Simple and Regioselective Bromination of 5,6-Disubstituted-indan-1-ones with Br2 Under Acidic and Basic Conditions
Abstract
:Introduction
Results and Discussion
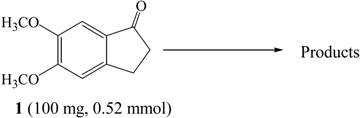 | |||
|---|---|---|---|
| Entry | Reaction conditions | Products (Yield) b | |
| I | Br2a (0.52 mmol)/CCl4/rt/2hrs |  | 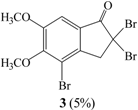 |
| III | Br2 (0.52 mmol)/CCl4/ice bath/2 hrs |  |  |
| II | Br2 (0.52 mmol)/CHCl3/rt/2 hrs | 2 (22%) | 3 (2%) |
| IV | Br2 (0.52 mmol)/CHCl3/ice bath/2 hrs | 4 (14%) | 5 (5%) |
| V | Br2 (0.52 mmol)/I2, Fe/CCl4/ice bath/6 hrs | 4 (32%) | |
| VI | Br2 (0.52 mmol)/AlCl3 (1.04 mmol)/CH2Cl2/ice bath/2 hrs |  | 5 (8%) |
| VII | KBr (0.57 mmol)/Oxone® (0.57 mmol)/MeOH/0→5°C/2 hrs | 4 (46%) | |
| VIII | NBS (0.57 mmol)/THF/rt/1 hr | No reaction | |
| IX | NH4Br (0.57 mmol)/H2O2 (0.57 mmol)/AcOH/rt/2 hrs | No reaction | |
| X | Pyridinium bromochromate (0.52 mmol)/glacial AcOH/30-40°C/ 20 min. | No reaction | |
| Entry | Starting materials | Reaction conditions | Products (Yield) b | |
|---|---|---|---|---|
| XI | 1 (100 mg, 0.52 mmol) | Br2a (0.57 mmol)/AcOH/rt/2 hrs | 6 (95%) | |
| XII | Br2 (1.04 mmol)/K2CO3 (1.56 mmol)/CH2Cl2/ rt/1hr | 6 (44%) | 5 (23%) | |
| XIII | Br2 (1.04 mmol)/K2CO3 (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (79%) |  | |
| XIV | Br2 (1.04 mmol)/KOH (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (81%) | 7 (7%) | |
| XV | Br2 (1.04 mmol)/Cs2CO3 (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (67%) | 7 (20%) | |
| XVI | 8 (100 mg, 0.61 mmol) | Br2 a (0.67 mmol)/AcOH/rt/3.5 hrs |  | |
| Br2 (1.22 mmol)/KOH (1.83 mmol)/CH2Cl2/ice bath/0.5 hrs | 9 (55%) | |||
| XVII | 10 (100 mg, 0.76 mol) | Br2 (0.84 mmol)/AcOH/rt/2.5 hrs |  | |
| Br2 (1.52 mmol)/KOH (2.28 mmol)/CH2Cl2/rt/ 20 hrs |  | |||
| XVIII | 13 (100 mg, 0.60 mol) | Br2 (0.66 mmol)/AcOH/rt/1 hr |  | |
| Br2 (1.20 mmol)/KOH (1.80 mmol)/CH2Cl2/rt/ 20 hrs |  | |||
Conclusions
Experimental
General
1. Bromination of 5,6-dimethoxyindan-1-one (1).
1.1. Bromination with bromine/CCl4 at room temperature.
1.2. Bromination with bromine/CCl4 in an ice bath.
1.3. Bromination with bromine/CHCl3 at room temperature.
1.4. Bromination with bromine/CHCl3 in an ice bath.
1.5. Bromination with bromine/I2, Fe in an ice bath.
1.6. Bromination with bromine/AlCl3 in an ice bath.
1.7. Bromination with potassium bromide/oxone℘/MeOH at room temperature.
1.8. Bromination with bromine/AcOH at room temperature.
1.9. Bromination with bromine/K2CO3 at room temperature.
1.10. Bromination with bromine/K2CO3 in an ice bath.
1.11. Bromination with bromine/KOH in an ice bath
1.12. Bromination with bromine/Cs2CO3 / in an ice bath
2. Bromination of 5,6-dihydroxyindan-1-one (8): preparation of the starting material.
2.1. Bromination with bromine/AcOH at room temperature.
2.2. Bromination with bromine/KOH in an ice bath.
3. Bromination of indan-1-one (10)
3.1. Bromination with bromine/AcOH at room temperature
3.2. Bromination with bromine/KOH at room temperature
4. Bromination of 5,6-difluoroindan-1-one (13)
4.1. Bromination with bromine/AcOH at room temperature
4.2. Bromination with bromine/KOH at room temperature
Acknowledgements
References
- Taylor, R. (Ed.) Electrophilic aromatic substitution; John Wiley & Sons: Chichester, 1990.
- Diederich, F.; Stang, P.J. Metal-catalyzed cross-coupling reactions; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Jacquesy, J.; Jouannetaud, M.; Makani, S. meta-Bromination of phenols in superacids. J. Chem. Soc. Commun. 1980, 110–111. [Google Scholar]
- Duan, J.; Zhang, L.H.; Dolbier, W.R., Jr. A convenient new methods for the bromination of deactivated aromatic compounds. Synlett. 1999, 1245–1246. [Google Scholar] [CrossRef]
- Auerbach, J.; Weissman, S.A.; Blacklock, T.J. N-Bromosuccinimide/dibromodimethylhydantoin in aqueous base: A practical method for the bromination of activated benzoic acids. Tetrahedron Lett. 1993, 34, 931–934. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Halogenation of aromatic compounds by N-chloro, N-bromo, and N-iodosuccinimide. Chem. Lett. 2003, 32, 932–933. [Google Scholar] [CrossRef]
- Barhate, N.B.; Gajare, A.S.; Wakharkar, R.D.; Bedekar, A.V. Simple and efficient chlorination and bromination of aromatic compounds using aqueous TBHP (or H2O2) and a hydrohalic acid. Tetrahedron Lett. 1998, 39, 6349–6350. [Google Scholar]
- Majetich, G.; Hicks, R.; Reister, S. Electrophilic aromatic bromination using bromodimethyl-sulfonium bromide generated in situ. J. Org. Chem. 1997, 62, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.V.V.K.; Narender, N.; Srinivasu, P.; Kulkarni, S.J.; Ragavan, K.V. Novel bromination method for aniline and anisole using NH4Br/H2O2 in CH3COOH. Synth. Commun. 2004, 34, 2143–2152. [Google Scholar] [CrossRef]
- Gnaim, J.M.; Sheldon, R.A. Regioselective bromination of aromatic compounds with Br2/SO2Cl2 over microporous catalysts. Tetrahedron Lett. 2005, 46, 4465–4468. [Google Scholar] [CrossRef]
- Chiappe, C.; Leandri, E.; Pieraccini, D. Highly efficient bromination of aromatic compounds using 3-methylimidazolium tribromide as reagent/solvent. Chem. Commun. 2004, 2536–2537. [Google Scholar]
- Narender, N.; Sriniva, P.; Ramakrishna, M.; Kilkarmi, S.J.; Reghaven, K.V. An efficient and regioselective oxybromination of aromatic compounds using potassium bromide and Oxone®. Synth. Commun. 2002, 32, 2313–2318. [Google Scholar] [CrossRef]
- Doyle, M.P.; Van Lente, M.A.; Mowat, R.; Fobare, W.F. Alkyl nitrite-metal halide deamination reactions. 7. Synthetic coupling of elctrophilic bromination with substitutive deamination 7. J. Org. Chem. 1980, 45, 2570–2575. [Google Scholar]
- Park, M.Y.; Yang, S.G.; Jadhav, V.; Kim, Y.H. Practical and regioselective brominations of aromatic compounds using tetrabutylammonium peroxydisulfate. Tetrahedron Lett. 2004, 45, 4887–4890. [Google Scholar] [CrossRef]
- Ozgun, B.; Degirmenbasi, N. Quinolium bromochromate − A new reagent for bromination and oxidation. Synth. Commun. 1996, 26, 3601–3601. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A mild and efficient procedure for alpha-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. commun. 2004, 470–471. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, J.Y.; Yoon, S.Y.; Bae, Y.H.; Bae, Y.H.; Lee, S.J. Efficient microwave induced direct α-bromination of carbonyl compounds. Tetrahedron Lett. 2004, 45, 191–193. [Google Scholar] [CrossRef]
- Lee, J.C.; Bae, Y.H.; Chang, S.-K. Efficient α-halogenation of carbonyl compounds by N-bromosuccinimide and N-chlorosuccinimides. Bull. Korea Chem. Soc. 2003, 24, 407–408. [Google Scholar] [CrossRef]
- Bertelsen, S.; Halland, N.; Bachmann, S.; Marigo, M.; Braunton, A.; Jorgensen, K.A. Organocatalytic asymmetric α-bromination of aldehydes and ketones. Chem Commun. 2005, 4821–4823. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chi, D.Y. Nonselective bromination-selective debromination strategy: Selective bromination of unsymmetrical ketones on singly activated carbon against doubly activated carbon. Org. Lett. 2003, 5, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, A.; Provot, O.; Rasolojaona, O.; Alami, M.; Brion, J.-D. N-Methylpyrrolidin-2-one hydrobromide (MPHT) a mild reagent for selective bromination of carbonyl compounds : Synthesis of substituted 2-bromo-1-naphthols. Tetrahedron Lett. 2005, 46, 4187–4191. [Google Scholar] [CrossRef]
- Jung, W.; Ma, E. Synthesis of 2-(5,6-dimethoxy-1-indenyl)ethylamine. Yakhak Hoeji 2003, 47, 1–4. [Google Scholar]
- Sonesson, C.; Barf, T.; Nilsson, J.; Dijkstra, D.; Carlsson, A.; Svensson, K.; Smith, M.W.; Martin, I.J.; Duncan, J.N.; King, L.J.; Wikstrom, H. Synthesis and evaluation of pharmacological and pharmacokinetic properties of monopropyl analogs of 5-, 7-, and 8-[[(trifluoromethyl)-sulfonyl]oxy]-2-aminotetralines: Central dopamine and serotonin receptor activity. J. Med. Chem. 1995, 38, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Ma, E. Synthesis of 2-amino-5,6-difluoroindanּHCl. Yakhak Hoeji 1999, 43, 751–755. [Google Scholar]
- Logan, R.T.; Redpath, J.; Roy, R.G. Indene and naphthalene derivatives. US Pat. 4705782, 1987. [Google Scholar]
- Johnson, W.S.; Shelberg, W.E. A plan for distinguishing between some five- and six-membered ring ketones. J. Am. Chem. Soc. 1945, 67, 1745–1754. [Google Scholar] [CrossRef]
- House, H.O.; McDaniel, W.C. Perhydroindan derivatives. 18. The use of indenone ketals as dienophiles. J. Org. Chem. 1977, 42, 2155–2163. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Choi, T.; Ma, E. Simple and Regioselective Bromination of 5,6-Disubstituted-indan-1-ones with Br2 Under Acidic and Basic Conditions. Molecules 2007, 12, 74-85. https://doi.org/10.3390/12010074
Choi T, Ma E. Simple and Regioselective Bromination of 5,6-Disubstituted-indan-1-ones with Br2 Under Acidic and Basic Conditions. Molecules. 2007; 12(1):74-85. https://doi.org/10.3390/12010074
Chicago/Turabian StyleChoi, Taeyoung, and Eunsook Ma. 2007. "Simple and Regioselective Bromination of 5,6-Disubstituted-indan-1-ones with Br2 Under Acidic and Basic Conditions" Molecules 12, no. 1: 74-85. https://doi.org/10.3390/12010074
APA StyleChoi, T., & Ma, E. (2007). Simple and Regioselective Bromination of 5,6-Disubstituted-indan-1-ones with Br2 Under Acidic and Basic Conditions. Molecules, 12(1), 74-85. https://doi.org/10.3390/12010074




