Targeted Synthesis of 1-(4-Hydroxyiminomethylpyridinium)-3-pyridiniumpropane Dibromide – A New Nerve Agent Reactivator
Abstract
:Introduction
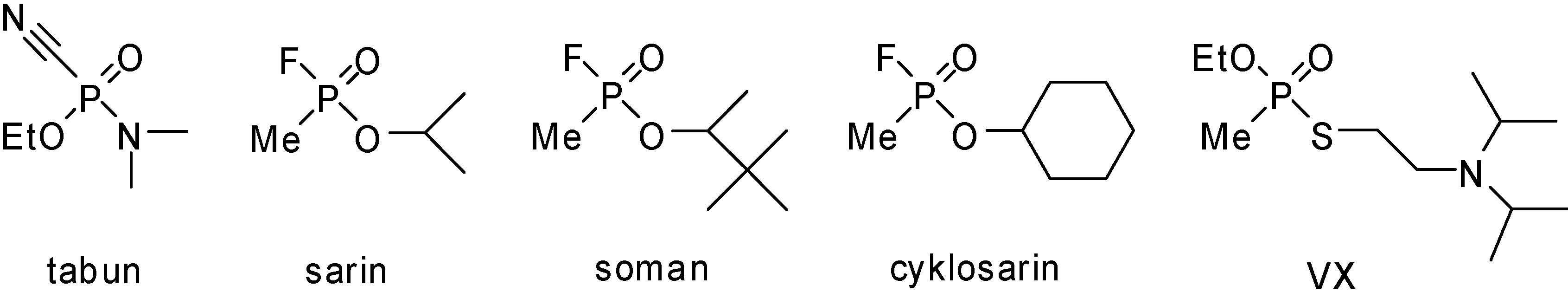
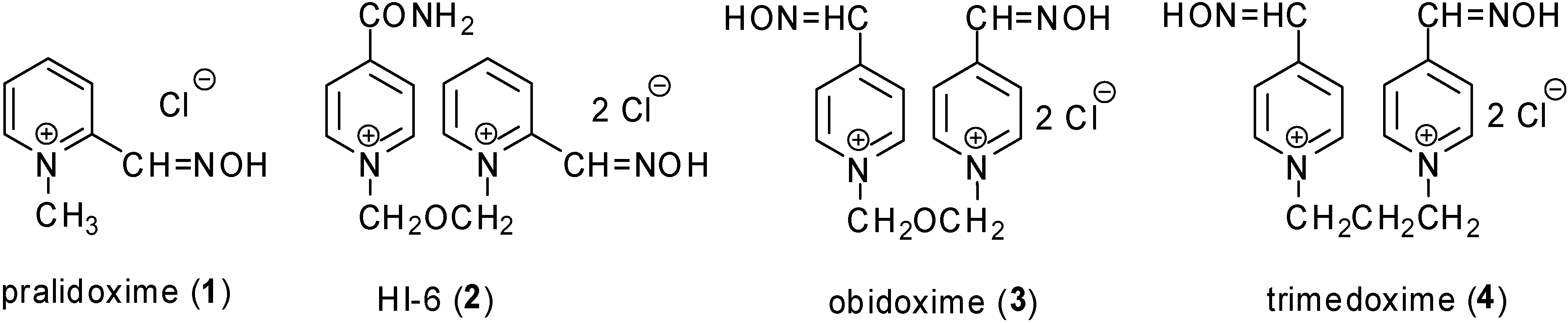
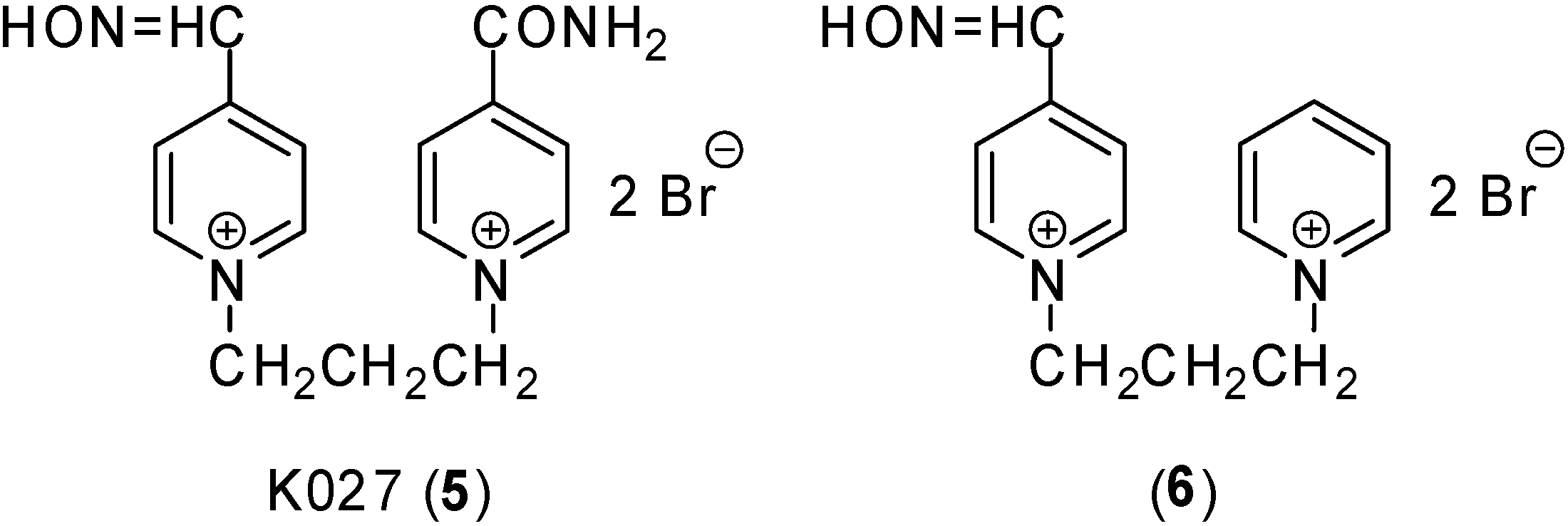
Results and Discussion
| Nerve agent | ||||
|---|---|---|---|---|
| tabun | cyclosarin | |||
| Oxime | Reactivation potency [%] ± SD | Reactivation potency [%] ± SD | ||
| 10-5 M | 10-3 M | 10-5 M | 10-3 M | |
| pralidoxime (1) | 0 | 4 ± 0 | 0 | 4 ± 0 |
| HI-6 (2) | 4 ± 0 | 2 ± 0 | 71 ± 3 | 70 ± 3 |
| obidoxime (3) | 3 ± 0 | 25 ± 1 | 2 ± 0 | 4 ± 0 |
| trimedoxime (4) | 6 ± 0 | 41 ± 2 | 0 | 0 |
| K027 (5) | 1 ± 0 | 11 ± 0 | 0 | 0 |
| 6 | 13 ± 0 | 13 ± 0 | 20 ± 1 | 21 ± 1 |
Conclusions
Experimental
General
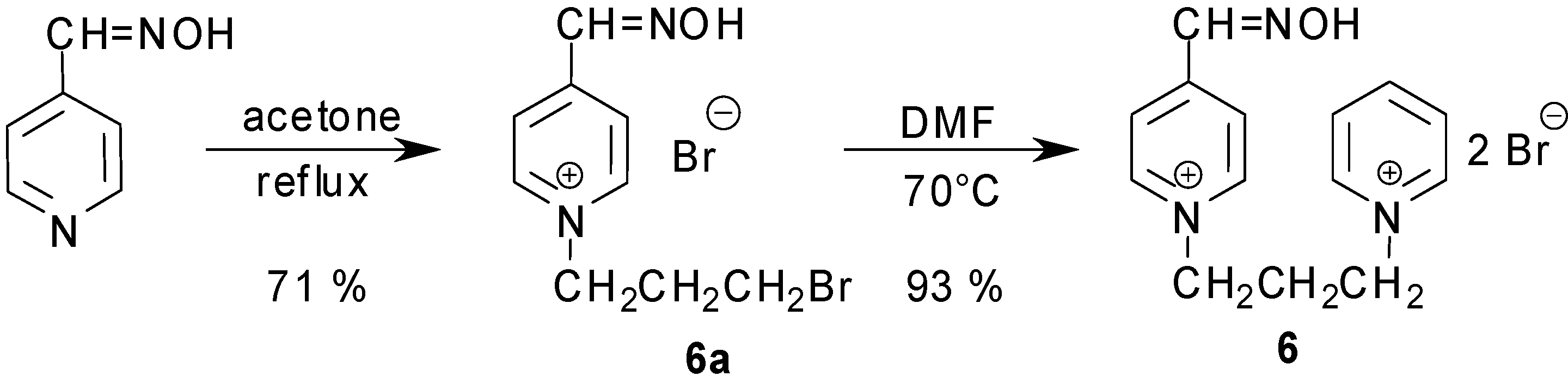
Synthesis
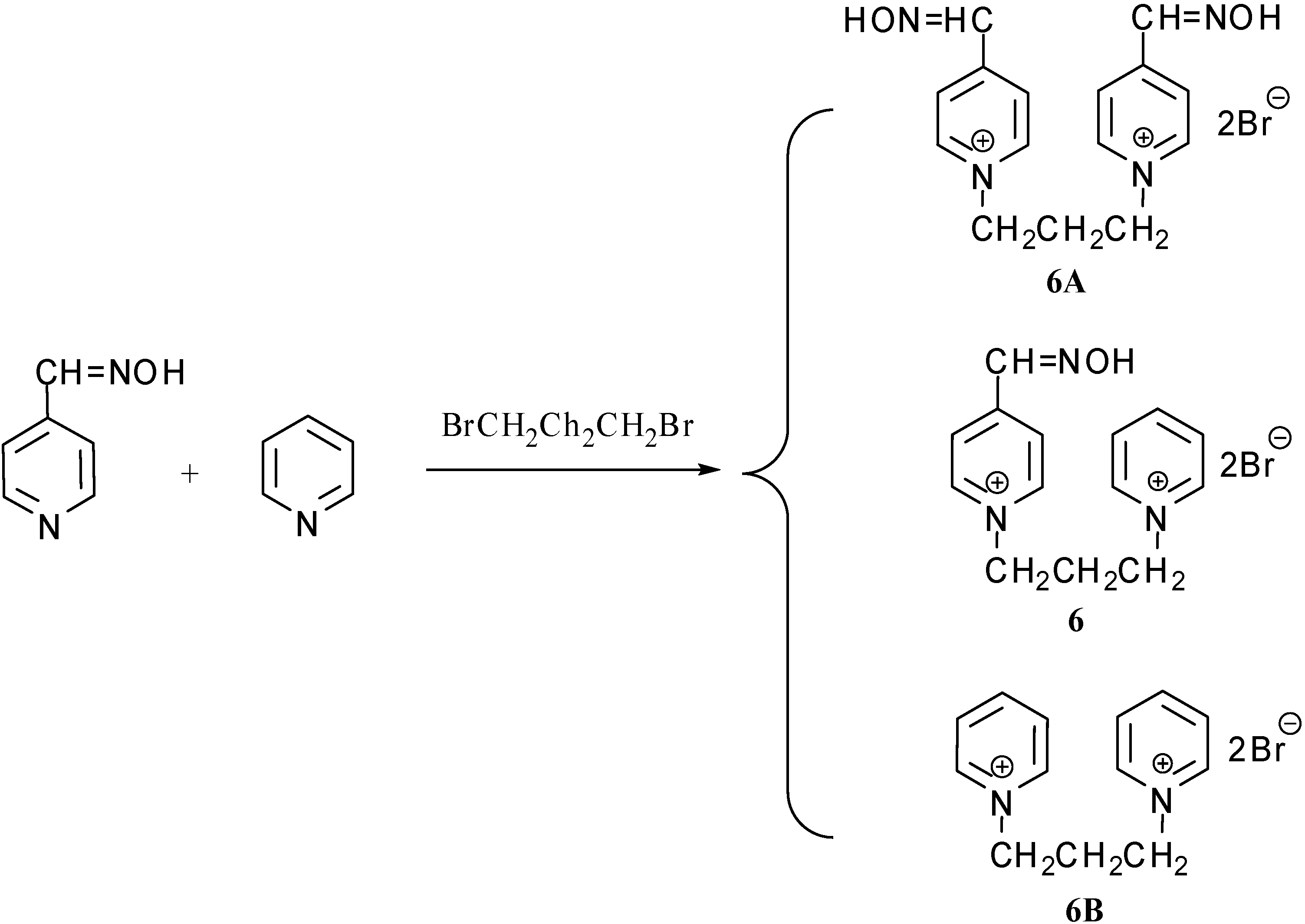
Characterization data
Biological activity
Acknowledgements
References and Notes
- Bajgar, J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar]
- Tu, A.T. Chemical Terrorism: Horrors in Tokyo Subway and Matsumoto City; Alaken Inc.: Fort Collins, CO, 2002. [Google Scholar]
- Matousek, J. Health and environmental threats associated with the destruction of chemical weapons. Ann. N. Y. Acad. Sci. 2006, 1076, 549–558. [Google Scholar]
- Poziomek, E.J.; Hackley, B.E.; Steinberg, G.M. J. Pyridinium aldoximes. J. Org. Chem. 1958, 23, 714–717. [Google Scholar]
- Lüttringhaus, A.; Hagedorn, I. Quaternary hydroxyiminomethylpyridinium salts. The dischloride of bis-(4-hydroxyiminomethyl-1-pyridinium-methyl)-ether (Lueh6), a new reactivator of acetylcholinesterase inhibited by organic phosphoric acid esters. Arzneimittelforschung 1964, 14, 1–5. [Google Scholar]
- Hagedorn, I.; Gündel, W.H.; Schoene, K. Reactivation of phosphorylated acetylcholine esterase with oximes: contribution to the study of the reaction course. Arzneimittelforschung 1969, 19, 603–606. [Google Scholar]
- Krejcova, G.; Kuca, K.; Sevelova, L. Cyclosarin-An Organophosphate Nerve Agent. Def. Sci. J. 2005, 55, 105–115. [Google Scholar]
- Musilek, K.; Kuca, K.; Jun, D.; Dolezal, M. Progress in synthesis of new acetylcholinesterase reactivators in period 1990-2004. Curr. Org. Chem. 2007, 11, 229–238. [Google Scholar]
- Musilek, K.; Lipka, L.; Racakova, V.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. New methods in synthesis of acetylcholinesterase reactivators and evaluation of their potency to reactivate cyclosarin-inhibited AChE. Chem. Pap. 2006, 60, 48–51. [Google Scholar] [CrossRef]
- Eyer, P.; Szinicz, L.; Thiermann, H.; Worek, F.; Zilker, T. Testing of antidotes for organophosphorus compounds: Experimental procedures and clinical reality. Toxicology 2007, 233, 108–119. [Google Scholar]
- Kuca, K.; Cabal, J.; Musilek, K.; Jun, D.; Bajgar, J. Effective bisquaternary reactivators of tabun-inhibited AChE. J. Appl. Toxicol. 2005, 25, 491–495. [Google Scholar]
- Szinicz, L.; Worek, F.; Thiermann, H.; Kehe, K.; Eckert, S.; Eyer, P. Development of antidotes: Problems and strategies. Toxicology 2007, 233, 23–30. [Google Scholar] [PubMed]
- Yang, G.Y.; Yoon, J.H.; Seong, C.M.; Park, N.S.; Jung, Y.S. Synthesis of bis-pyridinium oxime antidotes using bis(methylsulfonoxymethyl) ether for organophosphate nerve agents. Bull. Korean Chem. Soc. 2003, 24, 1368–1370. [Google Scholar] [CrossRef]
- Park, N.-J.; Jung, Y.-S.; Musilek, K.; Jun, D.; Kuca, K. Potency of several structurally different acetylcholinesterase reactivators to reactivate house fly and bovine acetylcholinesterases inhibited by paraoxon and DFP. B. Korean Chem. Soc. 2006, 27, 1401–1404. [Google Scholar] [CrossRef]
- Chennamaneni, S.R.; Vobalaboina, V.; Garlapati, A. Quaternary salts of 4,3' and 4,4' bis-pyridinium monooximes: synthesis and biological activity. Bioorg. Med. Chem. Lett. 2005, 15, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Srinivas Rao, C.; Venkateswarlu, V.; Achaiah, G. Quaternary salts of 4,3' and 4,4' bis-pyridinium monooximes. Part 2: synthesis and biological activity. Bioorg. Med. Chem. Lett. 2006, 16, 2134–2138. [Google Scholar]
- Musilek, K.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of a novel series of bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J. Enzym. Inhib. Med. Chem. 2005, 20, 409–415. [Google Scholar] [CrossRef]
- Musilek, K.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Bioorg. Med. Chem. Lett. 2006, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Holas, O.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of asymmetrical bispyridinium compounds bearing cyano-moiety and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase. Bioorg. Med. Chem. Lett. 2006, 16, 5673–5676. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Holas, O.; Kuca, K.; Jun, D.; Dohnal, V.; Opletalova, V.; Dolezal, M. Novel series of bispyridinium compounds bearing a (Z)-but-2-ene Linker – Synthesis and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase. Bioorg. Med. Chem. Lett. 2007, 17, 3172–3176. [Google Scholar]
- Oh, K.A.; Yang, G.Y.; Jun, D.; Kuca, K.; Jung, Y.S. Bis-pyridiumaldoxime reactivators connected with CH2O(CH2)n OCH2 linkers between pyridinium rings and their reactivity against VX. Biorg. Med. Chem. Lett. 2006, 16, 4852. [Google Scholar] [CrossRef]
- Odzak, R.; Calic, M.; Hrenar, T.; Primozic, I.; Kovarik, Z. Evaluation of monoquaternary pyridinium oximes potency to reactivate tabun-inhibited human acetylcholinesterase. Toxicology 2007, 233, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Bielavsky, J.; Cabal, J.; Bielavska, M. Synthesis of a potential reactivator of acetylcholinesterase-1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett. 2003, 44, 3123–3125. [Google Scholar]
- Kuca, K.; Kassa, J. In vitro reactivation of acetylcholinesterase using the oxime K027. Vet. Hum. Toxicol. 2004, 46, 15–18. [Google Scholar]
- Calic, M.; Lucic Vrdoljak, A.; Radic, B.; Jelic, D.; Jun, D.; Kuca, K.; Kovarik, Z. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology 2006, 219, 85–96. [Google Scholar]
- Lucic-Vrdoljak, A.; Calic, M.; Radic, B.; Berend, S.; Kuca, K.; Kovarik, Z. Pretreatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology 2006, 228, 41–50. [Google Scholar]
- Kassa, J.; Kuca, K.; Cabal, J.; Paar, M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vivo methods. J. Toxicol. Environ. Health A 2006, 69, 1875–1882. [Google Scholar]
- Kuca, K.; Cabal, J.; Jun, D.; Bajgar, J.; Hrabinova, M. Potency of new structurally different oximes to reactivate cyclosarin inhibited-human brain acetylcholinesterases. J. Enzyme Inhib. Med. Chem. 2006, 21, 6636–666. [Google Scholar] [CrossRef]
- Musilek, K.; Kuca, K.; Jun, D.; Dolezal, M. Synthesis of bispyridinium compounds bearing propane linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. Lett. Org. Chem. 2006, 3, 831–835. [Google Scholar]
- Kuca, K.; Cabal, J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin nerve agent. Toxicol. Mech. Meth. 2005, 15, 247–252. [Google Scholar]
- Bajgar, J.; Fusek, J.; Kuca, K.; Bartosova, L.; Jun, D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini-Rev. Med. Chem. 2007, 7, 461–466. [Google Scholar]
- Kuca, K.; Jun, D.; Musilek, K. Structural requirements of acetylcholinesterase reactivators. Mini-Rev. Med. Chem. 2006, 6, 269–277. [Google Scholar]
- Kuca, K.; Jun, D.; Musilek, K.; Bajgar, J. Reactivators of tabun-Inhibited tcetylcholinesterase: Structure biological activity relationship. Front. Drug Des. Discov. 2007, 3, 381–394. [Google Scholar]
- Ekstrom, F.; Akfur, C.; Tunemalm, A.K.; Lundberg, S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006, 45, 74–81. [Google Scholar]
- Ekstrom, F.; Pang, Y.P.; Boman, M.; Artursson, E.; Akfur, C.; Borjegren, S. Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol. 2006, 72, 597–607. [Google Scholar] [PubMed]
- Sample Availability: Samples of compounds 1-6 are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kuca, K.; Musilek, K.; Paar, M.; Jun, D.; Stodulka, P.; Hrabinova, M.; Marek, J. Targeted Synthesis of 1-(4-Hydroxyiminomethylpyridinium)-3-pyridiniumpropane Dibromide – A New Nerve Agent Reactivator. Molecules 2007, 12, 1964-1972. https://doi.org/10.3390/12081964
Kuca K, Musilek K, Paar M, Jun D, Stodulka P, Hrabinova M, Marek J. Targeted Synthesis of 1-(4-Hydroxyiminomethylpyridinium)-3-pyridiniumpropane Dibromide – A New Nerve Agent Reactivator. Molecules. 2007; 12(8):1964-1972. https://doi.org/10.3390/12081964
Chicago/Turabian StyleKuca, Kamil, Kamil Musilek, Martin Paar, Daniel Jun, Petr Stodulka, Martina Hrabinova, and Jan Marek. 2007. "Targeted Synthesis of 1-(4-Hydroxyiminomethylpyridinium)-3-pyridiniumpropane Dibromide – A New Nerve Agent Reactivator" Molecules 12, no. 8: 1964-1972. https://doi.org/10.3390/12081964
APA StyleKuca, K., Musilek, K., Paar, M., Jun, D., Stodulka, P., Hrabinova, M., & Marek, J. (2007). Targeted Synthesis of 1-(4-Hydroxyiminomethylpyridinium)-3-pyridiniumpropane Dibromide – A New Nerve Agent Reactivator. Molecules, 12(8), 1964-1972. https://doi.org/10.3390/12081964




