Isolation and Biological Activity of New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr.
Abstract
:Introduction
Results and Discussion
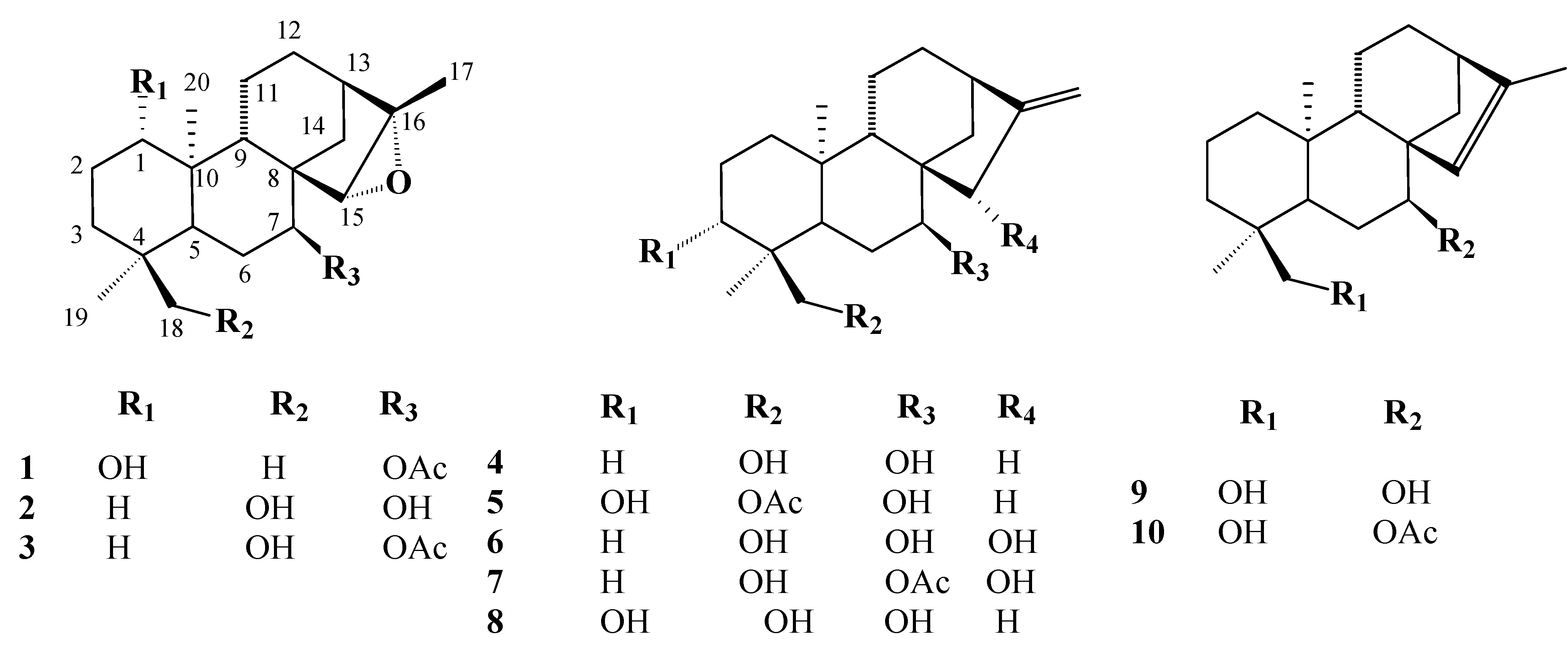
Biological activity
| Tested material | E. coli | S. aureus | K. pneumonia | C. albicans |
|---|---|---|---|---|
| S. stricta extract | 300 | 600 | 300 | NA |
| 1 | NA | 600 | NA | NA |
| 2 | NA | NA | NA | NA |
| 3 | NA | 600 | 600 | NA |
| 4 | 200 | 200 | NA | NA |
| 5 | 600 | 600 | 600 | NA |
| 8 | 600 | 600 | NT | NA |
| 9 | 300 | 600 | 600 | NA |
| 10 | NA | NT | 300 | NA |
| Gentamycin* | 0.97 | 0.48 | 0.48 | NT |
Conclusions
Experimental
General
Plant material
Extraction and isolation
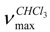 ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) IR
ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) IR 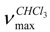 cm-1 : 3400, 1725 and 1270 (C=O), 1050 (C-O); 1H-NMR δ: 4.86 (1H, t, J=2.5, H-7), 3.32 (1H, dd, J=10 and 5 Hz, H-1), 2.97 (1H, s, H-15), 2.09 (3H,s, OAc), 2.98 (1H, s, H-15), 2.08 (3H, s, OAc),1.44 (3H, s, Me-17), 1.08 (3H, s, Me-20), 0.78 (3H,s, Me-18) 0.82 (3H,s, Me-19); 13C-NMR δ: 80.3 (C-1), 29.2 (C-2), 34.8 (C-3), 37.6 (C-4), 34.8 (C-5), 26.3 (C-6), 74.1 (C-7), 48.3 (C-8), 45.9 (C-9), 38.9 (C-10), 17.3 (C-11), 27.4 (C-12), 39.3 (C-13), 31.2 (C-14), 62.4 (C-15), 77.9 (C-16), 17.9 (C-17), 18.6 (C-18), 17.5 (C-19), 15.3 (C-20), 21.1 (OCOCH3), 178.6 (OCO-CH3); EIMS (rel.int.) m/z: 362.2 [M]+ (10), 344 [M-OH]+ (28) 302.2 [M-COOCH3]+(23), 288.2 (45), 254.1 (85), 225.1 (30), 201.1 (50), 131.0 (60), 120.0 (50), 108.9 (82), 95.1 (65), 80.0 (23), 69.0 (97); HRMS: 362.2560 (calcd for C22H34O4 362.2457).
cm-1 : 3400, 1725 and 1270 (C=O), 1050 (C-O); 1H-NMR δ: 4.86 (1H, t, J=2.5, H-7), 3.32 (1H, dd, J=10 and 5 Hz, H-1), 2.97 (1H, s, H-15), 2.09 (3H,s, OAc), 2.98 (1H, s, H-15), 2.08 (3H, s, OAc),1.44 (3H, s, Me-17), 1.08 (3H, s, Me-20), 0.78 (3H,s, Me-18) 0.82 (3H,s, Me-19); 13C-NMR δ: 80.3 (C-1), 29.2 (C-2), 34.8 (C-3), 37.6 (C-4), 34.8 (C-5), 26.3 (C-6), 74.1 (C-7), 48.3 (C-8), 45.9 (C-9), 38.9 (C-10), 17.3 (C-11), 27.4 (C-12), 39.3 (C-13), 31.2 (C-14), 62.4 (C-15), 77.9 (C-16), 17.9 (C-17), 18.6 (C-18), 17.5 (C-19), 15.3 (C-20), 21.1 (OCOCH3), 178.6 (OCO-CH3); EIMS (rel.int.) m/z: 362.2 [M]+ (10), 344 [M-OH]+ (28) 302.2 [M-COOCH3]+(23), 288.2 (45), 254.1 (85), 225.1 (30), 201.1 (50), 131.0 (60), 120.0 (50), 108.9 (82), 95.1 (65), 80.0 (23), 69.0 (97); HRMS: 362.2560 (calcd for C22H34O4 362.2457).Antibacterial and antifungal activity
Acknowledgements
References
- Mill, M.R. Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; University Press: Edinburgh, 1982; Vol.7, pp. 192–193. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present), 2; Nobel Tıp Kitabevleri: Istanbul, 1984. [Google Scholar]
- Yeşilada, E.; Ezer, N. Essential oil composition of four Turkish species of Sideritis. Phytochemistry 1996, 41, 203–205. [Google Scholar] [CrossRef]
- Kilic, T.; Yildiz, Y.K.; Topçu, G.; Gören, A.C.; Ay, M.; Bodige, S.; Watson, W.H. X-ray analysis of sideroxol from Sideritis leptoclada. J. Chem. Cryst. 2005, 35, 647–650. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A.; Piozzi, F. Diterpenes from Sideritis theezans. Phytochemistry 1975, 14, 1451–1452. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Fraga, B.M.; Hernandez, M.G.; Hanson, J.R. The 13C-NMR Spectra of Some ent-18-hydroxykaur-16-enes. Phytochemistry 1981, 20, 846–847. [Google Scholar] [CrossRef]
- Aljancic, I.; Macura, S.; Juranic, S.; Andjelkovic, N.; Randjelovic, N.; Milosavljevic, S. Diterpenes from Achillea clypeolata. Phytochemistry 1996, 43, 169–172. [Google Scholar]
- Başer, K.H.C.; Bondi, M.L.; Bruno, M.; Kırımer, N.; Piozzi, F.; Tümen, G.; Vasollo, N. An ent-kaurene from Sideritis Huber-Morathi. Phytochemistry 1996, 43, 1293–1296. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernandez, M.G.; Fernandez, C.; Arteaga, J.M. Diterpenes from Sideritis dendrochahorra and S. cystosiphon. Phytochemistry 1987, 26, 775–777. [Google Scholar] [CrossRef]
- Topçu, G.; Gören, A.C.; Kılıç, T.; Yıldız, Y.K.; Tümen, G. Diterpenes from Sideritis sipylea and S. dichotoma. Turk. J. Chem. 2002, 26, 189–194. [Google Scholar]
- Venturella, P.; Bellino, A. Eubotriol and Eupol, New Diterpenoids from Sideritis euboea. Experientia 1977, 33, 1270–1271. [Google Scholar] [CrossRef]
- Cabrera, E.; Garcia-Granados, A.; Buruaga, A.S.D.; Buruaga, J.M.S. Diterpenoids from Sideritis hirsuta ssp. Nivalis. Phytochemistry 1983, 22, 2779–2781. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernandez, M.G.; Santana, J.M.H.; Artega, J.M. Diterpenes from Sideritis ferrensis. Phytochemistry 1991, 30, 913–915. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A.; Marino, M.L. New Diterpenes from Sideritis sicula. Phytochemistry 1978, 17, 811–812. [Google Scholar]
- Queseda, T.G.D.; Rodrigez, B.; Valverde, S. Diterpenenes from Sideritis lagascana and sideritis valverd. Phytochemistry 1974, 13, 2008–2009. [Google Scholar]
- Fraga, B.M.; Hernandez, M.G.; Diaz, C.E. On the ent kaurene diterpenes from Sideritis athoa. Nat. Prod. Res. 2003, 17, 141–144. [Google Scholar] [CrossRef]
- Topçu, G.; Gören, A.C.; Kılıç, T.; Yıldız, Y.K.; Tümen, G. Diterpenes from Sideritis trojana. Nat. Prod. Lett. 2002, 16, 33–37. [Google Scholar] [CrossRef]
- Goren, A.C.; Topçu, G.; Bilsel, G.; Bilsel, M.; Wilkinson, J.M.; Cavanagh, H.M. Analysis of essential oil of Satureja thymbra by hydrodistillation, thermal desorber and headspace GC/MS techniques and its antimicrobial activity. Nat. Prod. Res. 2004, 18, 189–195. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Standard methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; NCCLS Approved Standard M7-A2; Villanova, PA, 1990; Vol. 10, No. 8. [Google Scholar]
- Goren, A. C.; Bilsel, G.; Bilsel, M.; Demir, H.; Kocabas, E.E. Analysis of Essential Oil of Coridothymus capitatus (L.) and Its Antibacterial and Antifungal Activity. Z. Naturforsch C 2003, 58, 687–690. [Google Scholar]
- Sample Availability: Available from the author.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kilic, T. Isolation and Biological Activity of New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr. Molecules 2006, 11, 257-262. https://doi.org/10.3390/11040257
Kilic T. Isolation and Biological Activity of New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr. Molecules. 2006; 11(4):257-262. https://doi.org/10.3390/11040257
Chicago/Turabian StyleKilic, Turgut. 2006. "Isolation and Biological Activity of New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr." Molecules 11, no. 4: 257-262. https://doi.org/10.3390/11040257
APA StyleKilic, T. (2006). Isolation and Biological Activity of New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr. Molecules, 11(4), 257-262. https://doi.org/10.3390/11040257




