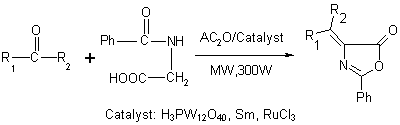Dodecatungstophosphoric Acid (H3PW12O40), Samarium and Ruthenium (ІІІ) Chloride Catalyzed Synthesis of Unsaturated 2-Phenyl-5(4H)-oxazolone Derivatives under Solvent-free Conditions
Abstract
:Introduction
Results and Discussion
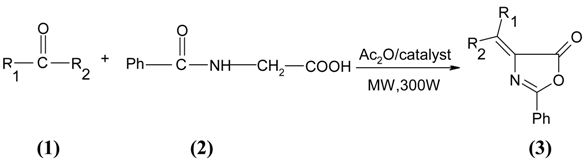
| 1a) Benzaldehyde | 1f) 4-(N,N-Dimethylamino)benzaldehyde |
| 1b) 4-Chlorobenzaldehyde | 1g) 4-Methylbenzaldehyde |
| 1c) 2,4-Dichlorobenzaldehyde | 1h) Furfural |
| 1d) 3-Nitrobenzaldehyde | 1i) Thiophenecarboxaldehyde |
| 1e) 4-Methoxybenzaldehyde | 1j) Cyclohexanone |
| Product | Y(%)(t)a | Y(%)(t)b | Y(%)(t)c | m.p.(◦C) | m.p.(Lit) |
|---|---|---|---|---|---|
| 3a | 87(2) | 80(3) | 92(5) | 160-161 | 158 [13] |
| 3b | 83(2.5) | 73(4) | 87(7) | 189-190 | 185 [14] |
| 3c | 93(3) | 86(5) | 96(8) | 181-182 | 183 [14] |
| 3d | 87(3) | 82(5) | 93(7) | 174-175 | 175-176 [15] |
| 3e | 95(1.5) | 87(2) | 97(4) | 158-159 | 159 [16] |
| 3f | 92(1.5) | 87(2) | 95(4) | 212-213 | 213-214 [15] |
| 3g | 89(2) | 82(3) | 93(5) | 146-147 | 145-146 [18] |
| 3h | 84(2) | 80(3) | 87(5) | 171-172 | 171[16] |
| 3i | 89(2.5) | 84(4) | 93(6) | 178-179 | 180 [16] |
| 3j | 77(2) | 71(3) | 82(4) | 138-139 | 137-138 [15] |
| Product | IR (KBr)/υ [cm-1] | 1H-NMR (CDCl3)/δ[ppm] | MS/m/z (%) |
|---|---|---|---|
| 3a | 1790, 1650 | 7.24 (s, 1H, vinyl); 7.44-8.12 (m, 10H, ArH) | 249 (M+, 15), 105 (100), 77 (70) |
| 3b | 1800,1650 | 7.44-8.12 (m, 10H, vinyl and ArH) | 285 (M+2), 283 (M+, 15), 105 (100), 77 (70) |
| 3c | 1800, 1660 | 7.36-8.08 (m, 9H, vinyl and ArH) | 319 (M+4, 2), 317 (M+2, 8), 318 (M+, 10), 105 (100), 77 (70) |
| 3d | 1800,1660 | 7.42-8.29 (m, 10H, vinyl and ArH) | 294 (M+, 15), 105 (100), 77 (70) |
| 3e | 1790,1660 | 4.02 (s, 3H, CH3); 7.07 (s, 1H, vinyl); 7.25-8.22 (m, 9H, ArH) | 279 (M+, 10), 105 (100), 77 (70) |
| 3f | 1810, 1660 | 3.12 (s, 6H, CH3); 6.86-8.12 (m, 10H, vinyl and ArH) | 292 (M+, 10), 105 (100), 77 (70) |
| 3g | 1800, 1660 | 2.35 (s, 3H, CH3); 7.07 (s, 1H, vinyl); 7.25-8.12 (m, 9H, ArH) | 263 (M+, 10), 105 (100), 77 (70) |
| 3h | 1790, 1660 | 6.66 (q, 1H, 2-furyl);7.17-8.28 (m, 8H, vinyl , furyl and ArH) | 239 (M+, 5), 105 (100), 77 (70) |
| 3i | 1810, 1660 | 6.89 (q, 1H, 2-thienyl);7.37-8.04 (m, 8H, vinyl, thienyl and ArH) | 255 (M+, 5), 105 (100), 77 (70 ) |
| 3j | 1780, 1650 | 1.34-2.13 (m, 10H, cyclohexyl); 7.34-7.88 (m, 5H, ArH) | 241 (M+, 10), 105 (100), 77 (70) |
Experimental
General
General Procedure for Synthesis of 2-phenyl-5(4H)-oxazolone derivatives 3a-j
Acknowledgments
References
- Martinez, A.P.; Lee, W.W.; Goodman, L. Some 2-fluoroethylamines derived from hydrocinnamic acid, phenylpyruvic acid and DL-phenylalanine. Tetrahedron 1964, 20, 2763–2771. [Google Scholar] [CrossRef]
- Gelmi, M.L.; Clerici, F.; Melis, A. 5(4H)-Oxazolone, part X. acid and base effects on the translactonization reaction of 4-(2-oxa-alkylidene)-5(4H)-oxazolones: new synthesis of 5- alkylidene-3-benzoylamino-2(5H)-furanones. Tetrahedron 1997, 53, 1843–1854. [Google Scholar] [CrossRef]
- Aaglawe, M.J.; Dhule, S.S.; Bahekar, S.S.; Wakte, P.S.; Shinde, D.B. Synthesis and antibacterial activity of some oxazolone derivatives. J. Kor. Chem. Soc. 2003, 47, 133–136. [Google Scholar] [CrossRef]
- Cativiela, C.; Fraile, J.M.; Garcia, J. I.; Lopez, M. P.; Mayoral, J.A.; Pires, E. Diels-Alder reaction of (E)-2-phenyl-4-[(s)-2,2-dimethyl-1,3-dioxolan 4-ylmethylen]-5(4H)-oxazolone with heterogeneous catalysts. Tetrahedron Asymmetry 1996, 7, 2391–2394. [Google Scholar]
- Singh, H.; Yadav, L.D.S.; Shukla, K.N.; Dwivedi, R. Ring transformation of Michael adducts of Benzylidene-5-oxazolones and 2-amino-1,3,4-thiadiazoles to antifungal 6,7-dihydro-5(H)- thiadiazolo[3,2- a] pyrimidin-5-ones. J. Agric. Food Chem. 1990, 38, 1962–1964. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Rahat, S.; Khan, M.K.; Choudhary, Z.M.; Murad, S.; Ismail, Z.; Rahman, A. Synthesis and immunomodulatory properties of selected oxazolone derivatives. Bioorg. Med. Chem. 2004, 12, 2049–2057. [Google Scholar] [CrossRef]
- Khan, K.M.; Mughal, U.R.; Khan, M.T.H.; Ullah, Z.; Perveen, S.; Choudhary, M.I. Oxazolones: new tyrosinase inhibitors; synthesis and their structure–activity relationships. Bioorg. Med. Chem. 2006, 14, 6027–6033. [Google Scholar] [CrossRef]
- Koczan, G.; Csik, G.; Csampai, A.; Balog, E.; Bosze, S.; Sohar, P.; Hudecz, F. Synthesis and characterization of 4-ethoxymethylene-2-[1]-naphthyl-5(4H)-oxazolone and its fluorescent amino acid derivatives. Tetrahedron 2001, 57, 4589–4598. [Google Scholar] [CrossRef]
- Avenoza, A.; Busto, J.H.; Cativiela, C.; Peregrina, J.M. Synthesis of conformationally constrained hydroxyl-α-amino acids by intramolecular conjucate addition. Aminoacids 2000, 18, 117–137. [Google Scholar]
- Krasnov, V.P.; Zhdanova, E.A.; Solieva, N.Z.; Sadretdinova, L.Sh.; Bukrina, I.M.; Demin, A.M.; Levit, G.L.; Ezhikova, M.A.; Kodess, M.I. Study of the effect of the nature of the side chain in esters of α-amino acids on the diastereoselectivity of condensation with 5(4H)-oxazolone in the synthesis of dipeptides with N-terminal N-acetylphenylalanine. Russ. Chem. Bull., Int. Ed. 2004, 53, 1331–1334. [Google Scholar] [CrossRef]
- Yadav, L.D.S.; Misra, A.R.; Singh, H. Ring transformation of Michael adducts of Benzylidene-5- oxazolones and 3-mercapto-s-triazoles to 2,3-dihydro-4H-s-triazolo[3,4-b][1,3] thiazin-4-ones with some antifungal activity. J. Agric. Food Chem. 1988, 36, 633–636. [Google Scholar] [CrossRef]
- Tripathy, P.K.; Mukerjee, A.K. A fast synthesis of 2-acylamino-2-alkenoic acids. Synthesis 1984, 418–422. [Google Scholar] [CrossRef]
- Crawford, M.; Little, W.T. The Erlenmeyer reaction with aliphatic aldehydes 2-phenyloxazol-5 one being used instead of hippuric acid. J. Chem. Soc. 1959, 729–731. [Google Scholar] [CrossRef]
- Rao, Y.S. A new stereospecific synthesis of the E Isomers of 2-phenyl-4-arylmethylene-2- oxazoline-5-ones. J. Org. Chem. 1976, 722–724. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, H.D.; Mukerjee, A.K. Condensation of 2-substituted 5-oxo-4,5dihydro-1,3- oxazoles with imines and their corresponding carbonyl compounds. Synthesis 1980, 836–839. [Google Scholar]
- Beccalli, E.M.; Clerici, F.; Gelmi, M.L. 5(4H)-Oxazolone. Part XΙΙΙ. A new synthesis of 4-arylidene-5(4H)-oxazolones by the stille reaction. Tetrahedron 1999, 55, 781–786. [Google Scholar] [CrossRef]
- Hamidian, H.; Momeni, A. Novel Synthesis of unsaturated 5(4H)-oxazolone derivatives with using palladium(II) acetate as a catalyst and microwave irradiation in solvent-free condition. Hetrocycl. Commun. 2006, 12, 29–34. [Google Scholar]
- Paul, S.; Nanda, P.; Gupta, R.; Loupy, A. Calcium acetate catalyzed synthesis of 4-arylidene-2- phenyl- 5(4H)-oxazolones under solvent-free conditions. Tetrahedron Lett. 2004, 45, 425–427. [Google Scholar]
- Chandrasekhar, S.; Karri, P. Erlenmeyer azlactone synthesis with aliphatic aldehydes under solvent-free microwave conditions. Tetrahedron Lett. 2007, 48, 785–786. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in organic synthesis. Wiley-VCH: New York, USA, 2006; Volume 1. [Google Scholar]
- Kidwai, M. Dry media reactions. Pure Appl. Chem. 2001, 73, 147–151. [Google Scholar]
- Firouzabadi, H.; Iranpoor, N.; Khoshnood, A. Dodecatungstophosphoric acid (H3PW12O40) as a highly efficient catalyst for the amidation of alcohols and protected alcohols with nitriles in water: a modified Ritter reaction. Catal. Commun. 2008, 9, 529–531. [Google Scholar] [CrossRef]
- Jermy, B.R.; Pandurangan, A. Efficient synthesis of diacetal of pentaerythritol under microwave irradiation using heteropoly acid H3PW12O40. Catal. Commun. 2006, 7, 921–925. [Google Scholar] [CrossRef]
- Davoodnia, A.; Bakavoli, M.; Barakouhi, Gh.; Tavakoli-Hoseini, N. A new route to the synthesis of thieno[2,3-d] pyrimidine-4(3H)-one derivatives catalysed by 12-tungstophosphoric acid (H3PW12O40). Chinese Chem. Lett. 2007, 18, 1483–1486. [Google Scholar] [CrossRef]
- Heravi, M.; Rajabzadeh, G.; Bamoharram, F.; Seifi, N. An eco-friendly catalytic route for synthesis of 4-amino-pyrazolo[3,4-d] pyrimidine derivatives by Keggin heteropolyacids under classical heating and microwave irradiation. J. Mol. Catal. Chem. A. 2006, 256, 238–241. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Neumann, R. Dehydration of alcohols. J. Org. Chem. 2002, 67, 7075–7079. [Google Scholar] [CrossRef]
- Heravi, M.M.; Motamedi, R.; Seifi, N.; Bamoharram, F. catalytic synthesis of 6-aryl-1H- pyrazolo[3,4-d] pyrimidine-4[5H]-ones by heteropolyacid: H14[NaP5W30O110] and H3PW12O40. J. Mol. Catal. Chem. A. 2006, 249, 1–3. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, X.; Zhang, Y.M. A new strategy for the synthesis of α, β-diaroylpropionates promoted by samarium metal in DMF. Tetrahedron Lett. 2003, 44, 1667–1670. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Y.M. Stereo- and regiospecific four-molecule reaction of aroyl chlorides with iso- pentylene: direct formation of (E)-β, γ-unsaturated carbonyl compounds promoted by samarium metal in DMF. Tetrahedron Lett. 2004, 45, 1295–1298. [Google Scholar] [CrossRef]
- Qi, J.Y.; Ji, J.X.; Yueng, C.H.; Kwong, H.L.; Chen, A.S.C. A convenient and highly efficient method for the protection of aldehydes using very low loading hydrous ruthenium trichloride as catalyst. Tetrahedron Lett. 2004, 45, 7719–7721. [Google Scholar] [CrossRef]
- Fabio, F.; Wong, C.H. RuCl3- Promoted amide formation from azides and thioacids. Tetrahedron Lett. 2003, 44, 9083–9085. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tikdari, A.M.; Fozooni, S.; Hamidian, H. Dodecatungstophosphoric Acid (H3PW12O40), Samarium and Ruthenium (ІІІ) Chloride Catalyzed Synthesis of Unsaturated 2-Phenyl-5(4H)-oxazolone Derivatives under Solvent-free Conditions. Molecules 2008, 13, 3246-3252. https://doi.org/10.3390/molecules13123246
Tikdari AM, Fozooni S, Hamidian H. Dodecatungstophosphoric Acid (H3PW12O40), Samarium and Ruthenium (ІІІ) Chloride Catalyzed Synthesis of Unsaturated 2-Phenyl-5(4H)-oxazolone Derivatives under Solvent-free Conditions. Molecules. 2008; 13(12):3246-3252. https://doi.org/10.3390/molecules13123246
Chicago/Turabian StyleTikdari, Ahmad Momeni, Samieh Fozooni, and Hooshang Hamidian. 2008. "Dodecatungstophosphoric Acid (H3PW12O40), Samarium and Ruthenium (ІІІ) Chloride Catalyzed Synthesis of Unsaturated 2-Phenyl-5(4H)-oxazolone Derivatives under Solvent-free Conditions" Molecules 13, no. 12: 3246-3252. https://doi.org/10.3390/molecules13123246
APA StyleTikdari, A. M., Fozooni, S., & Hamidian, H. (2008). Dodecatungstophosphoric Acid (H3PW12O40), Samarium and Ruthenium (ІІІ) Chloride Catalyzed Synthesis of Unsaturated 2-Phenyl-5(4H)-oxazolone Derivatives under Solvent-free Conditions. Molecules, 13(12), 3246-3252. https://doi.org/10.3390/molecules13123246