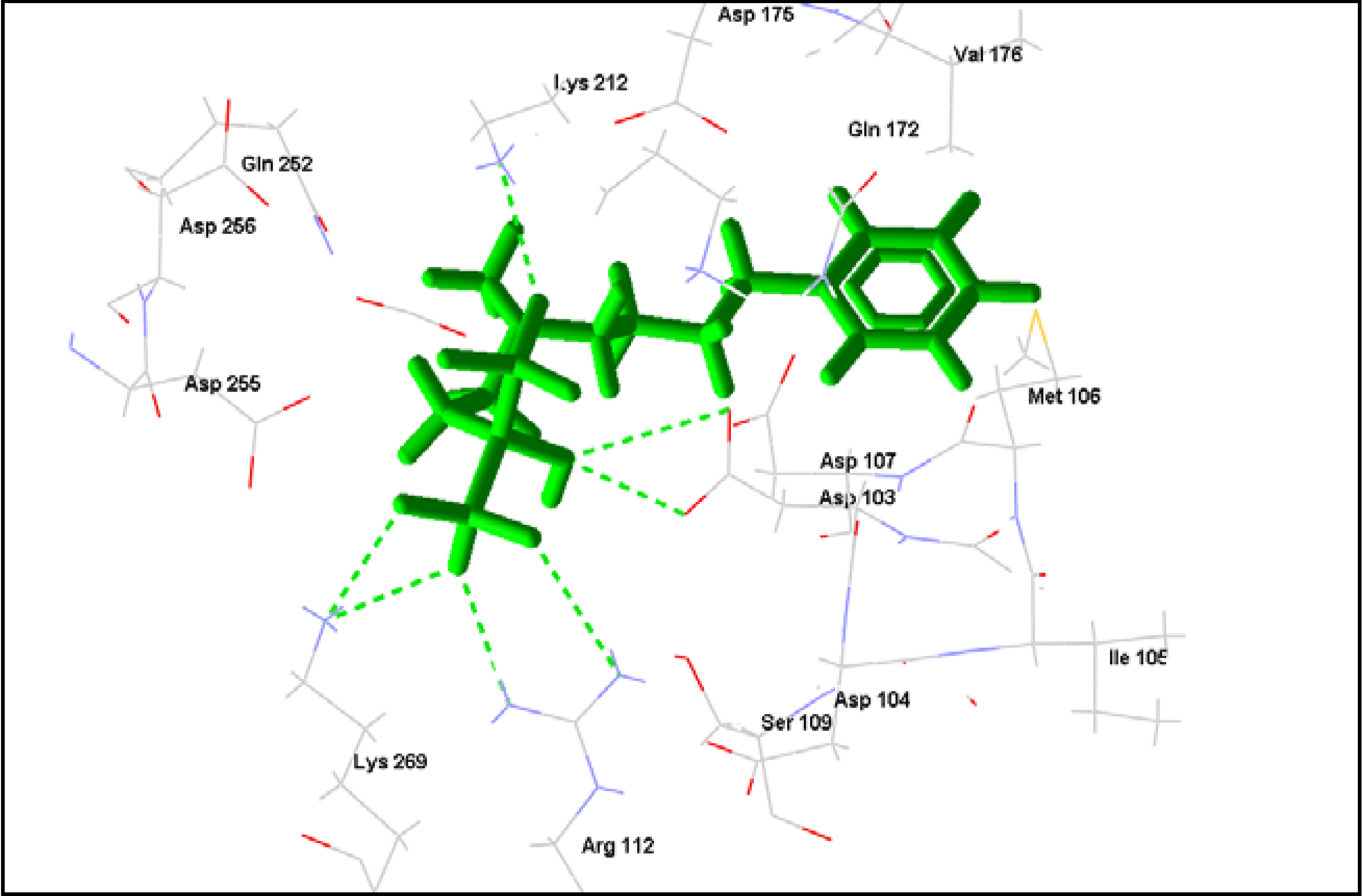
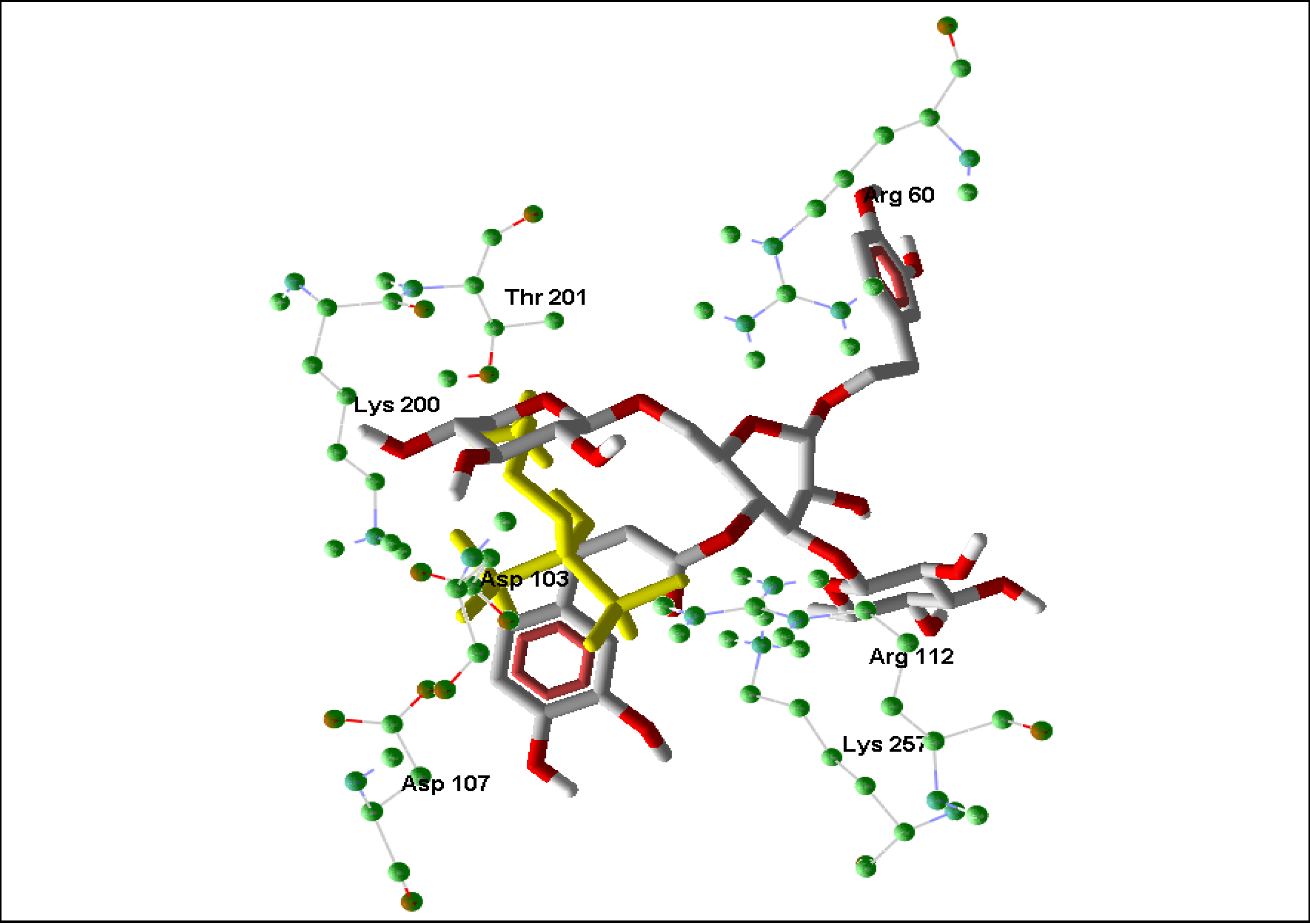
2.2. Rhodesain
| Compounds | Class of compound | Rerank Pose Score | H-bonding (kJ/mol) | ETotal (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| T. brucei | Human | T. brucei | Human | T. brucei | Human | ||
| 8-Hydroxylheptadeca-4,6-diyn-3-yl acetate | diacetylene | -105.81 | -92.08 | -2.77 | -2.70 | -134.90 | -109.49 |
| Cissampeloflavone | phenolic | -105.24 | -103.15 | -4.81 | -8.14 | -159.29 | -151.00 |
| Ningpogenin | iridoid | -101.68 | -66.52 | -12.27 | -8.05 | -138.13 | -81.82 |
| Vismione D | phenolic | -97.84 | -82.51 | -2.50 | -6.23 | -110.79 | -95.88 |
| 8-Hydroxyheptadeca-1-ene-4,6-diyn-3-yl acetate | diacetylene | -93.68 | -92.38 | -3.63 | -2.78 | -129.03 | -113.74 |
| K777a | --- | -87.75 | --- | -6.61 | --- | -135.61 | --- |
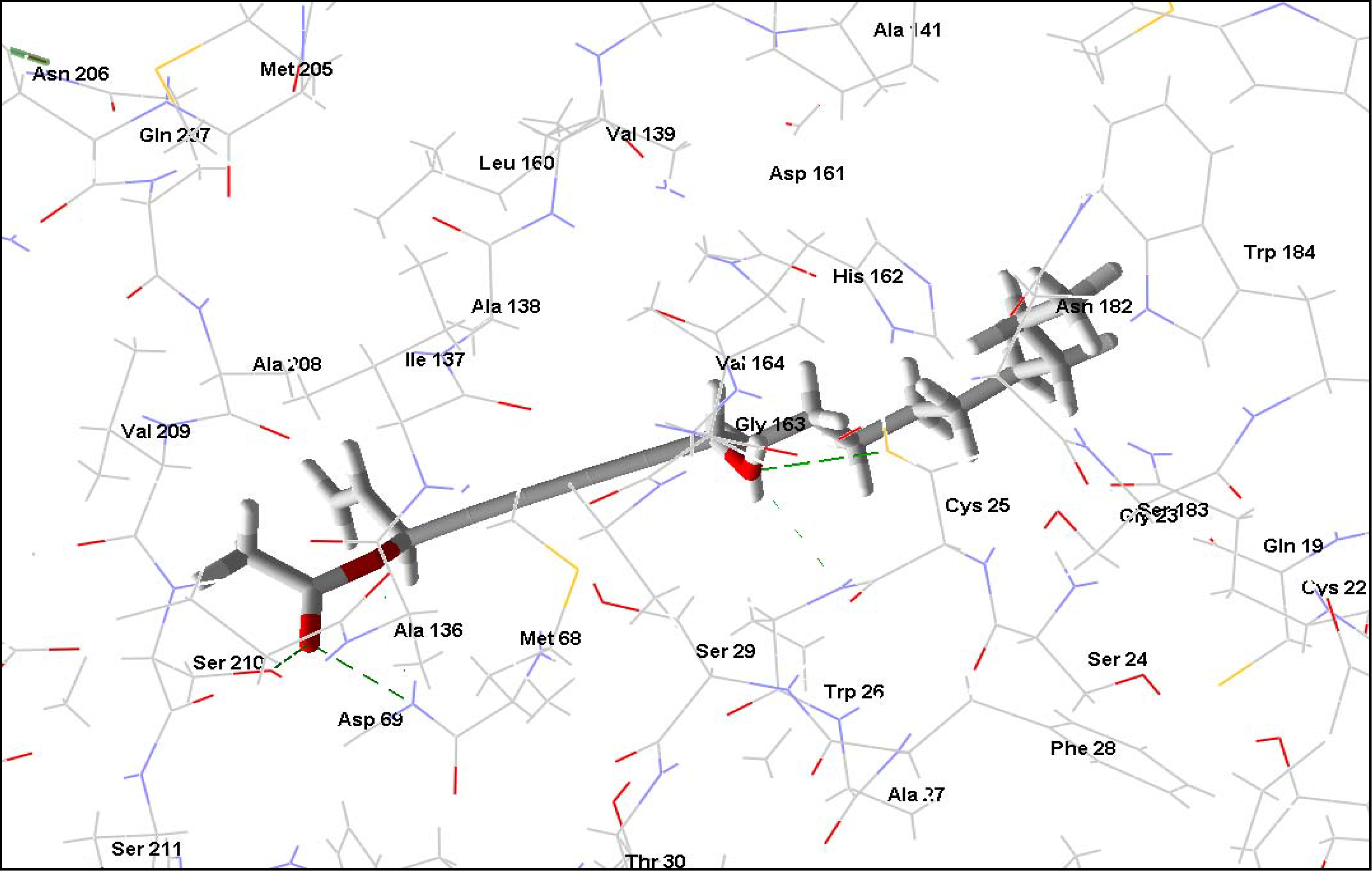
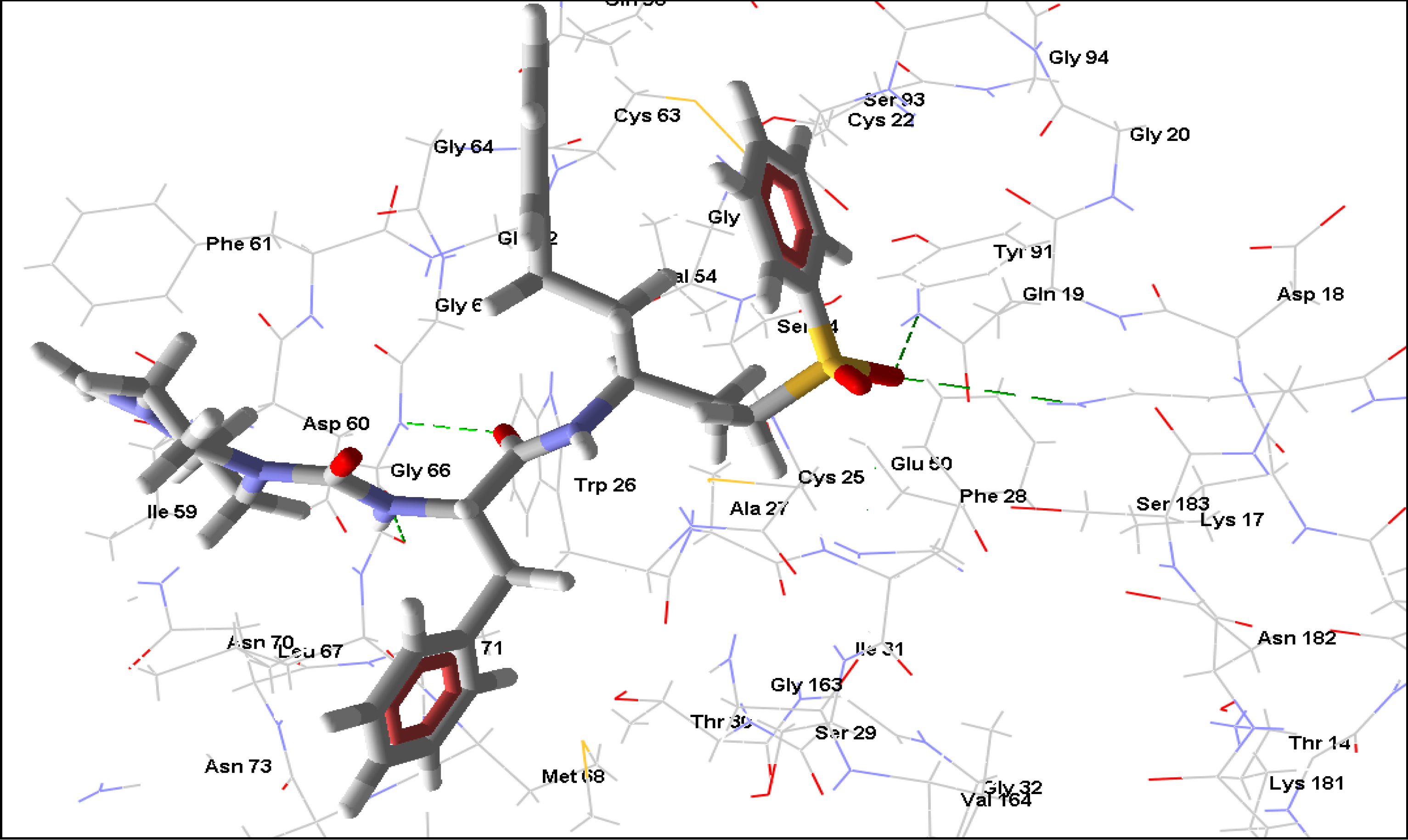
2.3. Trypanothione reductase
| Compounds | Class of compound | Rerank Pose Score | H-bonding (kJ/mol) | ETotal (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| T. brucei | Human | T. brucei | Human | T. brucei | Human | ||
| 3-Geranylemodin | quinone | -138.76 | -103.23 | -15.08 | -2.91 | -207.63 | -178.49 |
| Cissampeloflavone | phenolic | -135.28 | 20.09 | -5.62 | -2.06 | -159.57 | -166.18 |
| 8-Hydroxyheptadeca-1-ene-4,6-diyn-3-yl acetate | diacetylene | -132.24 | -155.71 | -11.30 | -2.72 | -197.78 | -181.85 |
| Aculeatin D | miscellaneous | -129.51 | -142.87 | -1.14 | 3.38 | -178.63 | -182.96 |
| Vismione D | phenolic | -127.88 | -155.17 | -6.95 | -4.75 | -155.87 | -187.92 |
| FADa | --- | -162.98 | -295.81 | -17.69 | -33.86 | -207.63 | -351.32 |
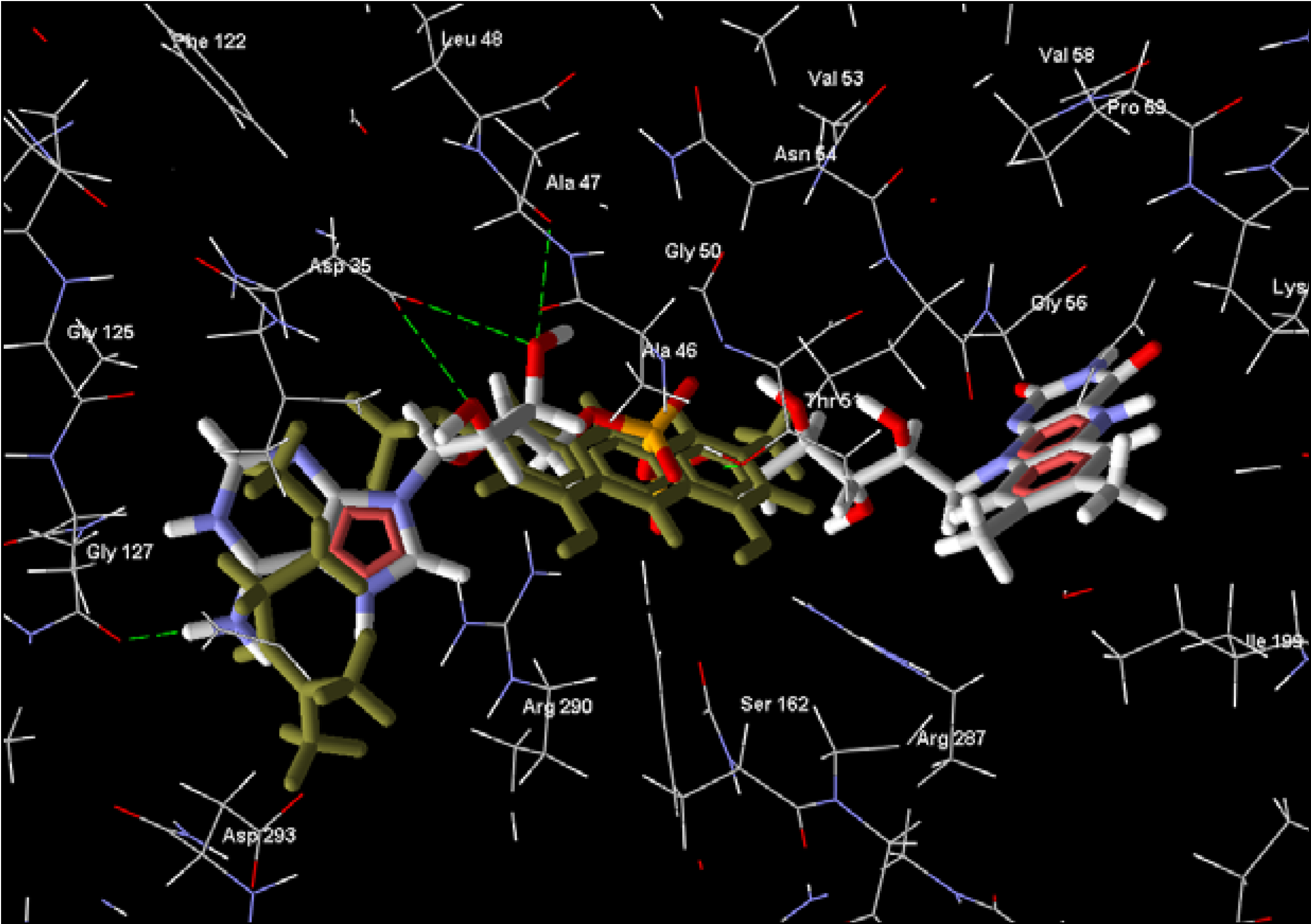

2.4. Triosephosphate Isomerase
| Compounds | Class of compound | Rerank Pose Score | H-bonding (kJ/mol) | ETotal (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| T. brucei | Human | T. brucei | Human | T. brucei | Human | ||
| Cissampeloflavone | phenolic | -114.60 | -9.91 | -7.97 | -2.50 | -168.78 | -21.80 |
| Piscatorin | phenolic | -110.52 | 8.07 | -6.70 | -2.42 | -144.90 | -41.04 |
| 8-Hydroxyheptadeca-1-ene-4,6-diyn-3-yl acetate | diacetylene | -109.03 | -34.68 | -8.24 | -1.10 | -140.88 | -52.57 |
| Vismione D | phenolic | -102.02 | -3.94 | -9.78 | 0.00 | -116.24 | -10.10 |
| 8-Hydroxylheptadeca-4,6-diyn-3-yl acetate | diacetylene | -100.58 | -38.15 | -10.54 | 0.00 | -132.00 | -44.82 |
| HPO42- | --- | -38.82 | --- | -8.23 | --- | -47.00 | --- |
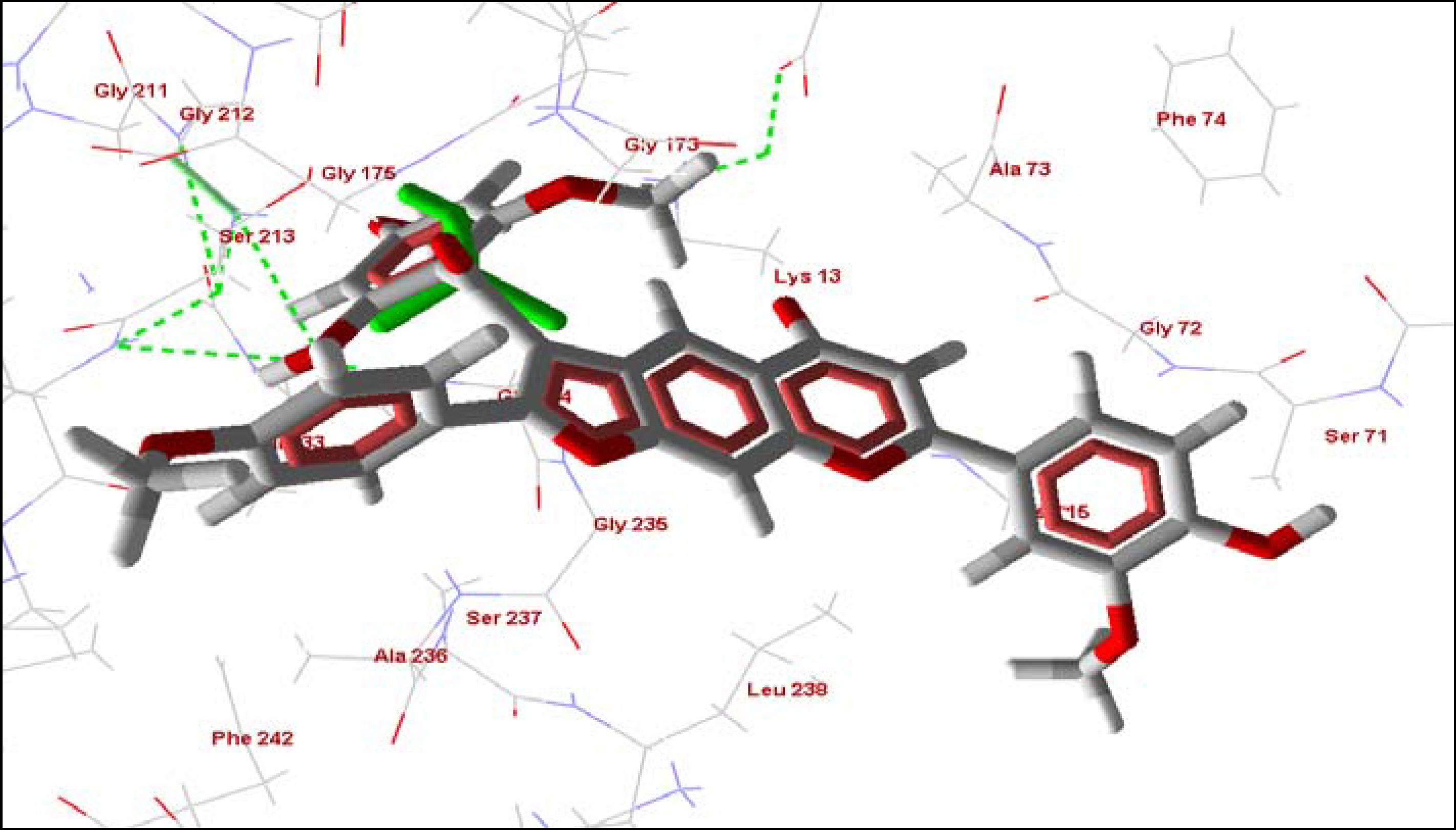
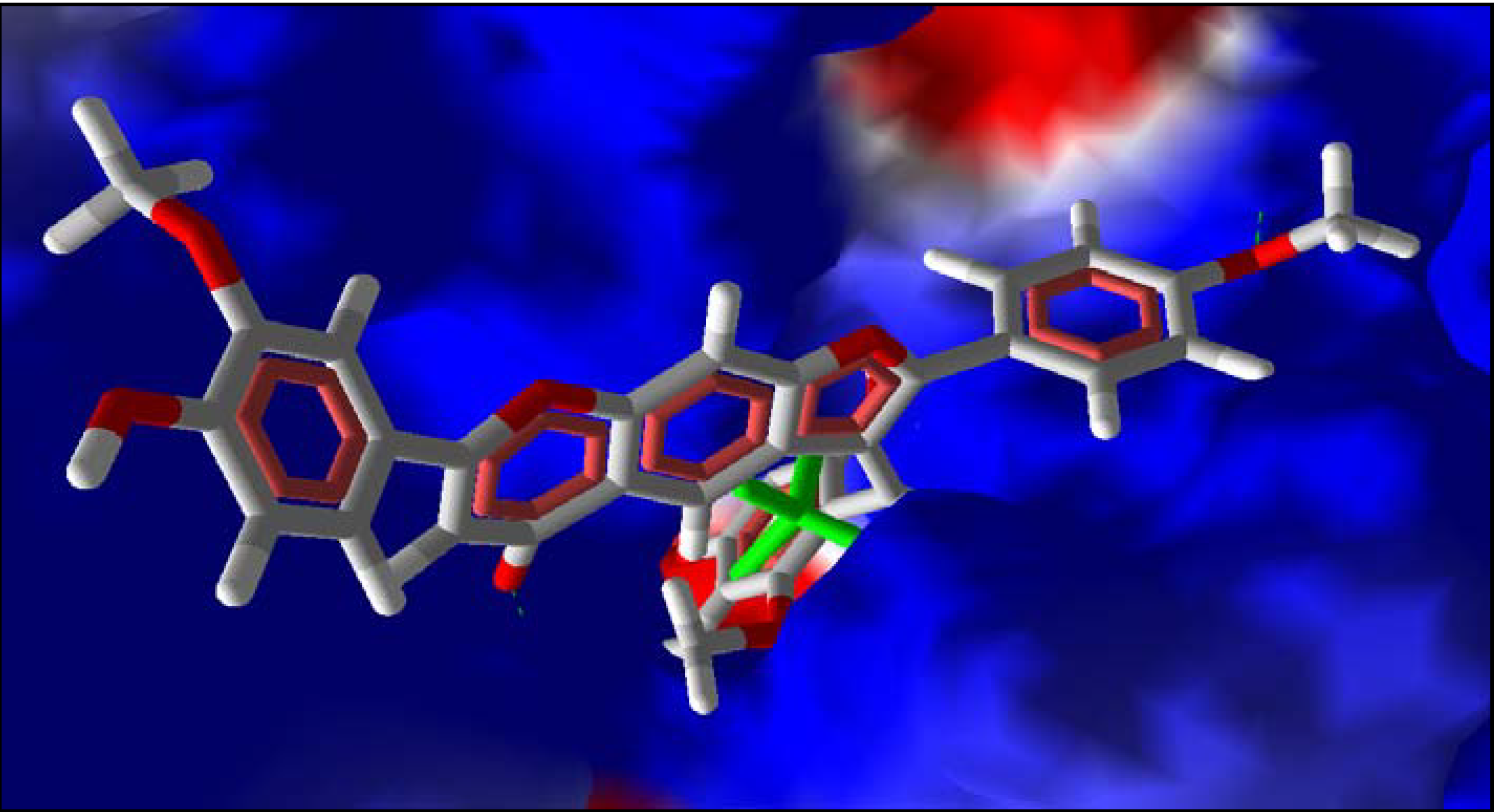
2.5. Summary
3. Experimental
3.1. Compound Dataset
3.2. Computation


References
- Barrett, M.P.; Boykin, D.W.; Brun, R.; Tidwell, R.R. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 2007, 152, 1155–1171. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Stuppner, H.; Langer, T. Virtual screening for the discovery of bioactive natural products. Prog. Drug Res. 2008, 65, 213–249. [Google Scholar]
- Omar, M.; Khan, F. Trypanothione Reductase: A viable chemotherapeutic target for antitrypanosomal and antileishmanial drug design. Drug Target Insights 2007, 1, 129–146. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry, 3rd Edition ed; Wiley and Sons: New York, 2005; pp. 945–947. [Google Scholar]
- Montalvetti, A.; Fernandez, A.; Sanders, J.M.; Ghosh, S.; Van Brussel, E.; Oldfield, E.; Docampo, R. Farnesyl pyrophosphate synthase is an essential enzyme in Trypanosoma brucei, in vitro RNA interference and in vivo inhibition studies. J. Biol. Chem. 2003, 278, 17075–17083. [Google Scholar]
- Linares, G.E.G.; Ravaschino, E.L.; Rodriguez, J.B. Progresses in the field of drug design to combat tropical protozoan parasitic diseases. Curr. Med. Chem. 2006, 13, 335–360. [Google Scholar] [CrossRef]
- Mao, J.; Mukherjee, S.; Zhang, Y.; Cao, R.; Sanders, J.M.; Song, Y.; Zhang, Y.; Meints, G.A.; Gao, Y.G.; Mukkamala, D.; Hudock, M.P.; Oldfield, E. Solid-state NMR, crystallographic, and computational investigation of bisphosphonates and farnesyl diphosphate synthase-bisphosphonate complexes. J. Am. Chem. Soc. 2006, 128, 14485–14497. [Google Scholar] [CrossRef]
- Docampo, R.; Moreno, S.N.J. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Curr. Drug Targets Infect. Disord. 2001, 11, 51–61. [Google Scholar] [CrossRef]
- Tasdemir, D.; Güner, N.D.; Perozzo, R.; Brun, R.; Dönmez, A.A.; Çalıs, I.C.; Rüedi, P. Anti-protozoal and plasmodial FabI enzyme inhibiting metabolites of Scrophularia lepidota roots. Phytochemistry 2005, 66, 355–362. [Google Scholar] [CrossRef]
- Bringmann, G.; Hamm, A.; Gunter, C.; Michel, M.; Brun, R.; Mudogo, V. Ancistroealaines A and B, two new bioactive naphthylisoquinolines and related naphthoic acids from Ancistrocladus ealaensis. J. Nat. Prod. 2000, 63, 1465–1470. [Google Scholar] [CrossRef]
- Bringmann, G.; Wohlfarth, M.; Rischer, H.; Schlauer, J.; Brun, R. Extract screening by HPLC coupled to MS-MS, NMR, and CD: A dimeric and three monomeric naphthylisoquinoline alkaloids from Ancistrocladus griffithi. Phytochemistry 2002, 61, 195–204. [Google Scholar] [CrossRef]
- Bringmann, G.; Saeb, W.; Rückert, M.; Mies, J.; Michel, M.; Brun, R. Ancistrolikokine D, a 5,8°-coupled naphthylisoquinoline alkaloid, and related natural products from Ancistrocladus likoko. Phytochemistry 2003, 62, 631–636. [Google Scholar] [CrossRef]
- Bringmann, G.; Dreyer, M.; Faber, J.H.; Dalsgaard, P.W.; Staerk, D.; Jaroszewski, J.W.; Ndangalasi, H.; Mbago, F.; Brun, R.; Reichert, M.; Maksimenka, K.; Christensen, S.B. Ancistrotanzanine A, the first 5,3°-coupled naphthylisoquinoline alkaloid, and two further, 5,8°-linked related compounds from the newly described plant species Ancistrocladus tanzaniensis. J. Nat. Prod. 2003, 66, 1159–1165. [Google Scholar] [CrossRef]
- Bringmann, G.; Dreyer, M.; Faber, J.H.; Dalsgaard, P.W.; Staerk, D.; Jaroszewski, J.W.; Ndangalasi, H.; Mbago, F.; Brun, R.; Maksimenka, K.; Christensen, S.B. Ancistrotanzanine C and related 5,1°- and 7,3°-coupled naphthylisoquinoline alkaloids from Ancistrocladus tanzaniensis. J. Nat. Prod. 2004, 67, 743–748. [Google Scholar] [CrossRef]
- Camacho, M.D.R.; Philipson, J.D.; Croft, S.L.; Rock, P.; Marshall, S.J.; Schiff, P.L. In vitro activity of Triclisia patens and some bisbenzylisoquinone alkaloids against Leishmania dovovani and Trypanosoma brucei brucei. Phytother. Res. 2002, 16, 432–436. [Google Scholar] [CrossRef]
- Merschjohann, K.; Sporer, F.; Steverding, D.; Wink, M. In vitro effect of alkaloids on bloodstream forms of Trypanosoma brucei and T. congolense. Planta Med. 2001, 67, 623–627. [Google Scholar] [CrossRef]
- Bringmann, G.; Messer, K.; Wolf, K.; Mühlbacher, J.; Grüne, M. Dioncophylline E from Dioncophyllum tholonnii, the first 7,3°-coupled dioncophyllaceous naphthylisoquinoline alkaloid. Phytochemistry 2002, 60, 389–397. [Google Scholar] [CrossRef]
- Bringmann, G.; Messer, K.; Brun, R.; Mudogo, V. Ancistrocongolines A-D, new naphthylisoquinoline alkaloids from Ancistrocladus congolensis. J. Nat. Prod. 2002, 65, 1096–1101. [Google Scholar] [CrossRef]
- Labraña, J.; Machocho, A.K.; Kricsfalusy, V.; Brun, R.; Codina, C.; Viladomat, F.; Bastida, J. Alkaloids from Narcissus angustifolius subsp. transcarpathicus. Phytochemistry 2002, 60, 847–852. [Google Scholar] [CrossRef]
- Machocho, A.K..; Bastida, J.; Codina, C.; Viladomat, F.; Brun, R.; Chhabra, S.C. Augustamine type alkaloids from Crinum kirkii. Phytochemistry 2004, 65, 847–852. [Google Scholar] [CrossRef]
- Senn, M.; Gunzenhauser, S.; Brun, R.; Séquin, U. Antiprotozoal polyacetylenes from the Tanzanian medicinal plant Cussonia zimmermannii. J. Nat. Prod. 2007, 70, 1565–1569. [Google Scholar] [CrossRef]
- Heilmann, J.; Brun, R.; Mayr, S.; Rali, T.; Sticher, O. Minor cytotoxic and antibacterial compounds from the rhizomes of Amomum aculeatum. Phytochemistry 2001, 57, 1281–1285. [Google Scholar] [CrossRef]
- Kamnaings, P.; Tsopmo, A.; Tanifum, E.A.; Tcheundem, M.H.K.; Tane, P.; Ayafor, J.F.; Sterner, O.; Rattendi, D.; Iwu, M.M.; Schuster, B.; Bacchi, C. Trypanocidal diarylheptanoids from Aframomum letestuianum. J. Nat. Prod. 2003, 66, 364–367. [Google Scholar] [CrossRef]
- Wright, A.D.; Papendorf, O.; König, G.M. Ambigol C and 2,4-Dichlorobenzoic Acid, new natural products produced by the terrestrial cyanobacterium Fischerella ambigua. J. Nat. Prod. 2005, 68, 459–461. [Google Scholar] [CrossRef]
- Pontius, A.; Krick, A.; Kehraus, S.; Brun, R.; König, G.M. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 2008, 71, 1579–1584. [Google Scholar] [CrossRef]
- Ramírez, I.; Carabot, A.; Meléndez, P.; Carmona, J.; Jimenez, M.; Patel, A.V.; Crabb, T.A.; Blunden, G.; Cary, P.D.; Croft, S.L.; Costa, M. Cissampeloflavone, a chalcone-flavone dimer from Cissampelos pareira. Phytochemistry 2003, 64, 645–647. [Google Scholar] [CrossRef]
- Gertsch, J.; Tobler, R.T.; Brun, R.; Sticher, O.; Heilmann, J. Antifungal, antiprotozoal, cytotoxic and piscicidal properties of Justicidin B and a new arylnaphthalide lignan from Phyllanthus piscatoru. Planta Med. 2003, 69, 420–424. [Google Scholar]
- Asres, K.; Bucar, F.; Knauder, E.; Yardley, V.; Kendrick, H.; Croft, S.L. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytother. Res. 2001, 15, 613–617. [Google Scholar] [CrossRef]
- Mbwanbo, Z.H.; Apers, S.; Moshi, M.J.; Kapingu, M.C.; Van Miert, S.; Claeys, M.; Brun, R.; Cos, P.; Pieters, L.; Vlietinck, A. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med. 2004, 70, 706–710. [Google Scholar] [CrossRef]
- Jonckers, T.H.M.; van Miert, S.; Cimanga, K.; Bailly, C.; Colson, P.; De Pauw-Gillet, M.C.; van den Heuvel, H.; Claeys, M.; Lemiere, F.; Esmans, E.L.; Rozenski, J.; Quirijnen, L. Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J. Med. Chem. 2002, 45, 3497–3508. [Google Scholar] [CrossRef]
- Moideen, S.V.K.; Houghton, P.J.; Rock, P.; Croft, S.L.; Aboagye-Nyame, F. Activity of extracts and naphthoquinones from Kigelia pinnata against Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. Planta Med. 1999, 65, 536–540. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Bezabih, M.; Msuta, T.; Brun, R.; Menche, D.; Mühlbacher, J.; Bringmann, G. Gaboroquinones A and B and 4°-O-demethylknipholone-4°-O-β-D-glucopyranoside, phenylanthraquinones from the roots of Bulbine frutescens. J. Nat. Prod. 2002, 65, 1117–1121. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Brun, R.; Willuhn, G.; Khalid, S.A. Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones. Planta Med. 2002, 68, 750–751. [Google Scholar] [CrossRef]
- Tchinda, A.T.; Tsopmo, A.; Tane, P.; Ayafor, J.F.; Conolly, J.D.; Sterner, O. Vernoguinosterol and vernoguinoside, trypanocidal stigmastane derivatives from Vernonia guineensis (Asteraceae). Phytochemistry 2002, 59, 371–374. [Google Scholar] [CrossRef]
- Cao, R.; Chen, C.K.M.; Guo, R.T.; Wang, A.H.J.; Oldfield, E. Structures of a potent phenylalkyl bisphosphonate inhibitor bound to farnesyl and geranylgeranyl diphosphate synthases. Proteins 2008, 73, 431–439. [Google Scholar] [CrossRef]
- Vermelho, A.B.; De Simone, S.G.; d’Avila-Levy, C.M.; Souza do Santos, A.L.; Nogueira de Melo, A.C. Trypanosomatidae Peptidases: A target for drugs development. Curr. Enzyme Inhibit. 2007, 3, 19–48. [Google Scholar] [CrossRef]
- Sajid, M.; Mckerrow, J.H. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 2002, 120, 1–21. [Google Scholar] [CrossRef]
- González, F.V.; Izquierdo, J.; Rodríguez, S.; McKerrow, J.H.; Hansell, E. Dipeptidyl-α,β-epoxyesters as potent irreversible inhibitors of the cysteine proteases cruzain and rhodesain. Bioorg. Med. Chem. Lett. 2007, 17, 6697–6700. [Google Scholar] [CrossRef]
- Brinen, L.S.; Hansell, E.; Cheng, J.; Roush, W.R.; McKerrow, J.H.; Fletterick, R.J. A target within the target: probing cruzain’s P1° site to define structural determinants for the Chagas’ disease protease. Structure 2000, 8, 831–840. [Google Scholar] [CrossRef]
- Krieger, S.; Schwarz, W.; Ariyanayagam, M.R.; Fairlamb, A.H.; Krauth-Siegel, R.L.; Clayton, C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2000, 35, 542–552. [Google Scholar]
- Jones, D.; Ariza, A.; Chow, W.H.A.; Oza, S.L.; Fairlamb, A.H. Properties of trypanothione reductase from T. brucei. Retrieved on 12/14/2008 from http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=68081.
- Michels, P.A.M. Compartmentation of glycolysis in trypanosomes: a potential target for new trypanocidal drugs. Biol. Cell 1988, 64, 157–164. [Google Scholar] [CrossRef]
- Bakker, B.M.; Michelsi, P.A.M.; Opperdoesi, F.R.; Hans, V.; Westerhoff, H.V. Metabolic control analysis of glycolysis in trypanosomes as an approach to improve selectivity and effectiveness of drugs. J. Biol. Chem. 1999, 274, 14551–14559. [Google Scholar]
- Olivares-Illana, V.; Pérez-Montfort, R.; López-Calahorra, F.; Costas, M.; Rodríguez-Romero, A.; Tuena de Gómez-Puyou, M.T.; Gómez Puyou, A. Structural differences in triosephosphate isomerase from different species and discovery of a multitrypanosomatid inhibitor. Biochemistry 2006, 45, 2556–2560. [Google Scholar] [CrossRef]
- Nickbarg, E.B.; Davenport, R.C.; Petsko, G.A.; Knowles, J.R. Triosephosphate isomerase: removal of a putatively electrophilic histidine residue results in a subtle change in catalytic mechanism. Biochemistry 1988, 27, 5948–5960. [Google Scholar] [CrossRef]
- Dictionary of Natural Products on DVD; CRC Press, Taylor and Francis LLC: Boca Raton, Florida, USA, 1992-2008.
- ACD/Chem. Sketch Freeware; Advanced Chemistry Development, Inc.: Toronto, Ontario, Canada, 1994-2006.
- Thomsen, R.; Christensen, M.H. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Sample availability: Not available.



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogungbe, I.V.; Setzer, W.N. Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets. Molecules 2009, 14, 1513-1536. https://doi.org/10.3390/molecules14041513
Ogungbe IV, Setzer WN. Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets. Molecules. 2009; 14(4):1513-1536. https://doi.org/10.3390/molecules14041513
Chicago/Turabian StyleOgungbe, Ifedayo V., and William N. Setzer. 2009. "Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets" Molecules 14, no. 4: 1513-1536. https://doi.org/10.3390/molecules14041513
APA StyleOgungbe, I. V., & Setzer, W. N. (2009). Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets. Molecules, 14(4), 1513-1536. https://doi.org/10.3390/molecules14041513






