13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species
Abstract
:1. Introduction
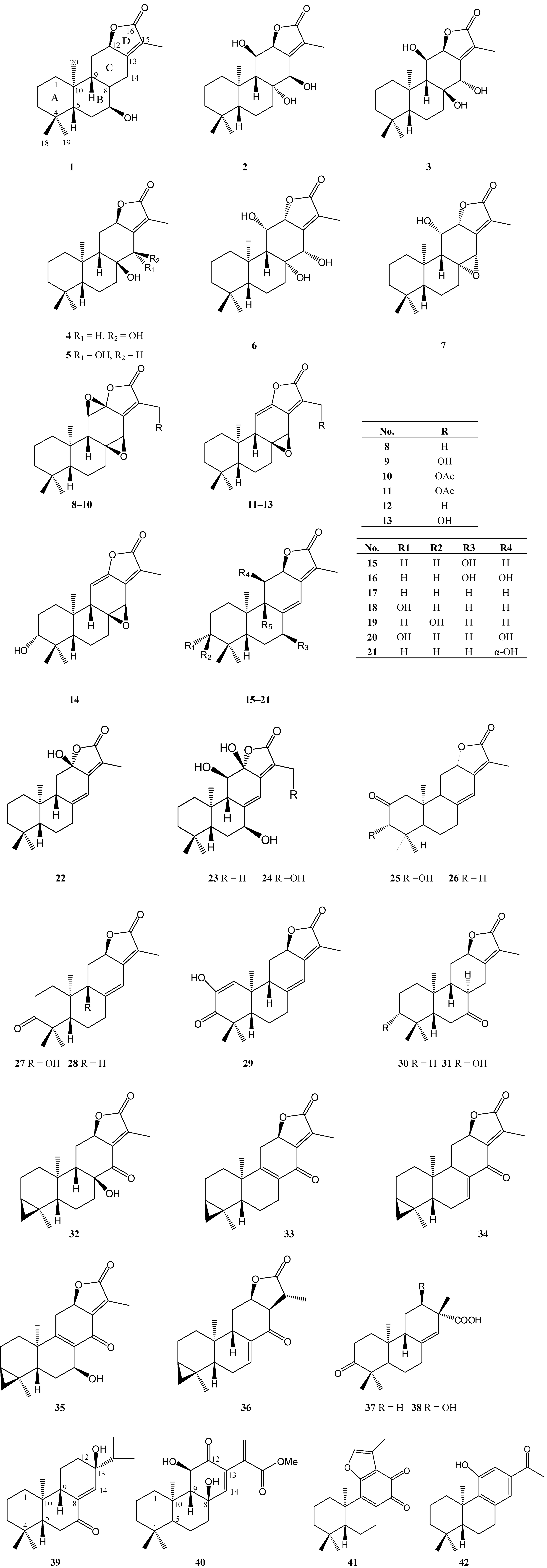
2. Abietane Derivates Isolated from Euphorbia Species (Table 1)
| No | Name | Species | Ref |
|---|---|---|---|
| 1 | 7β-Hydroxy-8α,14-dihydro jolkinolide E | E. terracina | [27] |
| 2 | Yuexiandajisu D | E. ebracteolata | [25] |
| 3 | Yuexiandajisu E | E. ebracteolata | [25] |
| 4 | ent-8β,14α-Dihydroxy-13(15)-ene-16(12β)-abietanolide | E. wallichii | [28] |
| 5 | ent-8β,14β-Dihydroxy-13(15)-ene-16(12β)-abietanolide | E. wallichii | [28] |
| 6 | Ebracteolatanolide B | E. ebracteolata | [25] |
| 7 | Ebracteolatanolide A | E. ebracteolata | [25] |
| 8 | Jolkinolide B | E. fischeriana, | [29] |
| E. sessiliflora | [30] | ||
| 9 | 17-Hydroxyjolkinolide B | E. fischeriana | [31] |
| 10 | 17-Acetoxyjolkinolide B | E. fischeriana | [31] |
| 11 | 17-Acetoxyjolkinolide A | E. fischeriana | [32] |
| 12 | Jolkinolide A | E. wallichii | [28] |
| E. fischeriana | [29] | ||
| E. fidjiana | [33] | ||
| E. guyoniana | [34] | ||
| 13 | 17-Hydroxyjolkinolide A | E. fischeriana | [32] |
| E. fidjiana | [33] | ||
| 14 | 3α-Hydroxyjolkinolide A | E. wallichii | [28] |
| 15 | 7β-Hydroxy-ent-abieta-8(14),13(15)-dien-12α,16-olide | E. seguieriana | [35] |
| 16 | 7β,9β-Dihydroxy-ent-abieta-8(14),13(15)-dien-12α,16-olide | E. seguieriana | [35] |
| 17 | ent-Abieta-8(14),13(15)-dien-16,12-olide [Jolkinolide E] | E. fidjiana | [33] |
| E. characias | [34] | ||
| E. guyoniana | [36] | ||
| 18 | Helioscopinolide A | E. pubescens | [37] |
| E. semiperfoliata | [38] | ||
| E. helioscopia | [39] | ||
| 19 | Helioscopinolide B | E. pubescens | [37] |
| E. semiperfoliata | [38] | ||
| E. helioscopia | [39] | ||
| E. calyptrata | [40] | ||
| 20 | Helioscopinolides H | E. calyptrata | [40] |
| 21 | ent-11α-Hydroxyabieta-8(14),13(15)-dien-16,12α-olide | E. ebracteolata | [25] |
| E. sessiliflora | [30] | ||
| E. fidjiana | [33] | ||
| 22 | ent-12-Hydroxy-12[R]-abieta-8(14 ),13(15)-dien-16,12-olide | E. sessiliflora | [30] |
| 23 | 7β,11β,12β-Trihydroxy-ent-abieta-8(14),13(15)-dien-16,12-olide | E. fischeriana | [31] |
| 24 | Langduin B | E. fischeriana | [32] |
| 25 | Helioscopinolide C | E. helioscopia | [39,41] |
| 26 | Helioscopinolides F | E. calyptrata | [40] |
| 27 | Helioscopinolide D | E. calyptrate | [42] |
| 28 | Helioscopinolide E | E. calyptrate | [42] |
| 29 | Helioscopinolides I | E. calyptrata | [40] |
| 30 | 8α,14-Dihydro-7-oxo-jolkinolide E | E. characias | [36] |
| 31 | 8α,14-Dihydro-7-oxohelioscopinolide A [caudicifolin] | E. sessiliflora | [30] |
| E. characias | [36] | ||
| E. semiperfoliata | [38] | ||
| 32 | 3,4,18β-Cyclopropa-8β-hydroxy-14-oxo-ent-abiet-13,15-en-16,12-olide | E. retusa | [43] |
| 33 | 3,4,18β-Cyclopropa-14-oxo-ent-abieta-8,9,13,15-dien-16,12-olide | E. retusa | [43] |
| 34 | 3,4,18β-Cyclopropa-14-oxo-ent-abieta-7,13,15-dien-16,12-olide | E. retusa | [43] |
| 35 | 3,4,18β-Cyclopropa-7-hydroxy-14-oxo-ent-abieta-8,9,13,15-dien-16,12-olide | E. retusa | [43] |
| 36 | 3,4,18β-Cyclopropa-14-oxo-ent-abiet-7-en-16,12-olide | E. retusa | [43] |
| 37 | ent-16-Hydroxy-13[R]-pimar-8(14) -ene-3,15-dione | E. fidjiana | [33] |
| 38 | ent-l2α,16-Dihydroxy-13[R]-pimar-8(14) -ene-3,15-dione | E. fidjiana | [33] |
| 39 | 13β-Hydroxy-ent-abiet-8(14)-en-7-one | E. fischeriana | [31] |
| 40 | Methyl 8β,11β-dihydroxy-12-oxo-ent-abieta-13, 15(17)-dien-16-oate | E. portulacoides | [44] |
| 41 | 11,16-Epoxy-ent-abieta-8,11,15-triene-13,14-dione | E. guyoniana | [34] |
| 42 | 11-Hydroxy-ent-abieta-8,11,13-trien-15-one | E. guyoniana | [34] |
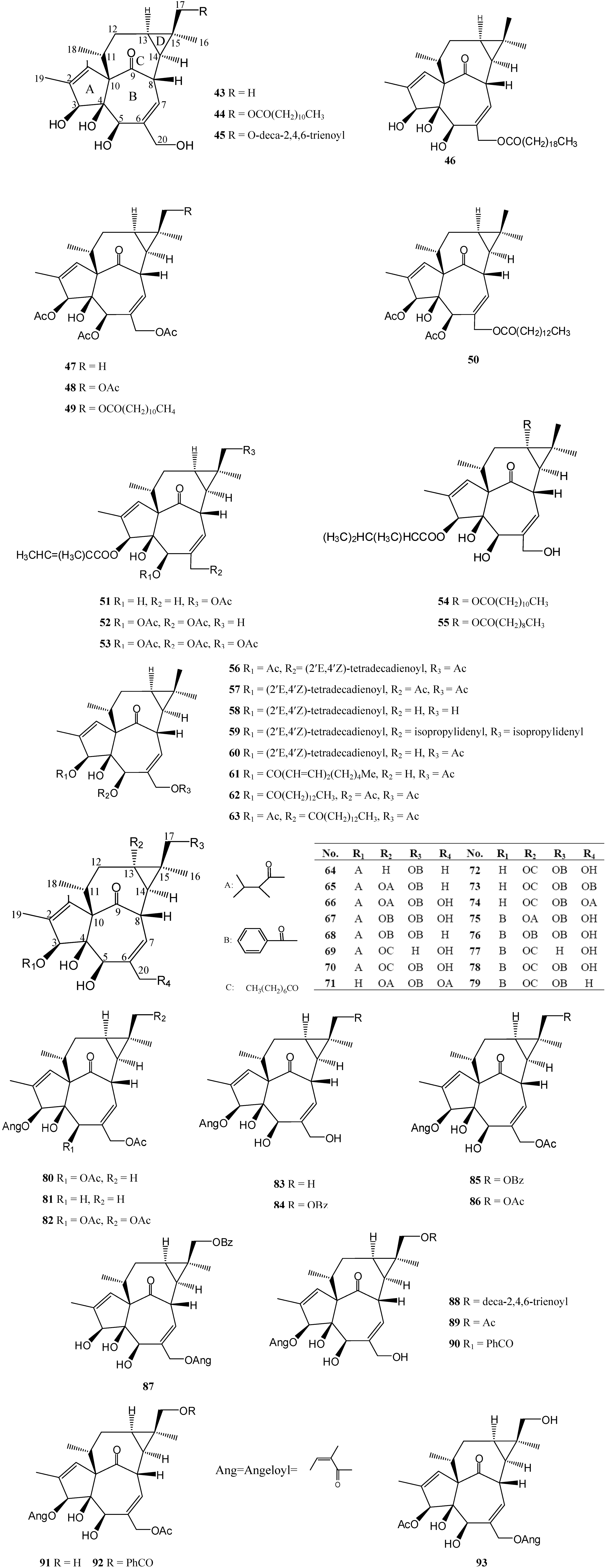
3. Ingenane Derivates Isolated from Euphorbia Species (Table 2)
| No | Name | Species | Ref |
|---|---|---|---|
| 43 | Ingenol | E.kansui | [45] |
| E. paralias | [48] | ||
| 44 | 13-O-Dodecanoylingenol | E. kansui | [45] |
| 45 | 17-[(2Z,4E,6Z)-Deca-2,4,6-trienoyloxy] [ingenol] | E. cauducifolia. | [21] |
| 46 | 20-Eicosanoate | E. iberica | [52] |
| 47 | 3,5,20-O-Triacetylingenol | E. kansui | [45] |
| 48 | 17-Hydroxyingenol tetraacetate | E. kamerunica | [53] |
| 49 | 5,20-O-Diacetyl-3-O-(2″,3″-dimethylbutanoyl)-13-O-dodecanoylingenol | E. kansui | [45] |
| 50 | 20-Tetradecanoate-ingenol-3,5-diacetate | E. broteri | [54] |
| 51 | 17-O-Acetyl-3-O-[(Z)-2-methyl-2-butenoyl]-20-deoxy-17-hydroxy-ingenol | E. trigona | [55] |
| 52 | 20-O-Acetyl-3-O-[(Z)-2-methyl-2-butenoyl]ingenol | E. trigona | [55] |
| 53 | 5,17,20-O-Triacetyl-3-O-[(Z)-2-methyl-2-butenoyl]-17-hydroxyingenol | E. trigona | [55] |
| 54 | 3-O-(2,3-Dimethylbutanoyl)-13-O-dodecanoylingenol | E. kansui | [45] |
| E. cyparissias | [46] | ||
| 55 | 3-O-(2,3-Dimethylbutanoyl)-13-O-decanoylingenol | E. kansui | [45] |
| E. cyparissias | [46] | ||
| 56 | 3,20-O-Diacetylingenol 5-O-(2'E,4'Z)-tetradecadienoate | E. petiolata | [56] |
| 57 | 5,20-O-Diacetylingenol 3-O-(2'E,4'Z)-tetradecadienoa | E. petiolata | [56] |
| 58 | Ingenol-3-O-(2'E,4'Z)-tetradecadieno | E. petiolata | [56] |
| 59 | 5,20-O-Isopropy1ideny1ingero1 3-O-(2'Z,4'Z)-tetradecadienoate | E. petiolata | [56] |
| 60 | 20-O-Acetylingenol-3-O-(2"E,4"Z)-decadienoate | E. petiolata | [57] |
| 61 | 20-Acetyl-ingenol-3-decadienoate | E. broteri | [54] |
| 62 | 3-Tetradecanoate-ingenol-5,20-diacetate | E. broteri | [54] |
| 63 | 5-Tetradecanoate-ingenol-3,20-diacetate | E. broteri | [54] |
| 64 | 17-Benzoyloxy-3-O-(2,3-dimethylbutanoyl)-20-deoxyingenol | E. esula | [58] |
| 65 | 17-Benzoyloxy-3-O-(2,3-dimethylbutanoyl)-13-(2,3-dimethylbutanoyloxy)-20-deoxyingenol | E. esula | [58] |
| 66 | 17-Benzoyloxy-3-O-(2,3-dimethylbutanoyl)-13-(2,3-dimethylbutanoyloxy) ingenol | E. esula | [58] |
| 67 | 13,17-Dibenzoyloxy-3-O-(2,3-dimethylbutanoyl)ingenol | E. esula | [58] |
| 68 | 13,17-Dibenzoyloxy-3-O-(2,3-dimethylbutanoyl)-20-deoxyingenol | E. esula | [58] |
| 69 | 3-O-(2,3-dimethylbutanoyl)-13-octanoyloxyingenol | E. esula | [58] |
| 70 | 17-Benzoyloxy-3-O-(2,3-dimethylbutanoyl)-13-octanoyloxyingenol | E. esula | [58] |
| 71 | 17-Benzoyloxy-20-O-(2,3-dimethylbutanoyl)-13-(2,3-dimethylbutanoyloxy)ingenol | E. esula | [58] |
| 72 | 17-Benzoyloxy-13-octanoyloxyingenol | E. esula | [58] |
| 73 | 20-O-Benzoyl-17-benzoyloxy-13-octanoyloxyingenol | E. esula | [58] |
| 74 | 17-Benzoyloxy-20-O-(2,3-dimethylbutanoyl)-13-octanoyloxyingenol | E. esula | [58] |
| 75 | 3-O-Benzoyl-17-benzoyloxy-13-(2,3-dimethylbutanoyloxy)ingenol | E. esula | [58] |
| 76 | 3-O-Benzoyl-13,17-dibenzoyloxyingenol | E. esula | [58] |
| 77 | 3-O-Benzoyl-13-octanoyloxyingenol | E. esula | [58] |
| 78 | 3-O-Benzoyl-17-benzoyloxy-13-octanoyloxyingenol | E. esula | [58] |
| 79 | 3-O-Benzoyl-17-benzoyloxy-13-octanoyloxy-20-deoxyingenol | E. esula | [58] |
| 80 | Ingenol-3-angelate-5,20-diacetate | E. canariensis | [59] |
| E. acrurensis | [60] | ||
| 81 | 5-Deoxyingenol-3-angelate-20-acetate | E. canariensis | [59] |
| 82 | 17-Acetoxyingenol-5,20-diacetate-3-angelate | E. kamerunica | [53] |
| 83 | Ingenol-3-angelate | E. canariensis | [59] |
| 84 | 17-Hydroxyingenol-3-angelate-17-benzoate | E. canariensis | [59] |
| 85 | 17-Hydroxyingenol-3-angelate-20-acetate-17-benzoate | E. canariensis | [59] |
| 86 | 17-Acetoxyingenol-20-acetate-3-angelate | E. canariensis | [59] |
| 87 | 17-Hydroxyingenol 17-benzoate 20-angelate | E. canariensis | [59] |
| 88 | 3-O-Angeloyl-17-[(2Z,4E,6Z)-deca-2,4,6-trienoyloxy]ingenol | E. cauducifolia | [21] |
| 89 | 17-Acetyloxy-3-O-angeloyl-ingenol | E. cauducifolia | [21] |
| 90 | 3-O-Angeloyl-17-(benzoyloxy)ingenol | E. cauducifolia | [21] |
| 91 | 20-O-Acetyl-3-O-angeloyl-17-hydroxyingenol | E. cauducifolia | [21] |
| 92 | 20-O-Acetyl-3-O-angeloyl-17-(benzoyloxy)ingenol | E. cauducifolia | [21] |
| 93 | 3-O-Acetyl-20-O-angeloyl-17-hydroxyingenol | E. cauducifolia | [21] |
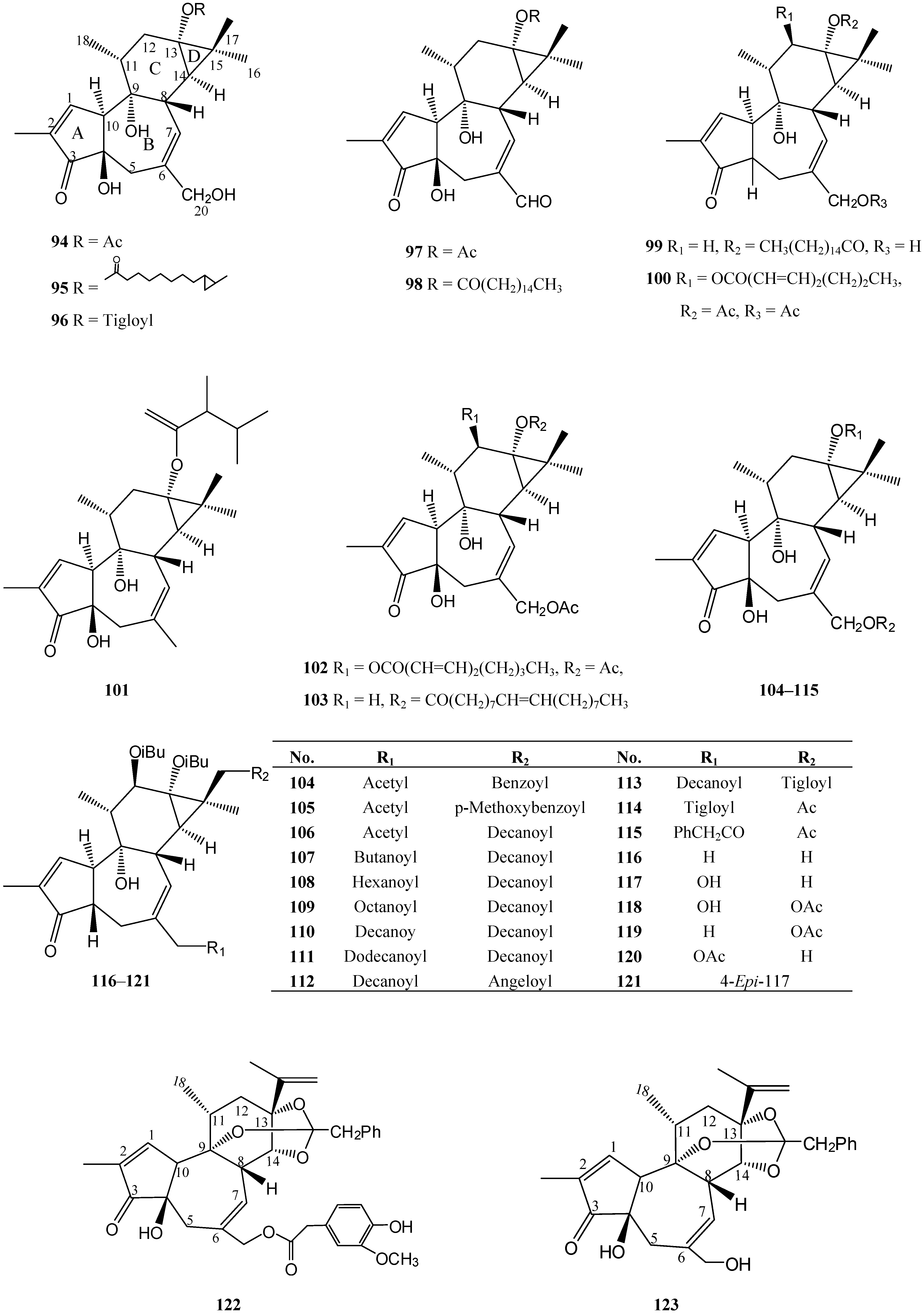
4. Tigliane Derivates Isolated from Euphorbia Species (Table 3)
| No. | Name | Species | Ref |
|---|---|---|---|
| 94 | 13-Acetoxy-12-deoxyphorbol [Prostratin] | E. fischeriana | [66] |
| 95 | 20-Hydroxy-12-deoxyphorbol 13-(cis-9,10-methylene)-undecanoate | E. poisonii | [14] |
| 96 | 20-Hydroxy-12-deoxyphorbol angelate | E. poisonii | [14] |
| 97 | 12-Deoxyphorbaldehyde-l3-acetate | E. fischeriana | [31] |
| 98 | 12-Deoxyphorbaldehyde-13-hexadecacetate | E. fischeriana | [31] |
| 99 | 4,12-Dideoxy(4α)phorbol-13-hexadecanoate | E. guyoniana | [67] |
| 100 | 12-O-(2Z,4E-Octadienoyl)-4-deoxyphorbol-13,20-diacetate | E. broteri | [54] |
| 101 | 4,12,20-Trideoxyphorbol-13-(2,3-dimethyl)butyrate | E. pithyusa subsp | [68] |
| 102 | 12-O-(2Z,4E-octadienoyl)-phorbol-13,20-diacetate | E. broteri | [54] |
| 103 | 12-Deoxyphorbol-13-(9Z)-octadecanoate-20-acetate | E. fischeriana | [31] |
| 104 | 13-O-Acetyl-20-O-benzoyl-12-deoxyphorbol | E. cornigera | [69] |
| 105 | 13-O-Acetyl-20-O-p-methoxybenzoyl-12-deoxyphorbol | E. cornigera | [69] |
| 106 | 13-O-Acetyl-20-O-decanoyl-12-deoxyphorbol | E. cornigera | [69] |
| 107 | 13-O-Butanoyl-20-O-decanoyl-12-deoxyphorbol | E. cornigera | [69] |
| 108 | 13-O-Hexanoyl-20-O-decanoyl-12-deoxyphorbol | E. cornigera | [69] |
| 109 | 13-O-Octanoyl-20-O-decanoyl-12-deoxyphorbol | E. cornigera | [69] |
| 110 | 13,20-Didecanoylphorbol | E. cornigera | [69] |
| 111 | 13-O-Dodecanoyl-20-O-decanoyl-12-deoxyphorbol | E. cornigera | [69] |
| 112 | 13-O-Decanoyl-20-O-angelyl-12-deoxyphorbol | E. cornigera | [69] |
| 113 | 13-O-Decanoyl-20-O-tiglyl-12-deoxyphorbol | E. cornigera | [69] |
| 114 | 12-Deoxyphorbol 20-acetate 13-angelate | E. poisonii | [14] |
| 115 | 12-Deoxyphorbol 20-acetate 13-phenylacetate | E. poisonii | [14] |
| 116 | 4,20-Dideoxyphorbol 12,13-bis(isobutyrate) | E. obtusifolia | [70] |
| 117 | 4-Deoxyphorbol 12,13-bis(isobutyrate) | E. obtusifolia | [70] |
| 118 | 17-Acetoxy-4-deoxyphorbol 12,13-bis(isobutyrate) | E. obtusifolia | [70] |
| 119 | 17-Acetoxy-4,20-dideoxyphorbol 12,13-bis(isobutyrate) | E. obtusifolia | [70] |
| 120 | 4-Deoxyphorbol 12,13-bis(isobutyrate) 20-acetate | E. obtusifolia | [70] |
| 121 | 4-Epi-4-Deoxyphorbol 12,13-bis(isobutyrate) | E. obtusifolia | [70] |
| 122 | 20-(4-Hydroxy-3-methoxyphenylacetate)9,13,14-orthophenylacetate | E. poisonii | [14] |
| 123 | 20-Hydroxyresiniferol 9,13,14-orthophenylacetate | E. poisonii | [14] |
5. 13C-NMR Data of Diterpenes
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| 1 | 38.0 | 40.9 | 38.3 | 43.2 | 40.5 | 41.9 | 41.6 | 41.4 | 41.4 | 41.3 | 40.0 | 41.4 | 39.3 | 37.6 |
| 2 | 18.4 | 18.0 | 17.3 | 20.1 | 18.7 | 17.4 | 17.1 | 18.5 | 18.5 | 18.5 | 18.4 | 18.4 | 18.4 | 27.0 |
| 3 | 42.0 | 40.9 | 41.3 | 43.2 | 43.1 | 39.9 | 38.6 | 40.0 | 39.2 | 39.0 | 41.5 | 39.8 | 41.5 | 78.2 |
| 4 | 32.7 | 32.3 | 32.4 | 34.2 | 34.1 | 37.8 | 37.6 | 33.3 | 33.5 | 33.5 | 33.5 | 33.4 | 33.5 | 39.2 |
| 5 | 46.8 | 54.7 | 55.3 | 56.6 | 57.0 | 55.6 | 55.4 | 53.2 | 53.6 | 53.5 | 53.5 | 53.4 | 53.5 | 52.9 |
| 6 | 30.2 | 19.8 | 16.7 | 22.0 | 19.2 | 18.0 | 17.7 | 21.2 | 21.0 | 20.9 | 20.8 | 20.8 | 20.9 | 20.5 |
| 7 | 68.9 | 39.9 | 34.9 | 43.1 | 36.3 | 35.5 | 35.4 | 36.5 | 36.6 | 35.7 | 33.8 | 34.0 | 34.0 | 33.9 |
| 8 | 35.1 | 75 | 74.4 | 75.7 | 77.6 | 75.0 | 74.8 | 60.8 | 66.9 | 67.4 | 61.3 | 61.1 | 61.3 | 61.0 |
| 9 | 42.5 | 62.6 | 56.3 | 58.0 | 47.4 | 72.0 | 72.1 | 66.6 | 47.0 | 47.8 | 51.9 | 51.7 | 51.8 | 51.6 |
| 10 | 38.0 | 36.9 | 37.2 | 40.3 | 39.0 | 40.5 | 40.5 | 48.2 | 39.1 | 39.3 | 41.6 | 41.3 | 41.4 | 41.1 |
| 11 | 27.5 | 67.3 | 65.0 | 29.9 | 29.0 | 57.2 | 65.4 | 61.3 | 61.6 | 61.9 | 107.6 | 104.1 | 106.4 | 103.4 |
| 12 | 78.5 | 79.0 | 79.7 | 79.0 | 79.9 | 79.7 | 79.8 | 85.4 | 85.5 | 85.3 | 149.5 | 147.4 | 147.3 | 147.6 |
| 13 | 163.4 | 157.5 | 160.3 | 165.3 | 166.5 | 161.2 | 160.3 | 148.4 | 150.8 | 154.5 | 147.2 | 144.9 | 146.5 | 144.8 |
| 14 | 26.7 | 71.9 | 71.8 | 73.5 | 74.3 | 65.2 | 57.4 | 55.6 | 53.6 | 55.3 | 54.3 | 54.4 | 54.4 | 54.3 |
| 15 | 120.5 | 124.2 | 125.9 | 123.2 | 125.5 | 125.0 | 126.0 | 130.1 | 150.8 | 128.3 | 122.3 | 125.1 | 127.4 | 125.4 |
| 16 | 175.4 | 175.4 | 176.2 | 178.0 | 177.8 | 175.4 | 175.3 | 169.8 | 168.2 | 167.4 | 170.5 | 170.6 | 169.2 | 170.4 |
| 17 | 8.4 | 6.7 | 7.9 | 8.2 | 9.4 | 9.2 | 9.0 | 8.6 | 56.5 | 54.9 | 55.4 | 8.6 | 56.3 | 8.6 |
| 18 | 33.1 | 33.0 | 32.4 | 34.6 | 34.1 | 16.7 | 16.4 | 33.2 | 33.5 | 33.5 | 33.4 | 33.4 | 33.5 | 28.3 |
| 19 | 21.6 | 20.8 | 20.6 | 22.4 | 22.4 | 21.8 | 21.3 | 22.1 | 21.9 | 21.9 | 21.9 | 21.9 | 21.9 | 15.5 |
| 20 | 12.6 | 16.8 | 15.7 | 18.1 | 15.4 | 33.6 | 33.7 | 15.4 | 15.6 | 15.1 | 15.0 | 14.9 | 15.1 | 15.0 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |
| 1 | 41.9 | 31.7 | 39.7 | 37.4 | 32.1 | 29.9 | 39.4 | 39.0 | 39.6 | 40.2 | 51.2 | 55.9* | 30.5 | 37.4 |
| 2 | 19.0 | 18.7 | 19.1 | 27.5 | 25.7 | 27.3 | 19.0 | 18.6 | 18.8 | 18.9 | 209.4 | 209.4 | 34.2 | 34.4 |
| 3 | 39.5 | 41.6 | 41.9 | 78.5 | 75.6 | 78.3 | 41.7 | 41.7 | 41.7 | 41.8 | 82.4 | 54.0* | 216.4 | 215.6 |
| 4 | 33.1 | 33.2 | 33.6 | 39.0 | 37.8 | 39.0 | 33.6 | 33.4 | 32.9 | 41.0 | 45.0 | 38.7 | 47.1 | 47.5 |
| 5 | 47.1 | 39.9 | 55.3 | 54.3 | 48.4 | 45.4 | 55.4 | 54.0 | 46.8 | 46.7 | 53.4 | 54.5 | 46.0 | 54.8 |
| 6 | 31.0 | 31.0 | 23.9 | 23.4 | 23.4 | 23.0 | 23.8 | 22.3 | 29.9 | 30.7 | 23.0 | 23.6 | 24.1 | 24.6 |
| 7 | 72.4 | 74.4 | 37.2 | 36.8 | 37.1 | 32.7 | 37.1 | 36.0 | 71.5 | 71.1 | 36.3 | 36.4 | 32.2 | 36.6 |
| 8 | 151.2 | 148.4 | 156.3 | 151.4 | 152.0 | 152.6 | 152.6 | 154.4 | 153.4 | 155.2 | 149.4 | 149.5 | 152.2 | 150.2 |
| 9 | 46.7 | 79.1 | 51.9 | 51.5 | 51.6 | 77.2 | 60.8 | 51.4 | 54.8 | 55.3 | 51.3 | 51.3 | 76.9 | 50.7 |
| 10 | 41.9 | 44.7 | 41.6 | 41.2 | 41.3 | 44.2 | 40.3 | 38.9 | 40.9 | 32.7 | 46.9 | 46.2 | 43.8 | 40.9 |
| 11 | 27.2 | 38.4 | 27.5 | 27.5 | 27.5 | 39.7 | 64.6 | 31.2 | 69.6 | 70.2 | 27.6 | 27.6 | 40.0 | 27.8 |
| 12 | 76.1 | 77.2 | 76.1 | 75.9 | 76.0 | 77.1 | 79.4 | 102.4 | 102.7 | 104.3 | 75.3 | 75.3 | 76.9 | 75.6 |
| 13 | 155.1 | 153.9 | 152.3 | 156.0 | 156.0 | 154.7 | 150.1 | 154.2 | 152.8 | 156.0 | 155.0 | 155.0 | 154.6 | 155.5 |
| 14 | 115.9 | 118.8 | 113.9 | 114.2 | 114.1 | 115.7 | 113.5 | 113.4 | 114.7 | 115.4 | 115.2 | 114.9 | 115.9 | 114.8 |
| 15 | 118.9 | 130.0 | 116.2 | 116.4 | 116.4 | 117.9 | 118.2 | 116.3 | 121.0 | 124.0 | 117.5 | 117.3 | 118.1 | 117.1 |
| 16 | 174.9 | 174.3 | 175.4 | 175.3 | 175.2 | 174.7 | 175.4 | 173.1 | 173.6 | 172.1 | 174.9 | 174.7 | 174.6 | 175.1 |
| 17 | 8.5 | 8.6 | 8.3 | 8.2 | 28.7 | 28.9 | 8.5 | 8.1 | 8.4 | 55.5 | 8.3 | 33.5 | 27.1 | 26.5 |
| 18 | 33.6 | 33.8 | 33.9 | 28.6 | 22.2 | 16.0 | 33.9 | 33.5 | 32.9 | 32.9 | 29.5 | 23.0 | 21.7 | 21.8 |
| 19 | 21.7 | 22.0 | 21.8 | 15.6 | 16.7 | 17.5 | 21.8 | 22.0 | 21.6 | 21.4 | 16.4 | 17.3 | 17.8 | 16.2 |
| 20 | 16.1 | 17.4 | 16.8 | 16.7 | 8.2 | 8.4 | 17.3 | 14.6 | 14.3 | 14.4 | 17.3 | 8.2 | 8.3 | 8.3 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | |
| 1 | 23.4* | 37.9 | 28.2 | 33.6 | 30.4 | 31.0 | 29.8 | 31.4 | 37.5 | 37.4 | 38.9 | 39.3 | 36.6 | 36.1 |
| 2 | 145.2 | 18.2 | 26.8 | 18.8 | 19.3 | 19.1 | 19.2 | 19.2 | 34.7 | 34.6 | 18.4 | 18.31 | 19.0 | 19.2 |
| 3 | 200.2 | 41.4 | 78.1 | 19.1 | 18.5 | 19.9 | 18.4 | 19.9 | 216.5 | 216 | 41.8 | 41.5 | 41.3 | 41.3 |
| 4 | 44.0* | 32.7 | 37.2 | 15.7 | 16.4 | 14.7 | 16.6 | 14.8 | 47.8 | 47.8 | 33.2 | 33.1 | 33.6 | 33.7 |
| 5 | 52.6 | 53.9 | 43.8 | 51.1 | 47.6 | 44.8 | 41.6 | 44.1 | 55.0 | 54.7 | 49.8 | 54.2 | 52.0 | 52.8 |
| 6 | 23.2 | 38.9 | 35.7 | 22.7 | 20.6 | 27.5 | 28.9 | 27.6 | 23.1 | 22.7 | 37.5 | 19.21 | 17.3 | 18.9 |
| 7 | 36.7 | 209.8 | 209.4 | 33.5 | 24.4 | 140.4 | 62.5 | 139.9 | 35.5 | 34.6 | 200.7 | 41.01 | 26.2 | 33.0 |
| 8 | 148.9 | 44.2 | 49.4 | 76.1 | 134.6 | 136.8 | 135.7 | 134.8 | 139.9 | 139.9 | 138.9 | 69.5 | 143.4 | 139.1 |
| 9 | 48.3 | 50.1 | 53.0 | 52.6 | 160.5 | 41.6 | 164.9 | 40.6 | 49.0 | 50.0 | 51.8 | 61.3 | 150.3 | 141.5 |
| 10 | 41.7* | 37.4 | 39.1 | 36.6 | 38.9 | 34.3 | 39.8 | 35.2 | 38.1 | 37.7 | 35.9 | 37.8 | 39.3 | 39.8 |
| 11 | 27.6 | 28.1 | 23.8 | 27 | 34.2 | 27.2 | 33.9 | 27.7 | 18.6 | 26.3 | 18.6 | 71.7 | 152.0 | 154.6 |
| 12 | 75.4 | 77.5 | 77.0 | 79.5 | 78.8 | 77.9 | 78.4 | 76.7 | 31.0 | 69.8 | 29.7 | 197.5 | 125.4 | 112.8 |
| 13 | 154.9 | 160.7 | 160.4 | 153.3 | 150.6 | 151.1 | 149.9 | 52.9 | 46.7 | 52.1 | 71.8 | 136.9 | 176.4 | 135.0 |
| 14 | 116.0 | 24.0 | 38.5 | 196 | 185.7 | 187.5 | 187.0 | 196.2 | 123.7 | 121.9 | 139.5 | 151.4 | 184.8 | 123.0 |
| 15 | 117.7 | 121.9 | 122.0 | 131.8 | 131.1 | 132.5 | 132.8 | 40.0 | 214.1 | 215.1 | 37.8 | 137.3 | 120.2 | 198.0 |
| 16 | 174.9 | 174.8 | 174.8 | 172.9 | 172.8 | 173.5 | 173.3 | 178.2 | 64.8 | 65.2 | 17.6 | – | 144.2 | 26.4 |
| 17 | 27.0 | 8.4 | 8.4 | 9.4 | 9.8 | 10.0 | 9.9 | 16.2 | 23.8 | 17.5 | 16.2 | 128.8 | 8.5 | 33.7 |
| 18 | 2.0 | 13.1 | 27.6 | 21.5 | 22.3 | 20.5 | 22.2 | 20.4 | 25.8 | 25.6 | 32.6 | 33.8 | 33.5 | 22.2 |
| 19 | 19.4 | 33.5 | 14.9 | 23.9 | 23.2 | 24.5 | 23.2 | 24.7 | 22.3 | 22.3 | 21.2 | 21.9 | 21.9 | 19.6 |
| 20 | 8.3 | 21.0 | 13.1 | 16.9 | 16.8 | 11.5 | 15.7 | 12.4 | 14.7 | 14.3 | 14.1 | 17.8 | 20.4 | – |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | |
| 1 | 130.0 | 129.0 | 131.7 | 130.0 | 132.2 | 131.8 | 130.5 | 132.2 | 132.1 | 132.2 | 131.6 | 131.4 | 131.4 | 132.1 |
| 2 | 138.8 | 139.3 | 136.3 | 138.8 | 133.2 | 133.6 | 136.0 | 133.4 | 135.8 | 135.8 | 136.0 | 136.2 | 136.2 | 133.3 |
| 3 | 80.5 | 80.3 | 80.2 | 80.6 | 82.2 | 82.0 | 81.7 | 82.1 | 82.9 | 82.7 | 81.6 | 82.5 | 82.5 | 82.1 |
| 4 | 84.3 | 84.0 | 74.7 | 84.4 | 85.8 | 85.7 | 85.7 | 86.0 | 85.0 | 84.9 | 85.8 | 84.5 | 84.4 | 86.0 |
| 5 | 75.3 | 75.2 | 75.0 | 73.8 | 74.8 | 74.6 | 74.8 | 75.0 | 77.3 | 74.8 | 74.8 | 76.7 | 76.7 | 75.0 |
| 7 | 127.4 | 126.2 | 128.4 | 128.3 | 131.9 | 131.0 | 129.7 | 131.8 | 123.1 | 129.5 | 130.9 | 127.2 | 127.3 | 128.2 |
| 8 | 44.0 | 43.2 | 42.9 | 44.1 | 43.6 | 43.1 | 42.9 | 43.7 | 43.0 | 43.6 | 43.2 | 42.6 | 42.6 | 43.7 |
| 9 | 207.8 | 206.5 | 205.2 | 208.0 | 205.4 | 204.6 | 204.7 | 205.4 | 206.0 | 206.2 | 204.7 | 205.9 | 205.9 | 205.6 |
| 10 | 72.4 | 72.6 | 72.0 | 72.6 | 71.9 | 71.8 | 71.8 | 72.1 | 72.0 | 72.1 | 72.0 | 71.8 | 71.8 | 72.1 |
| 11 | 39.8 | 38.6 | 37.7 | 39.6 | 38.6 | 38.6 | 37.9 | 38.7 | 38.7 | 38.5 | 38.6 | 37.4 | 37.4 | 38.6 |
| 12 | 30.8 | 35.0 | 35.2 | 31.0 | 31.1 | 30.8 | 35.0 | 31.1 | 30.8 | 31.2 | 30.7 | 35.0 | 35.0 | 31.2 |
| 13 | 23.1 | 68.8 | 29.1 | 23.9 | 23.1 | 23.2 | 69.1 | 23.1 | 24.0 | 24.0 | 24.1 | 69.0 | 69.0 | 23.2 |
| 14 | 22.9 | 28.2 | 29.4 | 23.2 | 22.9 | 24.0 | 28.0 | 23.3 | 23.7 | 23.3 | 23.2 | 28.2 | 28.2 | 23.0 |
| 15 | 24.0 | 30.2 | 30.1 | 22.7 | 24.4 | 27.7 | 30.7 | 24.4 | 27.4 | 28.5 | 27.6 | 30.3 | 30.3 | 24.3 |
| 16 | 28.5 | 22.5 | 24.7 | 28.5 | 28.4 | 24.2 | 22.2 | 28.5 | 24.3 | 23.1 | 24.2 | 22.4 | 22.5 | 28.4 |
| 17 | 15.4 | 16.7 | 66.8 | 15.5 | 15.5 | 65.7 | 16.8 | 15.4 | 65.6 | 15.5 | 65.7 | 16.7 | 16.7 | 15.5 |
| 18 | 17.3 | 18.4 | 18.2 | 17.4 | 17.0 | 16.6 | 18.0 | 17.1 | 16.7 | 17.3 | 16.6 | 18.2 | 18.2 | 17.1 |
| 19 | 15.5 | 15.4 | 15.5 | 15.4 | 15.4 | 15.4 | 15.3 | 15.6 | 15.6 | 15.6 | 15.4 | 15.4 | 15.5 | 15.4 |
| 20 | 67.2 | 66.8 | 62.3 | 66.4 | 65.8 | 65.7 | 65.7 | 65.9 | 21.9 | 66.8 | 65.7 | 67.1 | 67.2 | 65.9 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | |
| 1 | 132.2 | 132.0 | 132.2 | 132.2 | 132.2 | 132.0 | 132.3 | 132.1 | 131.5 | 131.0 | 131.2 | 131.8 | 131.5 | 131.1 |
| 2 | 133.4 | 135.9 | 135.9 | 136.1 | 136.1 | – | 133.5 | 136.2 | 136.3 | 136.8 | 136.8 | 136.4 | 136.2 | 136.7 |
| 3 | 82.5 | 82.7 | 81.8 | 82.8 | 82.5 | 82.4 | 82.4 | 82.0 | 82.7 | 82.1 | 82.2 | 82.9 | 82.6 | 82.0 |
| 4 | 86.0 | 84.8 | 84.1 | 85.0 | 85.1 | – | 85.9 | 85.2 | 84.9 | 84.8 | 84.9 | 85.0 | 84.5 | 84.8 |
| 5 | 78.5 | 76.7 | 74.0 | 74.9 | 75.0 | 75.0 | 75.1 | 76.7 | 77.0 | 76.0 | 76.5 | 77.4 | 76.9 | 76.0 |
| 6 | 135.8 | 139.4 | 136.6 | 136.1 | 136.2 | – | 135.5 | 138.4 | 138.2 | 140.2 | 140.1 | 138.3 | 139.6 | 140.1 |
| 7 | 128.4 | 128.2 | 128.3 | 129.3 | 129.4 | 132.0 | 131.7 | 122.8 | 122.0 | 125.6 | 126.1 | 122.2 | 127.3 | 125.7 |
| 8 | 43.7 | 43.5 | 43.6 | 43.9 | 43.8 | 43.7 | 43.7 | 42.8 | 42.6 | 42.7 | 43.0 | 42.9 | 42.7 | 42.6 |
| 9 | 205.7 | 207.0 | 207.6 | 206.2 | 206.1 | – | 205.3 | 206.7 | 205.1 | 205.2 | 204.9 | 205 | 205.8 | 205.1 |
| 10 | 72.2 | 72.0 | 72.3 | 72.1 | 72.3 | – | 72.1 | 71.5 | 71.9 | 71.9 | 71.9 | 72.0 | 71.9 | 71.7 |
| 11 | 38.5 | 38.4 | 37.6 | 38.8 | 38.6 | 38.6 | 38.7 | 38.5 | 38.1 | 37.8 | 38.0 | 38.4 | 37.5 | 37.8 |
| 12 | 31.1 | 31.2 | 31.2 | 31.2 | 31.3 | 31.2 | 31.2 | 30.5 | 35.6 | 35.6 | 35.3 | 35.3 | 34.4 | 35.0 |
| 13 | 23.2 | 23.3 | 23.5 | 23.3 | 23.4 | 23.1 | 23.1 | 23.8 | 68.3 | 68.3 | 69.3 | 69.3 | 69.0 | 68.5 |
| 14 | 23.0 | 23.0 | 23.0 | 23.1 | 23.3 | 23.3 | 23.3 | 23.4 | 28.7 | 28.5 | 28.6 | 29.0 | 28.3 | 28.4 |
| 15 | 24.3 | 24.0 | 24.0 | 24.0 | 24.0 | – | 24.4 | 27.5 | 33.9 | 34.0 | 34.5 | 34.4 | 30.3 | 34.0 |
| 16 | 28.4 | 28.5 | 28.5 | 28.5 | 28.5 | 28.5 | 28.5 | 24.2 | 18.7 | 18.5 | 18.7 | 18.7 | 22.5 | 18.5 |
| 17 | 15.6 | 15.5 | 15.6 | 15.5 | 15.5 | 15.4 | 15.4 | 66.2 | 65.5 | 65.5 | 65.6 | 65.7 | 16.7 | 65.5 |
| 18 | 17.1 | 17.3 | 17.5 | 17.3 | 17.3 | 17.1 | 17.1 | 16.4 | 18.0 | 18.1 | 18.0 | 17.9 | 18.2 | 17.9 |
| 19 | 15.4 | 15.5 | 15.5 | 15.6 | 15.5 | 15.6 | 15.6 | 15.3 | 15.5 | 15.4 | 15.6 | 15.6 | 15.4 | 15.5 |
| 20 | 66.1 | 67.1 | 64.3 | 66.8 | 66.7 | 65.9 | 65.6 | 21.6 | 21.7 | 66.6 | 67.0 | 21.8 | 67.2 | 66.7 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | |
| 1 | 128.6 | 128.6 | 128.4 | 128.8 | 131.4 | 131.5 | 132.0 | 131.5 | 132.0 | 132.0 | 132.0 | 131.5 | 132.1 | 131.6 |
| 2 | 139.7 | 139.8 | 139.7 | 139.6 | 136.8 | 136.8 | 136.0 | 136.8 | 136.0 | 136.0 | 136.0 | 133.7 | 135.7 | 136.2 |
| 3 | 80.4 | 80.2 | 80.1 | 80.4 | 83.0 | 83.0 | 83.3 | 83.0 | 83.6 | 81.8 | 85.7 | 81.5 | 82.5 | 82.2 |
| 4 | 84.3 | 84.3 | 84.3 | 84.3 | 84.9 | 85.1 | 84.6 | 84.9 | 85.1 | 85.8 | 80.5 | 85.7 | 84.7 | 84.9 |
| 5 | 73.8 | 75.2 | 74.0 | 73.8 | 77.3 | 76.3 | 76.9 | 77.2 | 77.2 | 74.9 | 43.7 | 74.6 | 77.1 | 76.6 |
| 6 | 137.7 | 141.2 | 137.5 | 137.8 | 139.8 | 140.2 | 139.0 | 139.8 | 138.0 | 133.0 | 132.0 | 135.9 | 139.0 | 139.7 |
| 7 | 126.8 | 125.2 | 125.5 | 126.7 | 126.4 | 126.0 | 128.0 | 126.4 | 122.0 | 132.0 | 129.0 | 130.8 | 128.6 | 127.2 |
| 8 | 43.2 | 43.3 | 43.3 | 43.3 | 42.9 | 43.0 | 42.7 | 42.9 | 42.8 | 43.6 | 44.2 | 43.1 | 43.5 | 43.2 |
| 9 | 204.9 | 205.4 | 205.3 | 204.8 | 205.0 | 205.1 | 206.0 | 204.9 | 205.0 | 206.0 | 208.0 | 204.6 | 206.6 | 205.9 |
| 10 | 72.9 | 72.7 | 72.8 | 72.7 | 72.2 | 72.0 | 72.0 | 72.0 | 72.1 | 72.0 | 75.0 | 71.9 | 72.0 | 72.0 |
| 11 | 38.8 | 38.8 | 38.8 | 38.9 | 38.0 | 38.2 | 37.6 | 38.1 | 38.5 | 38.6 | 37.0 | 38.5 | 38.3 | 38.4 |
| 12 | 35.9 | 35.4 | 35.6 | 35.4 | 35.9 | 35.3 | 35.2 | 35.4 | 35.4 | 31.1 | 31.5 | 30.6 | 31.1 | 30.9 |
| 13 | 68.1 | 68.4 | 68.3 | 68.3 | 69.4 | 69.3 | 69.0 | 68.6 | 68.6 | 23.1 | 23.7 | 23.1 | 23.3 | 24.3 |
| 14 | 28.7 | 28.7 | 28.6 | 28.7 | 28.6 | 28.6 | 28.3 | 28.6 | 28.8 | 22.9 | 23.2 | 23.9 | 23.0 | 23.5 |
| 15 | 33.8 | 33.9 | 33.9 | 33.9 | 34.0 | 34.4 | 30.3 | 34.1 | 34.1 | 24.3 | 23.6 | 27.5 | 24.0 | 27.7 |
| 16 | 18.8 | 18.7 | 18.6 | 18.7 | 18.9 | 18.6 | 22.5 | 18.7 | 18.7 | 28.4 | 28.6 | 24.1 | 28.5 | 24.6 |
| 17 | 65.4 | 65.7 | 65.5 | 65.5 | 65.5 | 65.6 | 16.7 | 65.5 | 65.6 | 15.4 | 15.5 | 65.6 | 15.5 | 66.2 |
| 18 | 18.5 | 18.2 | 18.2 | 18.2 | 18.4 | 18.1 | 18.4 | 18.1 | 18.1 | 17.0 | 18.2 | 16.5 | 17.3 | 16.9 |
| 19 | 15.2 | 15.4 | 15.3 | 15.3 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.5 | 15.6 | 15.3 | 15.5 | 15.6 |
| 20 | 65.7 | 66.6 | 66.4 | 65.7 | 67.1 | 66.9 | 67.4 | 67.1 | 21.8 | 65.9 | 68.5 | 65.6 | 67.5 | 67.2 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | |
| 1 | 131.6 | 131.7 | 131.3 | 131.3 | 131.7 | 131.7 | 131.7 | 131.7 | 131.7 | 160.6 | 161.4 | 161.3 | 160.4 | 160.5 |
| 2 | 136.2 | 136.4 | 136.8 | 136.8 | 136.3 | 136.3 | 136.7 | 136.3 | 136.3 | 132.9 | 132.7 | 132.7 | 133.5 | 133.5 |
| 3 | 82.3 | 82.5 | 84.7 | 84.7 | 84.2 | 84.4 | 83.6 | 83.9 | 84.1 | 209.2 | 209.3 | 209.4 | 208.3 | 208.4 |
| 4 | 84.9 | 84.9 | 74.2 | 74.2 | 74.2 | 74.5 | 74.3 | 74.4 | 74.1 | 73.8 | 73.8 | 73.8 | 72.8 | 72.8 |
| 5 | 74.7 | 74.9 | 74.0 | 74.0 | 74.5 | 74.2 | 74.3 | 74.3 | 75.0 | 38.7 | 38.6 | 38.5 | 34.4 | 34.6 |
| 6 | 136.4 | 136.3 | 136.8 | 136.8 | 136.7 | 136.5 | 136.7 | 136.3 | 136.6 | 140.4 | 139.8 | 140.0 | 142.9 | 142.9 |
| 7 | 128.2 | 128.7 | 128.5 | 128.5 | 128.3 | 128.4 | 128.0 | 128.1 | 128.4 | 130.4 | 130.6 | 130.4 | 158.1 | 158.2 |
| 8 | 43.2 | 43.3 | 42.1 | 42.1 | 42.9 | 42.9 | 42.4 | 42.8 | 42.9 | 39.1 | 39.2 | 39.1 | 41.4 | 41.5 |
| 9 | 205.5 | 205.5 | 207.2 | 207.2 | 206.0 | 205.7 | 205.0 | 205.4 | 205.2 | 76.0 | 76.0 | 76.2 | 77.1 | 77.1 |
| 10 | 72.0 | 72.1 | 71.7 | 71.7 | 71.9 | 71.9 | 71.7 | 71.8 | 71.9 | 56.2 | 55.8 | 55.7 | 55.8 | 55.8 |
| 11 | 38.5 | 38.5 | 37.2 | 37.2 | 37.7 | 37.6 | 37.6 | 37.6 | 37.7 | 36.6 | 36.3 | 36.3 | 36.5 | 36.5 |
| 12 | 30.9 | 30.9 | 34.9 | 34.9 | 35.1 | 35.1 | 35.2 | 35.3 | 35.2 | 32.3 | 32.0 | 31.9 | 31.7 | 31.8 |
| 13 | 24.3 | 24.3 | 29.4 | 29.4 | 29.4 | 29.3 | 29.5 | 29.8 | 29.2 | 63.8 | 63.2 | 63.2 | 63.0 | 63.0 |
| 14 | 23.5 | 23.5 | 29.1 | 29.1 | 29.6 | 29.4 | 29.8 | 29.6 | 29.7 | 32.8 | 32.6 | 32.8 | 32.0 | 32.1 |
| 15 | 27.7 | 27.5 | 29.7 | 29.7 | 30.2 | 29.8 | 30.0 | 30.0 | 30.0 | 22.5 | 26.6 | 22.9 | 22.9 | 22.7 |
| 16 | 24.5 | 24.4 | 24.8 | 24.8 | 24.8 | 24.9 | 24.7 | 24.9 | 24.7 | 23.2 | 23.1 | 23.6 | 23.1 | 23.2 |
| 17 | 66.1 | 65.6 | 66.2 | 66.2 | 66.3 | 66.3 | 62.5 | 66.3 | 62.2 | 15.3 | 15.4 | 15.4 | 15.3 | 15.3 |
| 18 | 16.9 | 17.0 | 18.5 | 18.5 | 18.6 | 18.7 | 18.8 | 18.2 | 18.4 | 18.8 | 18.6 | 18.6 | 18.5 | 18.6 |
| 19 | 15.6 | 15.6 | 16.1 | 16.1 | 15.3 | 16.0 | 16.0 | 15.9 | 15.5 | 9.9 | 10.1 | 10.1 | 10.1 | 10.1 |
| 20 | 66.5 | 66.7 | 62.2 | 62.2 | 62.4 | 62.3 | 65.5 | 66.3 | 66.4 | 67.9 | 68.3 | 68.2 | 193.8 | 193.8 |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 | 111 | ||
| 1 | 156.9 | 159.7 | 161.0 | 160.8 | 161.3 | 160.3 | 160.3 | 160.3 | 160.3 | 160.3 | 160.3 | 160.3 | 160.3 | |
| 2 | 143.0 | 137.3 | 138.3 | 135.7 | 132.9 | 136.3 | 136.3 | 136.2 | 136.2 | 136.3 | 136.3 | 136.2 | 136.3 | |
| 3 | 213.8 | 208.9 | 203.0 | 208.6 | 208.9 | 210.2 | 210.3 | 210.3 | 210.3 | 210.3 | 210.3 | 210.3 | 210.3 | |
| 4 | 50.1 | 42.6 | 44.4 | 73.6 | 73.7 | 44.5 | 44.5 | 44.5 | 44.5 | 44.6 | 44.5 | 44.6 | 44.6 | |
| 5 | 25.1 | 35.1 | 34.0 | 38.8 | 38.9 | 34.0 | 34.0 | 34.1 | 34.0 | 34.0 | 34.0 | 34.1 | 34.1 | |
| 6 | 136.3 | 136.5 | 136.2 | 132.9 | 135.2 | 139.6 | 139.6 | 139.7 | 139.7 | 139.6 | 139.6 | 139.6 | 139.7 | |
| 7 | 127.7 | 130.2 | 126.8 | 132.7 | 133.7 | 125.0 | 125.7 | 125.7 | 125.7 | 125.7 | 125.6 | 125.6 | 125.6 | |
| 8 | 41.0 | 42.3 | 41.9 | 39.4 | 39.5 | 42.2 | 42.2 | 42.2 | 42.2 | 42.2 | 42.2 | 42.2 | 42.2 | |
| 9 | 75.5 | 77.8 | 75.2 | 78.2 | 75.9 | 77.9 | 77.9 | 77.9 | 77.9 | 77.9 | 77.9 | 77.9 | 77.9 | |
| 10 | 47.1 | 54.1 | 53.9 | 56.2 | 55.8 | 54.3 | 54.3 | 54.3 | 54.4 | 54.3 | 54.3 | 54.3 | 54.3 | |
| 11 | 37.1 | 44.1 | 46.2 | 43.2 | 36.3 | 42.3 | 42.3 | 42.3 | 42.3 | 42.3 | 42.3 | 42.4 | 42.4 | |
| 12 | 30.5 | 76.1 | 31.8 | 76.1 | 31.9 | 56.7 | 56.7 | 54.8 | 56.8 | 56.7 | 56.7 | 56.7 | 52.7 | |
| 13 | 62.7 | 65.4 | 62.8 | 65.7 | 63.6 | 65.0 | 65.0 | 65.0 | 65.1 | 65.0 | 65.0 | 65.0 | 65.0 | |
| 14 | 33.1 | 35.4 | 32.0 | 36.1 | 32.4 | 35.9 | 35.8 | 35.9 | 36.0 | 35.9 | 35.9 | 36.0 | 36.0 | |
| 15 | 22.5 | 25.7 | 22.5 | 25.7 | 22.6 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | |
| 16 | 23.7 | 23.8 | 15.2 | 23.8 | 23.2 | 23.9 | 23.9 | 23.9 | 24.0 | 23.9 | 24.0 | 23.9 | 23.9 | |
| 17 | 15.2 | 16.7 | 22.9 | 16.7 | 15.3 | 17.0 | 17.0 | 16.9 | 16.9 | 17.0 | 16.9 | 16.9 | 16.8 | |
| 18 | 15.9 | 15.1 | 19.0 | 14.4 | 18.6 | 15.1 | 15.1 | 15.1 | 15.2 | 15.1 | 15.2 | 15.2 | 15.0 | |
| 19 | 10.4 | 10.2 | 10.0 | 10.1 | 10.1 | 10.2 | 10.2 | 10.3 | 10.3 | 10.2 | 10.3 | 10.3 | 10.3 | |
| 20 | 69.5 | 68.9 | 25.2 | 69.4 | 69.4 | 62.2 | 66.4 | 67.4 | 68.2 | 66.9 | 66.7 | 66.5 | 65.9 | |
| Carbon | Compound / δC (in ppm) | |||||||||||||
| 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 | 121 | 122 | 123 | |||
| 1 | 160.3 | 160.2 | 161.5 | 161.4 | 160.2 | 159.8 | 160.0 | 159.4 | 159.8 | 156.2 | 158.2 | 158.3 | ||
| 2 | 136.2 | 136.2 | 132.8 | 132.8 | 136.3 | 136.4 | 136.6 | 136.5 | 136.5 | 143.3 | 136.6 | 136.5 | ||
| 3 | 210.3 | 210.3 | 209.1 | 209.0 | 210.2 | 209.7 | 209.7 | 210.1 | 209.6 | 213.3 | 208.4 | 209.0 | ||
| 4 | 44.5 | 44.5 | 73.6 | 73.6 | 44.5 | 44.2 | 44.0 | 44.3 | 44.1 | 49.6 | 73.3 | 73.5 | ||
| 5 | 34.1 | 34.1 | 39.0 | 39.0 | 34.0 | 29.6 | 29.0 | 33.6 | 30.0 | 25.1 | 40.0 | 39.8 | ||
| 6 | 139.6 | 139.7 | 134.8 | 134.8 | 139.0 | 142.0 | 142.4 | 139.3 | 137.2 | 137.0 | 135.0 | 138.9 | ||
| 7 | 125.7 | 125.6 | 134.1 | 133.2 | 125.8 | 126.5 | 125.3 | 125.0 | 130.3 | 126.5 | 130.8 | 130.8 | ||
| 8 | 42.2 | 42.2 | 39.5 | 39.4 | 42.2 | 42.1 | 42.4 | 42.6 | 42.2 | 40.7 | 39.1 | 38.9 | ||
| 9 | 77.9 | 77.9 | 76.0 | 75.9 | 77.9 | 77.8 | – | 77.5 | 77.8 | 78.1 | 81.1 | 81.2 | ||
| 10 | 54.3 | 54.3 | 55.7 | 56.7 | 54.3 | 54.2 | 53.8 | 53.9 | 54.0 | 47.4 | 55.4 | 55.5 | ||
| 11 | 42.4 | 42.4 | 36.4 | 36.3 | 42.3 | 42.4 | 42.4 | 42.5 | 42.3 | 43.2 | 33.0 | 33.1 | ||
| 12 | 53.8 | 56.8 | 31.9 | 31.7 | 76.7 | 76.7 | 76.1 | 76.2 | 76.4 | 75.3 | 35.7 | 35.7 | ||
| 13 | 65.0 | 65.1 | 63.1 | 63.9 | 65.0 | 65.0 | 65.2 | 65.3 | 64.8 | 64.8 | 84.4 | 84.5 | ||
| 14 | 36.0 | 35.9 | 32.7 | 32.3 | 35.9 | 35.8 | 36.4 | 36.5 | 35.5 | 37.1 | 80.6 | 80.8 | ||
| 15 | 25.8 | 25.8 | 22.9 | 23.0 | 25.8 | 25.9 | 30.0 | 29.8 | 25.8 | 25.3 | 146.5 | 146.4 | ||
| 16 | 23.9 | 24.0 | 23.6 | 23.0 | 23.9 | 23.8 | – | 19.5 | 23.8 | 24.2 | 110.7 | 110.7 | ||
| 17 | 17.0 | 16.9 | 15.4 | 15.3 | 16.9 | 16.9 | 63.3 | 63.5 | 16.8 | 16.5 | 18.8 | 18.8 | ||
| 18 | 15.2 | 15.2 | 18.6 | 18.5 | 15.1 | 15.1 | 15.2 | 15.2 | 15.0 | 11.9 | 19.8 | 19.9 | ||
| 19 | 10.3 | 10.3 | 10.1 | 10.1 | 10.2 | 10.2 | 10.3 | 10.3 | 10.3 | 10.5 | 10.2 | 10.2 | ||
| 20 | 67.2 | 65.9 | 69.8 | 69.7 | 25.4 | 67.5 | 67.1 | 25.4 | 68.9 | 69.3 | 70.4 | 69.3 | ||
Acknowledgements
References and Notes
- Jassbi, A.R. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry 2006, 67, 1977–1984. [Google Scholar] [CrossRef]
- Hohmann, J.; Molnár, J. Euphorbiaceae diterpenes: Plant toxins or promising molecules for the therapy? Acta Pharm. Hung. 2004, 74, 149–157. [Google Scholar]
- Ahmad, W.; Nazir, M.; Khan, S.A. The chemical composition of various Euphorbia species for industrial applications. Part II. Neutral lipids of Euphorbia cauducifolia. Pak. J. Sci. Res. 1988, 31, 85–89. [Google Scholar]
- Duarte, N.; Gyémánt, N.; Abreu, P.M.; Molnár, J.; Ferreira, M.J.U. New macrocyclic lathyrane diterpenes, from Euphorbia lagascae, as inhibitors of multidrug resistance of tumour cells. Planta Med. 2006, 72, 162–168. [Google Scholar] [CrossRef]
- Yan, S.S.; Li, Y.; Wang, Y.; Shen, S.S.; Gu, Y.; Wang, H.B.; Qin, G.W.; Yu, Q. 17–Acetoxyjolkinolide B irreversibly inhibits I-kappaB kinase and induces apoptosis of tumor cells. Mol. Cancer Ther. 2008, 7, 1523–1532. [Google Scholar] [CrossRef]
- Liu, W.K.; Ho, J.C.K.; Qin, G.W.; Che, C.T. Jolkinolide B induces neuroendocrine differentiation of human prostate LNCaP cancer cell line. Biochem. Pharmacol. 2002, 63, 951–957. [Google Scholar]
- Luo, H.Y.; Wang, A.Q. Induction of apoptosis in K562 cells by jolkinolide B. Can. J. Physiol. Pharmacol. 2006, 84, 959–965. [Google Scholar] [CrossRef]
- Duarte, N.; Loge, H.; Ferreira, M.J.U. Antiproliferative activity of ent-abietane lactones against resistant human cancer cell lines. Planta Med. 2008, 74, 984–985. [Google Scholar]
- Kigoshi, H.; Ichino, T.; Yamada, K.; Ijuin, Y.; Makita, S.F.; Uemura, D. Stereostructure and bioactivities of jolkinolide D. Chem. Lett. 2001, 6, 518–519. [Google Scholar]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Singla, A.K.; Pathak, K. Phytoconstituents of Euphorbia species. Fitoterapia 1990, 61, 483–516. [Google Scholar]
- Appendino, G.; Szallasi, A. Euphorbium: Modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997, 60, 681–696. [Google Scholar] [CrossRef]
- Xu, Z.H.; Sun, J.; Xu, R.S.; Qin, G.W. Casbane diterpenoids from Euphorbia ebracteolata. Phytochemistry 1998, 49, 149–151. [Google Scholar]
- Fatope, M.O.; Zeng, L.; Ohayaga, J.E.; Shi, G.; McLaughlin, J.L. Selectively cytotoxic diterpenes from Euphorbia poisonii. J. Med. Chem. 1996, 39, 1005–1008. [Google Scholar] [CrossRef]
- Murugan, S.; Anand, R.; Uma-Devi, P.; Vidhya, N.; Rajesh, K.A. Efficacy of Euphorbia milli and Euphorbia pulcherrima on aflatoxin producing fungi (Aspergillus flavus and Aspergillus parasiticus). Afr. J. Biotechnol. 2007, 6, 718–719. [Google Scholar]
- Ma, Q.G.; Liu, W.Z.; Wu, X.Y.; Zhou, T.X.; Qin, G.W. Chemical studies of Lang-Du, a traditional Chinese medicine. 1. Diterpenoids from Euphorbia fischeriana. Phytochemistry 1997, 44, 663–666. [Google Scholar]
- Hezareh, M. Prostratin as a new therapeutic agent targeting HIV viral reservoirs. Drug News Perspect. 2005, 18, 496–500. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Palomares, E.; Marco, J.A.; Estornell, E. Tigliane diterpenes from the latex of Euphorbia obtusifolia with inhibitory activity on the mammalian mitochondrial respiratory chain. J. Ethnopharmacol. 2003, 85, 279–282. [Google Scholar] [CrossRef]
- Singh, A.; Agarwal, R.A. Possibility of using latex of euphorbiales for snail control. Sci. Total Environ. 1988, 77, 231–236. [Google Scholar] [CrossRef]
- Vogg, G.; Mattes, E.; Rothenburger, J.; Hertkorn, N.; Stefan, A.; Sandermann, J.H. Tumor promoting diterpenes from Euphorbia leuconeura L. Phytochemistry 1999, 51, 289–295. [Google Scholar]
- Baloch, I.B.; Baloch, M.K.; Saqib, Q.N. Tumor-Promoting Diterpene Esters from Latex of Euphorbia cauducifolia L. Helv. Chim. Acta. 2005, 88, 3145–3150. [Google Scholar] [CrossRef]
- Kedei, N.; Lundberg, D.J.; Toth, A.; Welburn, P.; Garfield, S.H.; Blumberg, P.M. Characterization of the Interaction of Ingenol 3-Angelate with Protein Kinase C. Cancer Res. 2004, 64, 3243–3255. [Google Scholar] [CrossRef]
- Cateni, F.; Falsone, G.; Zilic, J. Terpenoids and glycolipids from Euphorbiaceae. Mini-Rev. Med. Chem. 2003, 3, 425–437. [Google Scholar] [CrossRef]
- Zayed, S.; Farghaly, M.; Soliman, S.M.; Gotta, H.; Sorg, B.; Hecker, E. Dietary cancer risk from conditional cancerogens (tumor promoters) in produce of livestock fed on species of spurge (Euphorbiaceae) V. Skin irritant and tumor-promoting diterpene ester toxins of the tigliane and ingenane type in the herbs Euphorbia nubica and Euphorbia helioscopia contaminating fodder of livestock. J. Cancer Res. Clin. Oncol. 2001, 127, 40–47. [Google Scholar] [CrossRef]
- Shi, H.M.; Williams, I.D.; Sung, H.H.Y.; Zhu, H.X.; Ip, N.Y.; Min, Z.D. Cytotoxic diterpenoids from the roots of Euphorbia ebracteolata. Planta Med. 2005, 71, 349–354. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Gyémánt, N.; Madureira, A.M.; Molnár, J. Inhibition of P-glycoprotein transport activity in a resistant mouse lymphoma cell line by diterpenic lactones. Anticancer Res. 2005, 25, 3259–3262. [Google Scholar]
- Marco, J.A.; Sanz-Cervera, J.F.; Yuste, A.; Jakupovic, J. Isoterracinolides A and B, novel bishomoditerpene lactones from Euphorbia terracina. J. Nat. Prod. 1999, 62, 110–113. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.F.; Cai, X.H.; Ma, Y.B.; Luo, X.D. Three new diterpenoids from Euphorbia wallichii. Chin. J. Chem. 2004, 22, 199–202. [Google Scholar]
- Pan, Q.; Shi, M.F.; Min, Z.D. Studies on the 2D NMR spectra of Jolkinolide diterpenoids from Euphorbia fischriana. J. Chin. Pharm. Univ. 2004, 35, 16–19. [Google Scholar]
- Sutthivaiyakit, S.; Thapsut, M.; Prachayasittikul, V. Constituents and bioactivity of the tubers of Euphorbia sessiliflora. Phytochemistry 2000, 53, 947–950. [Google Scholar] [CrossRef]
- Wang, Y.B.; Huang, R.; Wang, H.B.; Jin, H.Z.; Lou, L.G.; Qin, G.W. Diterpenoids from the roots of Euphorbia fischeriana. J. Nat. Prod. 2006, 69, 967–970. [Google Scholar] [CrossRef]
- Che, C.T.; Zhou, T.X.; Ma, Q.G.; Qin, G.W.; Williams, I.D.; Wu, H.M.; Shi, Z.S. Diterpenes and aromatic compounds from Euphorbia fischeriana. Phytochemistry 1999, 52, 117–21. [Google Scholar]
- Lal, A.R.; Cambie, R.C.; Rutledge, P.S.; Woodgate, P.D. Chemistry of fijian plants. 6. Ent-pimarane and ent-abietane diterpenes from Euphorbia fidjiana. Phytochemistry 1990, 29, 2239–2246. [Google Scholar]
- Haba, H.; Lavaud, C.; Marcourt, L.; Long, C.; Harkat, H.; Benkhaled, M. Ent-abietane diterpenoids from Euphorbia guyoniana Boiss. & Reut. Biochem. Syst. Ecol. in press.
- Jeske, F.; Jakupovic, J.; Berendsohn, W. Diterpenes from Euphorbia seguieriana. Phytochemistry 1995, 40, 1743–1750. [Google Scholar]
- Appendino, G.; Belloro, E.; Cesare-Tron, G.; Jakupovic, J.; Ballero, M. Polycyclic diterpenoids from Euphorbia characias. Fitoterapia 2000, 71, 134–142. [Google Scholar] [CrossRef]
- Valente, C.; Pedro, M.; Duarte, A.; Nascimento, M.S.J.; Abreu, P.M.; Ferreira, M.J.U. Bioactive diterpenoids, a new jatrophane and two ent-abietanes, and other constituents from Euphorbia pubescens. J. Nat. Prod. 2004, 67, 902–904. [Google Scholar] [CrossRef]
- Appendino, G.; Jakupovic, S.; Tron, G.C.; Jakupovic, J.; Milon, V.; Ballero, M. Macrocyclic diterpenoids from Euphorbia semiperfoliata. J. Nat. Prod. 1998, 61, 749–756. [Google Scholar] [CrossRef]
- Shizuri, Y.; Kosemura, S.; Yamamura, S.; Ohba, S.; Ito, M.; Saito, Y. Isolation and structures of helioscopinolides, new diterpenes from Euphorbia helioscopia L. Chem. Lett. 1983, 12, 65–68. [Google Scholar]
- Crespi-Perellino, N.; Garofano, L.; Arlandini, E.; Pinciroli, V.; Minghetti, A.; Vincieri, F.F.; Danieli, B. Identification of new diterpenoids from Euphorbia calyptrata cell cultures. J. Nat. Prod. 1996, 59, 773–776. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.W. Chemical Studies on the constituents of the Chinese medicinal herb Euphorbia helioscopia L. Chem. Pharm. Bull. 2006, 54, 1037–1039. [Google Scholar] [CrossRef]
- Borghi, D.; Baumer, L.; Ballabio, M.; Arlandini, E.; Perellino, N.C.; Minghetti, A.; Vincieri, F.F. Structure elucidation of helioscopinolides D and E from Euphorbia calyptrata cell cultures. J. Nat. Prod. 2004, 54, 1503–1508. [Google Scholar]
- Haba, H.; Lavaud, C.; Magid, A.A.; Benkhaled, M. Diterpenoids and triterpenoids from Euphorbia retusa. J. Nat. Prod. 2009, 72, 1258–1264. [Google Scholar] [CrossRef]
- Morgenstern, T.; Bittner, M.; Silva, M.; Aqueveque, P.; Jakupovic, J. Diterpenes and phloracetophenones from Euphorbia portulacoides. Phytochemistry 1996, 41, 1149–1153. [Google Scholar]
- Shi, J.S.; Li, Z.X.; Nitoda, T.; Minoru, I.; Hiroshi, K.; Naomichi, B.; Kazuyoshi, K.; Shuhei, N. Antinematodal activities of ingenane diterpenes from Euphorbia kansui and their derivatives against the pine wood nematode. Z. Naturforsch., C: Biosci. 2008, 63, 59–65. [Google Scholar]
- Shi, J.X.; Li, Z.X.; Izumi, M.; Baba, N.; Nakajima, S. Termiticidal activity of diterpenes from the roots of Euphorbia kansui. Z. Naturforsch., C: Biosci. 2008, 63, 51–58. [Google Scholar]
- Matsumoto, T.; Cyong, J.C.; Yamada, H. Stimulatory effects of ingenols from Euphorbia kansui on the expression of macrophage Fc receptor. Planta Med. 1992, 58, 255–258. [Google Scholar] [CrossRef]
- Sayed, M.D.; Riszk, A.; Hammouda, F.M.; Elmissiry, M.M.; Williamson, E.M.; Evans, F.J. Constituents of Egyptian Euphorbiaceae. 9. Irritant and cyto-toxic ingenane esters from Euphorbia paralias L. Experientia 1980, 36, 1206–1207. [Google Scholar] [CrossRef]
- Marston, A.; Hecker, E. On the active principles of the Euphorbiaceae. 6. Isolation and biological-activities of seven milliamines from Euphorbia milii. Planta Med. 1983, 47, 141–147. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, N.L.; Yao, X.S.; Miyata, S.; Kitanaka, S. Studies on the bioactive constituents in Euphorbiaceae, part 3. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. Chem. Pharm. Bull. 2003, 51, 935–941. [Google Scholar] [CrossRef]
- Sorg, B.; Schmidt, R.; Hecker, E. Structure activity relationships of polyfunctional diterpenes of the ingenane type. I. Tumor-promoting activity of homologous, aliphatic 3-esters of ingenol and of Δ7,8-isoingenol-3-tetradecanoate. Carcinogenesis 1987, 8, 1–4. [Google Scholar] [CrossRef]
- Oksuz, S.; Ulubelen, A.; Barla, A.; Kohlbau, H.J.; Voelter, W. Triterpenoids and a diterpene from Euphorbia iberica. Planta Med. 1999, 65, 475–477. [Google Scholar] [CrossRef]
- Connoly, J.D.; Facunle, C.O.; Rycroft, D.S. Five ingol esters and a 17-hydroxyingenol ester from the latex of Euphorbia kamerunica. Assignment of esters using 13C-NMR. methods. Tetrahedron Lett. 1984, 25, 3773–3776. [Google Scholar] [CrossRef]
- Urones, J.G.; Barcala, P.B.; Cuadrado, M.J.S.; Marcos, I.S. Diterpenes from the Latex of Euphorbia broteri. Phytochemistry 1988, 27, 207–212. [Google Scholar]
- Tada, M.; Seki, H. Toxic diterpernes from Euphorbia trigona (Saiunkaku: an indoor foliage plant in Japan). Agric. Biol. Chem. 1989, 53, 425–430. [Google Scholar] [CrossRef]
- Shi, Y.P.; Jia, Z.J.; Ma, B.; Saleh, S.D.; Lahham, J. Ingenane diterpenes from Euphorbia petiolata. Planta Med. 1996, 62, 260–262. [Google Scholar] [CrossRef]
- Shi, J.G.; Jia, Z.J.; Yang, L. Lathyrane and ingenane diterpenoids from Euphorbia micractina. Planta Med. 1994, 60, 588–589. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Yang, M.; Zhang, J.Q.; Chen, G.T.; Huang, H.L.; Guan, S.H.; Ma, C.; Liu, X.; Guo, D.A. Ingenane diterpenoids from Euphorbia esula. Phytochemistry 2008, 69, 812–819. [Google Scholar]
- Marco, J.A.; Sanz–Cervera, J.F.; Yuste, A. Ingenane and lathyrane diterpenes from the latex of Euphorbia canariensis. Phytochemistry 1997, 45, 563–570. [Google Scholar]
- Marco, J.A.; Sanz-Cervera, J.F.; Ropero, F.J.; Checa, J.; Fraga, B.M. Ingenane and lathyrane diterpenes from the latex of Euphorbia acrurensis. Phytochemistry 1998, 49, 1095–1099. [Google Scholar]
- Ott, H.H.; Hecker, E. Highly irritant ingenane type diterpene esters from Euphorbia cyparissias L. Experientia 1981, 37, 88–91. [Google Scholar] [CrossRef]
- Wu, D.G.; Sorg, B.; Hecker, E. Oligocyclic and macrocyclic diterpenes in thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China). 6. Tigliane type diterpene esters from latex of Euphorbia prolifera. Phytother. Res. 1994, 8, 95–99. [Google Scholar] [CrossRef]
- Marston, A.; Hecker, E. Active principles of the Euphorbiaceae. 7. Milliamine H and milliamine I, peptide esters of 20-deoxy-5x-hydroxyphorbol from Euphorbia milii. Planta Med. 1984, 50, 319–322. [Google Scholar] [CrossRef]
- Wu, D.G.; Sorg, B.; Adolf, W.; Seip, E.H.; Hecker, E. Oligo- and macrocyclic diterpenes in thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China). 2. Ingenane type diterpene esters from Euphorbia nematocypha Hand.-Mazz. Phytother. Res. 1992, 6, 237–240. [Google Scholar] [CrossRef]
- Furstenberger, G.; Hecker, E. On the active principles of the Euphorbiaceae, XII. Highly unsaturated irritant diterpene esters from Euphorbia tirucalli originating from Madagascar. J. Nat. Prod. 1986, 49, 386–397. [Google Scholar] [CrossRef]
- Liu, W.Z.; Wu, X.Y.; Yang, G.J.; Ma, Q.G.; Zhou, T.X.; Tang, X.C.; Qin, G.W. 12-deoxyphorbol esters from Euphorbia fischeriana. Chin. Chem. Lett. 1996, 7, 917–918. [Google Scholar]
- Haba, H.; Lavaud, C.; Harkat, H.; Alabdul-Magid, A.; Marcourt, L.; Benkhaled, M. Diterpenoids and triterpenoids from Euphorbia guyoniana. Phytochemistry 2007, 68, 1255–1260. [Google Scholar]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J; Ballero, M. Diterpenoids from Euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef]
- Baloch, I.B.; Baloch, M.K.; Saqib, Q. Anti-tumor 12-deoxyphorbol esters from Euphorbia cornigera. Eur. J. Med. Chem. 2008, 43, 274–281. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Checa, J.; Palomares, E.; Fraga, B.M. Jatrophane and tigliane diterpenes from the latex of Euphorbia obtusifolia. Phytochemistry 1999, 52, 479–485. [Google Scholar]
- Sample Availability: Samples of the compounds 8, 12, 61 and 94 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, Q.-C.; Tang, Y.-P.; Ding, A.-W.; You, F.-Q.; Zhang, L.; Duan, J.-A. 13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species. Molecules 2009, 14, 4454-4475. https://doi.org/10.3390/molecules14114454
Wu Q-C, Tang Y-P, Ding A-W, You F-Q, Zhang L, Duan J-A. 13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species. Molecules. 2009; 14(11):4454-4475. https://doi.org/10.3390/molecules14114454
Chicago/Turabian StyleWu, Qi-Cheng, Yu-Ping Tang, An-Wei Ding, Fen-Qiang You, Li Zhang, and Jin-Ao Duan. 2009. "13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species" Molecules 14, no. 11: 4454-4475. https://doi.org/10.3390/molecules14114454
APA StyleWu, Q.-C., Tang, Y.-P., Ding, A.-W., You, F.-Q., Zhang, L., & Duan, J.-A. (2009). 13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species. Molecules, 14(11), 4454-4475. https://doi.org/10.3390/molecules14114454




