Efficient TCT-catalyzed Synthesis of 1,5-Benzodiazepine Derivatives under Mild Conditions
Abstract
:Introduction
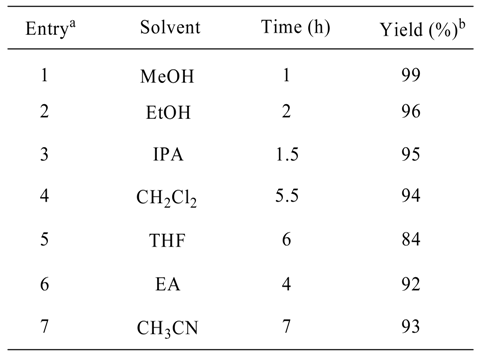
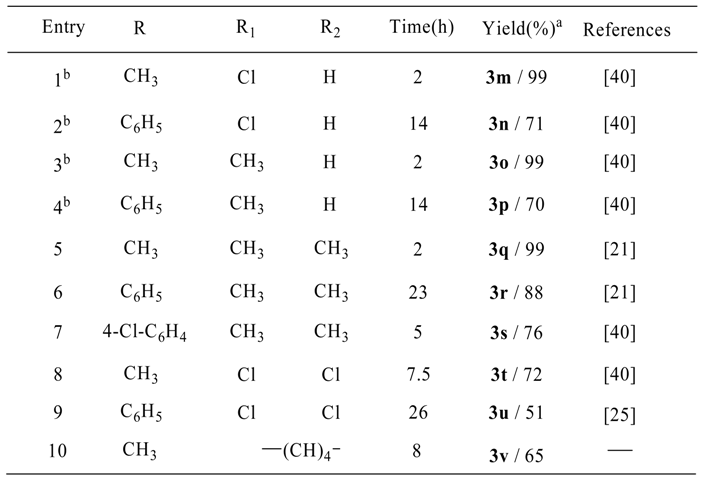
Results and Discussion
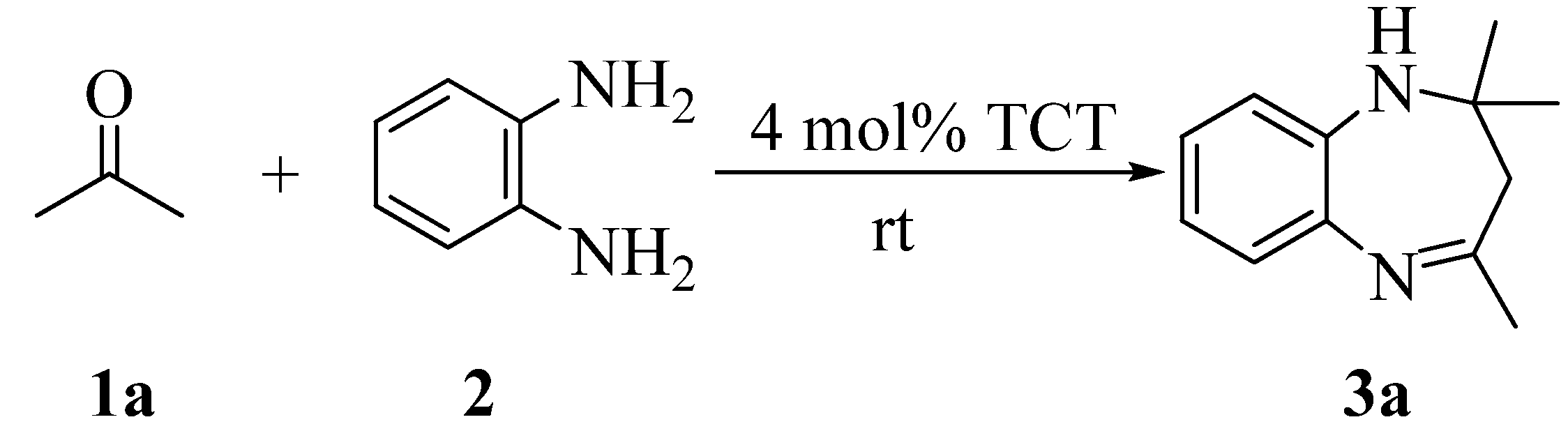
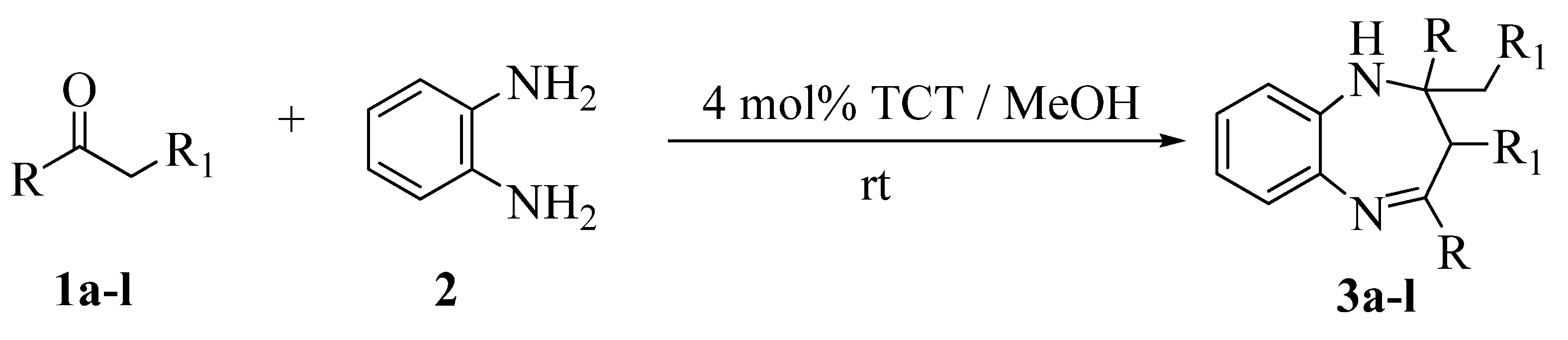
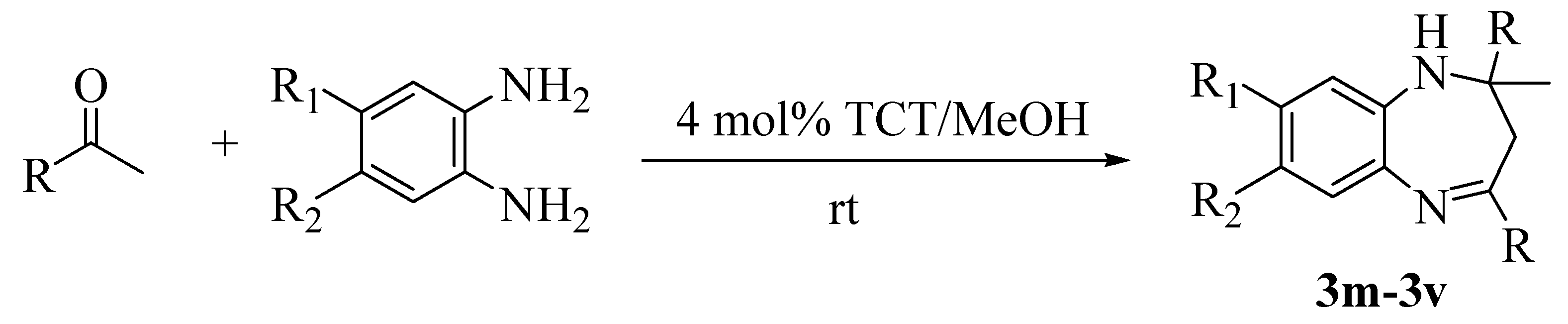
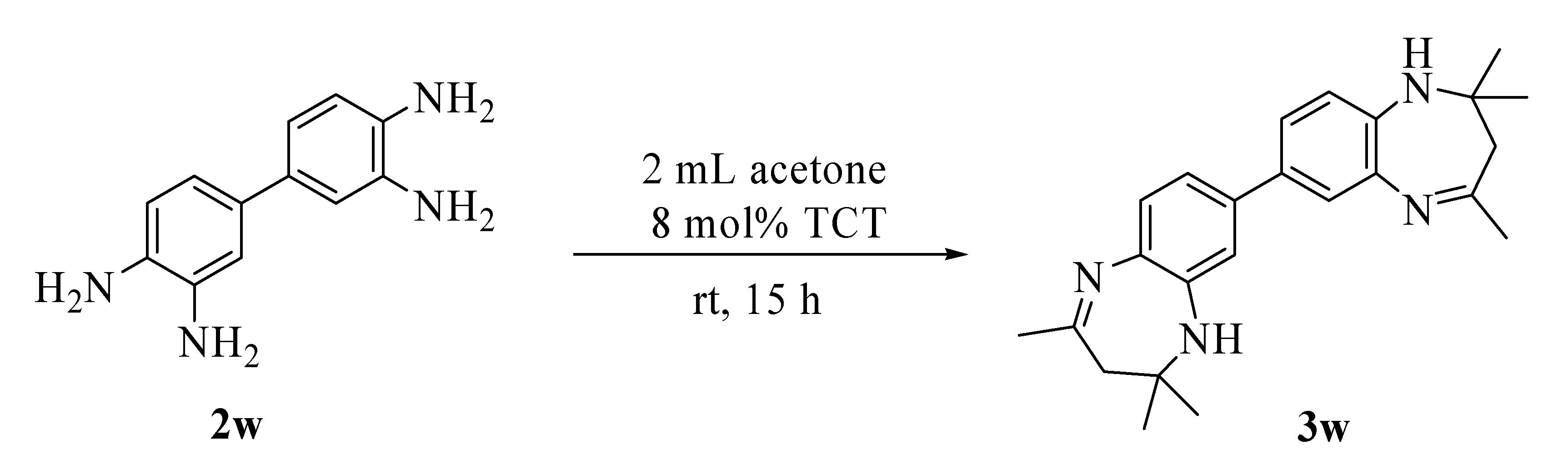
Conclusions
Experimental
General
General procedure for the preparation of 1,5-benzodiazepines
Acknowledgements
References
- Schutz, H. Benzodiazepines; Springer: Heidelberg, Germany, 1982. [Google Scholar]
- Randall, L. O.; Kamel, B. Benzodiazepines; Garattini, S., Mussini, E., Randall L., O., Eds.; Raven Press: New York, 1973; p. 27. [Google Scholar]
- Merluzzi, V.; Hargrave, K. D.; Labadia, M.; Grozinger, K.; Skoog, M.; Wu, J. C.; Shih, C.-K.; Eckner, K.; Hattox, S.; Adams, J.; Rosenthal, A. S.; Faanes, R.; Eckner, R. J.; Koup, R. A.; Sullivan, J. L. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science 1990, 250, 1411–1413. [Google Scholar]
- Di Braccio, M.; Grossi, G.; Romoa, G.; Vargiu, L.; Mura, M.; Marongiu, M. E. 1,5-Benzodiazepines. Part XII. Synthesis and biological evaluation of tricyclic and tetracyclic 1,5-benzodiazepine derivatives as nevirapine analogues. Eur. J. Med. Chem. 2001, 36, 935–949. [Google Scholar] [CrossRef]
- El-Sayed, A. M.; Khodairy, A.; Salah, H.; Abdel-Ghany, H. Part 7: Synthesis of some new 1,5-benzodiazepines fused with different heterocyclic moieties. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 711–722. [Google Scholar] [CrossRef]
- Nagaraja, G. K.; Vaidya, V. P.; Rai, K. S.; Mahadevan, K. M. An efficient synthesis of 1,5-thiadiazepines and 1,5-benzodiazepines by microwave-assisted heterocyclization. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2797–2806. [Google Scholar]
- Nabih, K.; Baouid, A.; Hasnaoui, A.; Kenz, A. Highly regio- and diastereoselective 1,3-dipolar cycloaddition of nitrile oxides to 2,4-dimethyl-3H-1,5-benzodiazepines: Synthesis of bis[1,2,4-oxadiazolo]-[1,5]benzodiazepine derivatives. Synth. Commun. 2004, 34, 3565–3572. [Google Scholar] [CrossRef]
- Reddy, K. V. V.; Rao, P. S.; Ashok, D. Facile synthesis of 2-benzoyl- 6-hydroxy-3-methyl-5- (2-substituted-2,3-dihydro-1H-1,5- benzodiazepin -4-yl) benzo[b]furans. Synth. Commun. 2000, 30, 1825–1836. [Google Scholar] [CrossRef]
- Haris, R. C.; Straley, J. M. Cationic polymethine dyes for acrylic fibers. U.S. Patent 1,537,757, 1968. [Chem. Abstr. 1970, 73, 100054w]. [Google Scholar]
- Claramunt, R. M.; Sanz, D.; Aggarwal, S.; Kumar, A.; Prakash, O.; Singh, S. P.; Elgueroc, J. The reaction of o-phenylenediamine with α,β-unsaturated carbonyl compounds. Arkivoc 2006, 35–45. [Google Scholar]
- Herbert, J. A. L.; Suschitzky, H. Syntheses of heterocyclic compounds. Part XXIX. Substituted 2,3-dihydro-1H-1,5-benzodiazepines. J. Chem. Soc. Perkin Trans. 1 1974, 2657–2661. [Google Scholar] [CrossRef]
- Morales, H. R.; Ulbarela, B. A.; Contreras, R. New synthesis of dihydro- and tetrahydro-1,5-benzodiazepines by reductive condensation of o-phenylenediamine and ketones in the presence of sodium borohydride. Heterocycles 1986, 24, 135–139. [Google Scholar] [CrossRef]
- Jung, D. I.; Choi, T. W.; Kim, Y. Y.; Kim, I. S.; Park, Y. M.; Lee, Y. G.; Jung, D. H. Synthesis of 1,5-benzodiazepine derivatives. Synth. Commun. 1999, 29, 1941–1951. [Google Scholar] [CrossRef]
- Balakrishna, M. S.; Kaboudin, B. A simple and new method for the synthesis of 1,5-benzodiazepine derivatives on a solid surface. Tetrahedron Lett. 2001, 42, 1127–1129. [Google Scholar] [CrossRef]
- Curini, M.; Epifano, F.; Marcotullio, M. C.; Rosati, O. Ytterbium triflate promoted synthesis of 1,5-benzodiazepine derivatives. Tetrahedron Lett. 2001, 42, 3193–3195. [Google Scholar] [CrossRef]
- Kaboudin, B.; Navaee, K. Alumina/phosphorus pentoxide (APP) as an efficient reagent for the synthesis of 1,5-benzodiazepines under microwave irradiation. Heterocycles 2001, 55, 1443–1446. [Google Scholar] [CrossRef]
- Pozarentzi, M.; Stephanatou, J. S.; Tsoleridis, C. A. An efficient method for the synthesis of 1,5-benzodiazepine derivatives under microwave irradiation wihout solvent. Tetrahedron Lett. 2002, 43, 1755–1758. [Google Scholar] [CrossRef]
- Yadav, J. S.; Reddy, B. V. S.; Eshwaraiah, B.; Anuradha, K. Amberlyst-15®: a novel and recyclable reagent for the synthesis of 1,5-benzodiazepines in ionic liquids. Green Chem. 2002, 4, 592–594. [Google Scholar] [CrossRef]
- Jarikote, D. V.; Siddiqui, S. A.; Rajagopal, R.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V. Room temperature ionic liquid promoted synthesis of 1,5-benzodiazepine derivatives under ambient conditions. Tetrahedron Lett. 2003, 44, 1835–1838. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G. S. K. K.; Reddy, K. B.; Reddy, N. M.; Yadav, J. S. A new, efficient and environmentally benign protocol for the synthesis of 1,5-benzodiazepines using cerium (III) chloride/sodium iodide supported on silica gel. Adv. Synth. Catal. 2004, 346, 921–923. [Google Scholar] [CrossRef]
- Yadav, J. S.; Reddy, B. V. S.; Kumar, S. P.; Nagaiah, K. Indium(III) bromide: A novel and efficient reagent for the rapid synthesis of 1,5-benzodiazepines under solvent-free conditions. Synthesis 2005, 480–484. [Google Scholar]
- De, S. K.; Gibbs, R. A. Scandium(III) triflate as an efficient and reusable catalyst for synthesis of 1,5-benzodiazepine derivatives. Tetrahedron Lett. 2005, 46, 1811–1813. [Google Scholar] [CrossRef]
- Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation reactions. J. Mol. Catal. A: Chem. 2005, 237, 93–100. [Google Scholar] [CrossRef]
- Yadav, J. S.; Reddy, B.V.S.; Satheesh, G.; Srinivasulu, G.; Kunwar, A. C. InCI3-catalyzed stereoselective synthesis of optically pure 1,5-benzodiazepines. Arkivoc 2005, (iii), 221–227. [Google Scholar]
- Varala, R.; Ramu, E.; Sreelatha, N.; Adapa, S. R. Ceric ammonium nitrate (CAN) promoted efficient synthesis of 1,5-benzodiazepine derivatives. Synlett 2006, 1009–1014. [Google Scholar]
- Pasha, M. A.; Jayashankara, V. P. Synthesis of 1,5-benzodiazepine derivatives catalyzed by zinc chloride. Heterocycles 2006, 68, 1017–1023. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Lu, J. Molecular iodine catalyzed one-pot synthesis of 1,5-benzodiazepine derivatives under solvent-free conditions. Synlett 2005, 1337–1339. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, P.; Nimesh, S.; Verma, A. K.; Chandra, R. An efficient synthesis of 1,5-benzadiazepine derivatives catalyzed by silver nitrate. Green Chem. 2006, 8, 519–521. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Ren, X.; Li, W.; Shi, Y.; Ouyang, P. Efficient synthesis of 1,5-benzodiazepines mediated by sulfamic acid under neat condition or in solution. Synth. Commun. 2007, 37, 1609–1615. [Google Scholar] [CrossRef]
- An, L.-T.; Ding, F.-Q.; Zou, J.-P.; Lu, X.-H. Montmorillonite K10: An efficient catalyst for solvent-free synthesis of 1,5-benzodiazepine derivatives. Synth. Commun. 2008, 38, 1259–1267. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A. An efficient route to alkyl chlorides from alcohols using the complex TCT/DMF. Org. Lett. 2002, 4, 553–555. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A. Beckmann rearrangement of oximes under very mild conditions. J. Org. Chem. 2002, 67, 6272–6274. [Google Scholar] [CrossRef]
- Kangani, C. O.; Day, B. W. Mild, efficient Friedel−Crafts acylations from carboxylic acids using cyanuric chloride and AlCl3. Org. Lett. 2008, 10, 2645–2648. [Google Scholar] [CrossRef]
- Das, B.; Kumar, R. A.; Thirupathi, P. One-pot three-component synthesis of α-amino nitriles catalyzed by 2,4,6-trichloro-1,3,5-triazine. Helv. Chim. Acta. 2007, 90, 1206–1210. [Google Scholar] [CrossRef]
- Blotny, G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Sharma, G. V. M.; Reddy, J. J.; Lakshmi, P. S.; Krishna, P. R. A versatile and practical synthesis of bis(indolyl)methanes/bis(indolyl)glycoconjugates catalyzed by trichloro-1,3,5-triazine. Tetrahedron Lett. 2004, 45, 7729–7732. [Google Scholar] [CrossRef]
- Bigdeli, M. A.; Mahdavinia, G. H.; Jafari, S.; Hazarkhani, H. Wet 2,4,6-trichloro[1,3,5]triazine (TCT) an efficient catalyst for synthesis of α,α′-bis(substituted-benzylidene) cycloalkanones under solvent-free conditions. Catal. Commun. 2007, 8, 2229–2231. [Google Scholar] [CrossRef]
- Yan, M.-C.; Tu, Z.; Lin, C.; Ko, S.; Hsu, J.; Yao, C.-F. An investigation of the reaction of 2-aminobenzaldehyde derivatives with conjugated nitro-olefins: An easy and efficient synthesis of 3-nitro-1,2-dihydroquinolines and 3-nitroquinolines. J. Org. Chem. 2004, 69, 1565–1570. [Google Scholar] [CrossRef]
- Kuo, C. -W.; More, S.; Yao, C. -F. NBS as an efficient catalyst for the synthesis of 1,5-benzodiazepine derivatives under mild conditions. Tetrahedron Lett. 2006, 47, 8523–8588. [Google Scholar] [CrossRef]
- Lin, C.; Fang, H.; Tu, Z.; Liu, J.-T.; Yao, C.-F. Stereoselective three-component synthesis of trans-endo-decahydroquinolin-4-one derivatives from aldehydes, aniline, and acetylcyclohexene. J. Org. Chem. 2006, 71, 6588–6591. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.-F. Ceric ammonium nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron 2006, 62, 7293–7299. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.-F. Heterogeneous catalyst: Amberlyst-15 catalyzes the synthesis of 14-substituted-14H-dibenzo[a,j]xanthenes under solvent-free conditions. Tetrahedron Lett. 2006, 47, 8827–8829. [Google Scholar] [CrossRef]
- More, S.; Sastry, M. N. V.; Yao, C.-F. Cerium (IV) ammonium nitrate (CAN) as a catalyst in tap water: A simple, proficient and green approach for the synthesis of quinoxalines. Green Chem. 2006, 8, 91–95. [Google Scholar] [CrossRef]
- More, S.; Sastry, M. N. V.; Yao, C.-F. TMSCl-catalyzed aza-Diels-Alder reaction: A simple and efficient synthesis of pyrano and furanoquinolines. Synlett 2006, 1399–1403. [Google Scholar]
- Khodaei, M. M.; Bahrami, K.; Nazarian, Z. TCT as a rapid and efficient catalyst for the synthesis of 1,5-benzodiazepines. Bull. Korean Chem. Soc. 2008, 29, 1280–1282. [Google Scholar] [CrossRef]
- Sample availability: Available from the authors
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-W.; Wang, C.-C.; Kavala, V.; Yao, C.-F. Efficient TCT-catalyzed Synthesis of 1,5-Benzodiazepine Derivatives under Mild Conditions. Molecules 2008, 13, 2313-2325. https://doi.org/10.3390/molecules13092313
Kuo C-W, Wang C-C, Kavala V, Yao C-F. Efficient TCT-catalyzed Synthesis of 1,5-Benzodiazepine Derivatives under Mild Conditions. Molecules. 2008; 13(9):2313-2325. https://doi.org/10.3390/molecules13092313
Chicago/Turabian StyleKuo, Chun-Wei, Chun-Chao Wang, Veerababurao Kavala, and Ching-Fa Yao. 2008. "Efficient TCT-catalyzed Synthesis of 1,5-Benzodiazepine Derivatives under Mild Conditions" Molecules 13, no. 9: 2313-2325. https://doi.org/10.3390/molecules13092313
APA StyleKuo, C.-W., Wang, C.-C., Kavala, V., & Yao, C.-F. (2008). Efficient TCT-catalyzed Synthesis of 1,5-Benzodiazepine Derivatives under Mild Conditions. Molecules, 13(9), 2313-2325. https://doi.org/10.3390/molecules13092313