Antibacterial Thymol Derivatives Isolated from Centipeda minima
Abstract
:Introduction
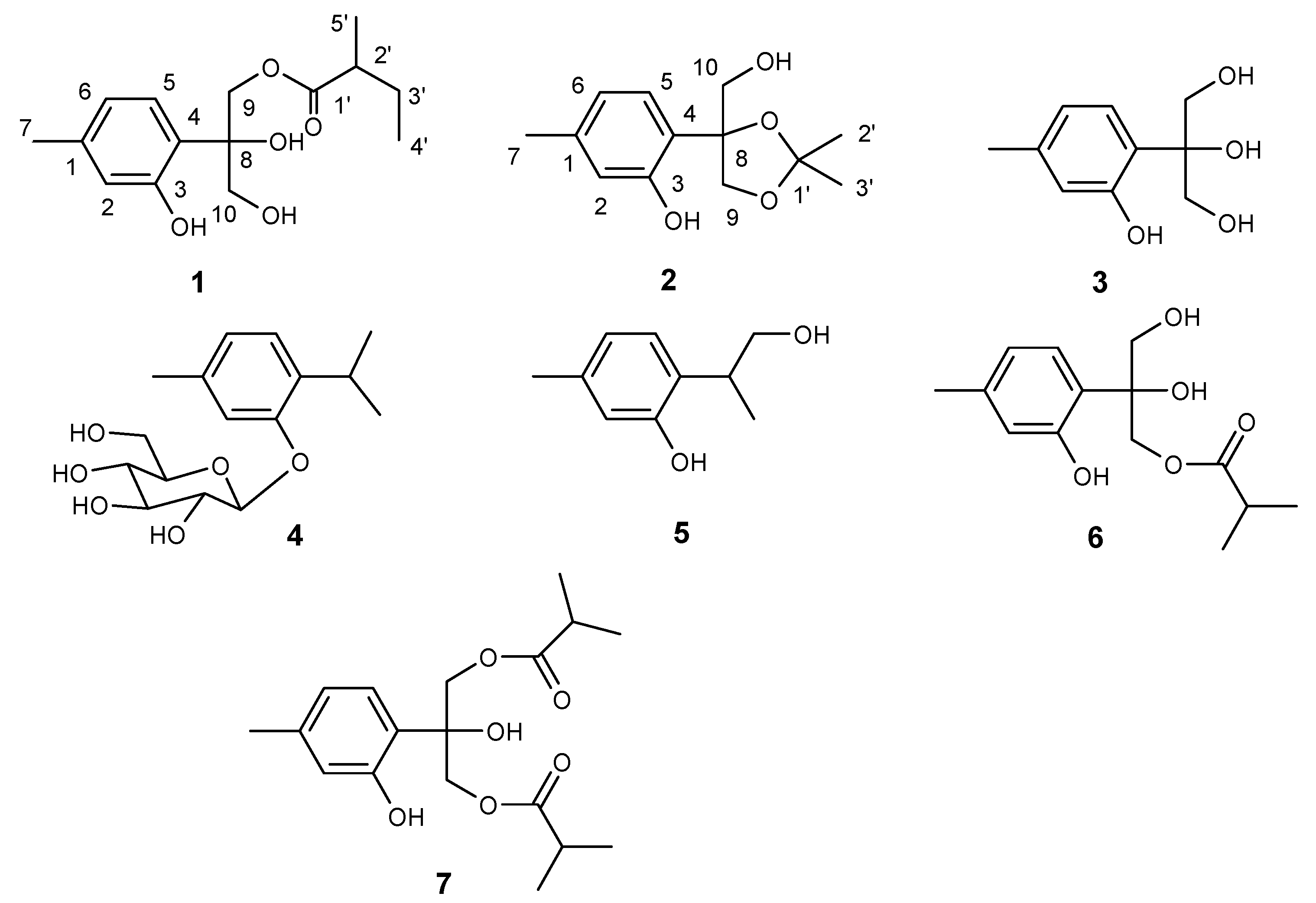
Results and Discussion
Experimental
General
Plant Material
Extraction and Isolation
| position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J=Hz) | δC | δH (J=Hz) | δC | |
| 1 | 139.9 | 139.5 | ||
| 2 | 6.72 br. s | 118.5 | 6.57 br. s | 117.1 |
| 3 | 156.4 | 154.6 | ||
| 4 | 119.6 | 127.3 | ||
| 5 | 6.94 d (7.9) | 126.3 | 7.32 d (7.8) | 128.5 |
| 6 | 6.67 br. d (7.9) | 120.5 | 6.62 br. d (7.8) | 120.9 |
| 7 | 2.29 s | 20.9 | 2.23 s | 21.1 |
| 8 | 78.9 | 86.5 | ||
| 9a9b | 4.54 d (12.0)4.46 d (12.0) | 67.3 | 4.40 d (9.0)4.16 d (9.0) | 72.3 |
| 10a10b | 3.90 d (12.0)3.81 d (12.0) | 65.9 | 3.73 br. d (11.5)3.60 br. d (11.5) | 67.3 |
| 1’ | 177.9 | 110.6 | ||
| 2’ | 2.40 m | 41.0 | 1.27 s | 25.9 |
| 3’a3’b | 1.62 m1.51 m | 26.6 | 1.52 s | 27.2 |
| 4’ | 0.84 m | 11.4 | ||
| 5’ | 1.11 d (7.7) | 16.4 | ||
Antibacterial Assay
| Pathogen | 1 | 2 | 3 | 4 | 5 | 6 | 7 | standards b | |
|---|---|---|---|---|---|---|---|---|---|
| Reference strains | |||||||||
| Staphylococcus aureus CMCC26001 | >100 | 12.5 | >100 | >100 | >100 | 100 | 6.25 | 15 | 7.5 |
| Escherichia coli CMCC44103 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 7.5 | 7.5 |
| Salmonella typhimurium CMCC80087 | >100 | >100 | 6.25 | 25 | 50 | 25 | >100 | 7.5 | 7.5 |
| Shigella flexneri CMCC51335 | >100 | 12.5 | >100 | >100 | >100 | >100 | 6.25 | 3.25 | 3.25 |
| Clinically isolated strains | |||||||||
| Staphylococcus epidermidis | >100 | 25 | >100 | 50 | >100 | 100 | 50 | 3.25 | 7.5 |
| Bacillus subtilis | >100 | 6.25 | >100 | >100 | >100 | 50 | >100 | 3.25 | 3.25 |
| Salmonella paratyphi-A | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 3.25 | 3.25 |
| Salmonella paratyphi-B | >100 | >100 | >100 | >100 | >100 | >100 | 6.25 | 3.25 | 3.25 |
Acknowledgements
References and Notes
- Tauxe, R. V. Emerging foodborne diseases: An evolving public health challenge. Dairy Food Environm. Sanitation 1990, 17, 788–795. [Google Scholar]
- Beović, B. The issue of antimicrobial resistance in human medicine. Int. J. Food Microbiol. 2006, 112, 280–287. [Google Scholar]
- Smid, E. J.; Gorris, L. G. M. Natural antimicrobials for food preservation. In Handbook of Food Preservation; Rahman, M. S., Ed.; New York: Marcel Dekker, 1999; pp. 285–308. [Google Scholar]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Reische, D. W.; Lillard, D. A.; Eintenmiller, R. R. Antioxidants in food lipids. In Chemistry, Nutrition and Biotechnology; Ahoh, C. C., Min, D. B., Eds.; New York: Marcel Dekker, 1998; pp. 423–448. [Google Scholar]
- Shi, Z.; Fu, G. X. Flora of China; Science Publishing House: Beijing, People’s Republic of China, 1983; Vol. 76, no. 1, pp. 132–133. [Google Scholar]
- Jiangsu New Medical College. Zhongyao Dacidian (Dictionary of Traditional Chinese Medicine); Shanghai Science and Technology Publishing House: Shanghai, People’s Republic of China, 1992. [Google Scholar]
- Wu, J. B.; Chun, T. T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Isolation of a new plenolin ester and the anti-allergy activity of sesquiterpene lactones. Chem. Pharm. Bull. 1985, 33, 4091–4094. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. B.; Chun, T. T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991, 39, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Wu, J. B.; Ebizuka, Y.; Sankawa, U. Platelet activating factor (PAF) antagonists contained in medicinal plants: Lignans and sesquiterpenes. Chem. Pharm. Bull. 1992, 40, 1196–1198. [Google Scholar]
- Taylor, R. S. L.; Towers, G. H. N. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Grenz, M.; Jakupovic, J.; King, R. M.; Robinson, H. Four heliangolides and other sesquiterpenes from Brasilia sickii. Phytochemistry 1983, 22, 1213–1218. [Google Scholar] [CrossRef]
- Ahmed, A. A.; Jakupovic, J. Sesqui- and monoterpenes from Jasonia montana. Phytochemistry 1990, 29, 3658–3661. [Google Scholar] [CrossRef]
- Passreiter, C. M.; Matthiesen, U.; Willuhn, G. 10-Acetoxy-9-chloro-8,9-dehydrothymol and further thymol derivatives from Arnica sachalinensis. Phytochemistry 1998, 49, 777–781. [Google Scholar] [CrossRef]
- Gonzalez, A. G.; Barrera, J. B.; Rosas, F. E.; Hernandez, A. Y.; Espineira, J.; Joseph-Nathan, P. Thymol derivatives from Schizogyne glaberrima. Phytochemistry 1986, 25, 2889–2891. [Google Scholar] [CrossRef]
- Gonzalez, A. G.; Bermejo, J. Sesquiterpene lactones and other constituents from Allagopappus species. J. Nat. Prod. 1995, 3, 432–437. [Google Scholar] [CrossRef]
- Uchiyama, T.; Miyase, T.; Ueno, A.; Usmanghani, K. Terpene and lignan glycosides from Pluchea indica. Phytochemistry 1991, 2, 655–657. [Google Scholar] [CrossRef]
- Shi, Y. P.; Guo, W.; Yang, C.; Jia, Z. J. Two new aromatic monoterpene derivatives from Carpesium lipskyi. Planta Med. 1998, 64, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Tori, M.; Ohara, Y.; Nakashima, K.; Sono, M. Thymol dervivatives from Eupatorium fortunei. J. Nat. Prod. 2001, 64, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Marco, J. A.; Sanz-Cervera, J. F.; Manglano, E. Chlorinated thymol derivatives from Inula Crithmoides. Phytochemistry 1993, 4, 875–878. [Google Scholar] [CrossRef]
- Yoshida, T.; Mori, K.; He, G. X. Inulavosin, a new thymol dimmer with piscicidal activity from Inula nervosa. Heterocycles 1995, 41, 1923–1926. [Google Scholar] [CrossRef]
- Baker, C. N.; Banerjee, S. N.; Tenover, F. C. Evolution of Alamar colorimetric MIC method for antimicrobial susceptibility testing of Gram-negative bacteria. J. Clinic Microbiol. 1994, 32, 1261–1267. [Google Scholar]
- Sample Availability: Samples of compounds 4 and 6 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial Thymol Derivatives Isolated from Centipeda minima. Molecules 2007, 12, 1606-1613. https://doi.org/10.3390/12081606
Liang H, Bao F, Dong X, Tan R, Zhang C, Lu Q, Cheng Y. Antibacterial Thymol Derivatives Isolated from Centipeda minima. Molecules. 2007; 12(8):1606-1613. https://doi.org/10.3390/12081606
Chicago/Turabian StyleLiang, Hengxing, Fukai Bao, Xiaoping Dong, Rui Tan, Caijun Zhang, Qing Lu, and Yongxian Cheng. 2007. "Antibacterial Thymol Derivatives Isolated from Centipeda minima" Molecules 12, no. 8: 1606-1613. https://doi.org/10.3390/12081606
APA StyleLiang, H., Bao, F., Dong, X., Tan, R., Zhang, C., Lu, Q., & Cheng, Y. (2007). Antibacterial Thymol Derivatives Isolated from Centipeda minima. Molecules, 12(8), 1606-1613. https://doi.org/10.3390/12081606




