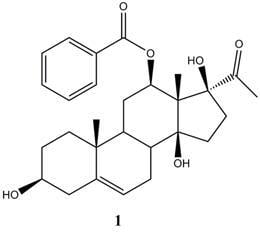
A New Cytotoxic Pregnanone from Calotropis gigantea
Abstract
:Introduction
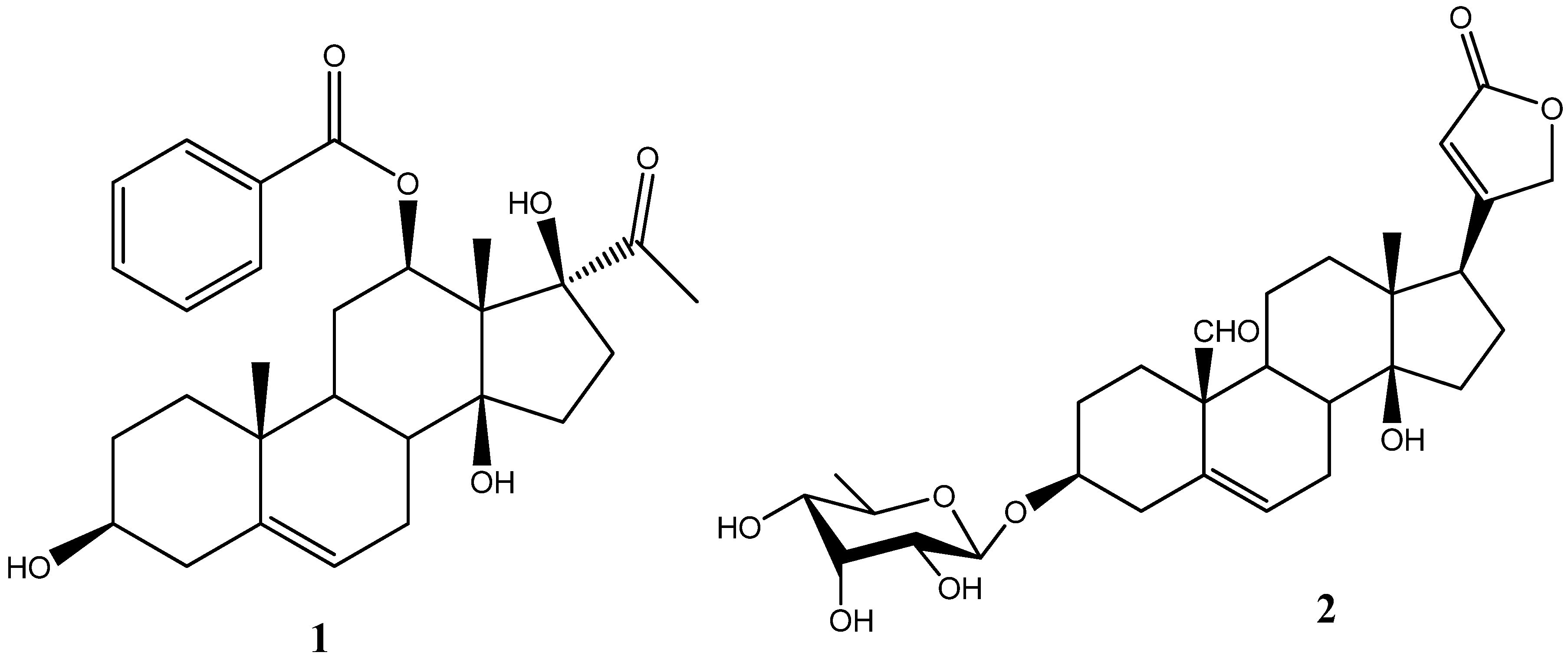
Results and Discussion

| Position | δC | δH | HMBC |
|---|---|---|---|
| 1 | 37.0 | 1.75 (1H, m, H-1a), 1.13 (1H, m, H-1b) | C-2, 3, 5, 10 |
| 2 | 31.4 | 1.81, 1.46 (each 1H, m) | C-3, 4, 10 |
| 3 | 71.4 | 3.53 (1H, m) | C-1, 2, 5 |
| 4 | 41.9 | 2.33 (1H, dd, 12.8, 3.6 Hz), 2.25 (1H, m, overlapped) | C-2, 6, 5, 10 |
| 5 | 139.5 | ||
| 6 | 121.1 | 5.41 (1H, m) | C-4, 5, 7, 10 |
| 7 | 26.0 | 2.20, 1.91 (each 1H, m) | C-5, 6, 9, 14 |
| 8 | 37.0 | 1.80 (1H, m) | C-7, 10, 14 |
| 9 | 42.6 | 1.32 (1H, m) | C-1, 5, 11, 12, 14 |
| 10 | 36.7 | ||
| 11 | 26.5 | 2.06, 1.45 (each 1H, m, overlapped) | C-8, 10, 13 |
| 12 | 73.1 | 4.80 (1H, dd, 11.3, 4.5 Hz) | C-9, 14, 17, 19, 7' |
| 13 | 57.5 | ||
| 14 | 88.5 | ||
| 15 | 31.7 | 2.12 (1H, m, H-15a), 1.92 (1H, m, H-15b) | C-8, 13, 17 |
| 16 | 31.8 | 2.90 (1H, m, H-16a), 1.88 (1H, m, H-16b) | C-13, 14, 20 |
| 17 | 91.2 | ||
| 18 | 7.7 | 1.41 (3H, s) | C-12, 13, 14, 17 |
| 19 | 19.4 | 0.98 (3H, s) | C-1, 5, 9, 10 |
| 20 | 209.3 | ||
| 21 | 27.4 | 2.06 (3H, overlapped) | C-17, 20 |
| 1' | 129.9 | ||
| 2' | 128.4 | 7.93 (1H, d, 7.5 Hz) | C-1', 3', 4', 6' |
| 3' | 129.5 | 7.43 (1H, t, 7.5 Hz) | C-1', 2', 4', 5' |
| 4' | 133.2 | 7.56 (1H, t, 7.5 Hz) | C-2', 3', 5', 6' |
| 5' | 129.5 | 7.43 (1H, t, 7.5 Hz) | C-1', 3', 4', 6' |
| 6' | 128.4 | 7.93 (1H, d, 7.5 Hz) | C-1', 2', 4', 5', 7' |
| 7' | 165.3 |
| Compounds (IC50, μg/mL) | |||
|---|---|---|---|
| 1 | 2 | Mitomycin C* | |
| K562 | 9.2 | 4.7 | 7.1 |
| SGC-7901 | 91.3 | 14.1 | 8.8 |
Conclusions
Experimental
General
Plant material
Extraction and isolation
 −89.7° (c 0.26, MeOH); HR-ESI-MS: m/z [M+Na]+ 491.2409( calcd. For C28H36O6Na, 491.2404); IRν
−89.7° (c 0.26, MeOH); HR-ESI-MS: m/z [M+Na]+ 491.2409( calcd. For C28H36O6Na, 491.2404); IRν  (cm-1): 3431, 2918, 2849, 1712, 1629, 1463, 1275, 1110; UVλmax nm (CHCl3): 241, 267, 284; 1H-NMR (400 MHz, CDCl3), 13C-NMR (100 MHz, CDCl3 ): Table 1.
(cm-1): 3431, 2918, 2849, 1712, 1629, 1463, 1275, 1110; UVλmax nm (CHCl3): 241, 267, 284; 1H-NMR (400 MHz, CDCl3), 13C-NMR (100 MHz, CDCl3 ): Table 1.Cytotoxicity bioassay
Acknowledgements
References and Notes
- Delectis Florae Reipublicae Popularis Sinicae Academiae Sinicae Edita: Flora Reipublicae Popularis Sinicae, Tomus 63; Science Press: Beijing, P.R. China, 1977; pp. 384–386.
- Mueen Ahmed, K.K.; Rana, A.C.; Dixit, V.K. Calotropis species (Ascelpediaceae)- A comprehensive review. Pharm. Mag. 2005, 1, 48–52. [Google Scholar]
- Singh, B.; Rastogi, R.P. Structure of asclepin and some observations on the NMR spectra of Calotropis glycosides. Phytochemistry 1972, 11, 757–762. [Google Scholar] [CrossRef]
- Lhinhatrakool, T.; Sutthivaiyakit, S. 19-Nor- and 18,20-Epoxy-cardenolides from the leaves of Calotropis gigantea. J. Nat. Prod. 2006, 69, 1249–1251. [Google Scholar] [CrossRef]
- Kiuchi, F.; Fukao, Y.; Maruyama, T.; Obata, T. Cytotoxic principles of a Bangladeshi crude drug, Akond Mul (Roots of Calotropis gigantea L.). Chem. Pharm. Bull. 1998, 46, 528–530. [Google Scholar] [CrossRef]
- Sen, S.; Sahu, N.P.; Mahato, S.B. Flavonol glycosides from Calotropis gigantea. Phytochemistry 1992, 31, 2919–2921. [Google Scholar] [CrossRef]
- Anjaneyulu, V.; Ramachandra Row, L. The triterpenes of Calotropis gigatea Linn. Curr. Sci. 1968, 6, 156–157. [Google Scholar]
- Thakur, S.; Das, P.; Itoh, T.; Imai, K.; Matsumoto, T. Latex extractables of Calotropis gigantea. Phytochemistry 1984, 23, 2085–2087. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Gupta, D.K.; Kapil, R.S. Occurrence of D/E trans stereochemistry isoreric to ursane (cis) series in a new pentacyclic triterpene from Calotropis procera. Tetrahedron Lett. 1992, 33, 7593–7596. [Google Scholar] [CrossRef]
- Ali, M.; Gupta, J. New pentacyclic triterpenic esters from the roots of Calotropis procera. Indian J. Chem. 1999, 38B, 877–881. [Google Scholar]
- Kitagawa, I.; Zhang, R.; Park, J.D.; Baek, N.I.; Takeda, Y.; Yoshikawa, M.; Shibuya, H. Indonesian medicinal plants. I. Chemical structures of calotroposides A and B, two new oxypregnane-oligoglycosides from the root of Calotropis gigantea (Asclepiadaceae). Chem. Pharm. Bull. 1992, 40, 2007–2013. [Google Scholar]
- Shibuya, H.; Zhang, R.; Park, J.D.; Baek, N.I.; Takeda, Y.; Yoshikawa, M.; Kitagawa, I. Indonesian medicinal Plants. V. Chemical structures of calotroposides C, D, E, F, and G, five additional new oxypregnane-oligoglycosides from the roots of Calotropis gigantea (Asclepiadaceae). Chem. Pharm. Bull. 1992, 40, 2647–2653. [Google Scholar]
- Pari, K.; Rao, P.J.; Devakumar, C.; Rastogi, J.N. A novel insect antifeedant nonprotein amino acid from Calotropis gigantea. J. Nat. Prod. 1998, 61, 102–104. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Elgamal, M.H.A.; Hanna, A.G.; Morsy, N.A.M.; Duddeck, H.; Simon, A.; Gati, T.; Toth, G. Complete 1H and 13C signal assignments of 5α-cardenolides isolated from Calotropis procera R. BR. J. Mol. Struct. 1999, 477, 201–208. [Google Scholar] [CrossRef]
- Pauli, G.F.; Matthiesen, U.; Fronczek, F.R. Sulfates as novel steroid metabolites in higher plants. Phytochem. 1999, 52, 1075–1084. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the co-author Hao-fu Dai (hfdai@yahoo.cn).
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.-N.; Wang, M.-Y.; Mei, W.-L.; Han, Z.; Dai, H.-F. A New Cytotoxic Pregnanone from Calotropis gigantea. Molecules 2008, 13, 3033-3039. https://doi.org/10.3390/molecules13123033
Wang Z-N, Wang M-Y, Mei W-L, Han Z, Dai H-F. A New Cytotoxic Pregnanone from Calotropis gigantea. Molecules. 2008; 13(12):3033-3039. https://doi.org/10.3390/molecules13123033
Chicago/Turabian StyleWang, Zhu-Nian, Mao-Yuan Wang, Wen-Li Mei, Zhuang Han, and Hao-Fu Dai. 2008. "A New Cytotoxic Pregnanone from Calotropis gigantea" Molecules 13, no. 12: 3033-3039. https://doi.org/10.3390/molecules13123033
APA StyleWang, Z.-N., Wang, M.-Y., Mei, W.-L., Han, Z., & Dai, H.-F. (2008). A New Cytotoxic Pregnanone from Calotropis gigantea. Molecules, 13(12), 3033-3039. https://doi.org/10.3390/molecules13123033