Experimental
General
1H- and 13C-NMR spectra were obtained using a NMR Bruker Avance spectrometer and were recorded at 300 and 75.5 MHz respectively. Mass spectra (EI, 70 eV) were determined on a FISONS TRIO 1000. Melting points were measured on a Boetius Rapido PHMK 73/2106 (Wägetechnik) instrument equipped with a TM-1300K thermometer. TLC was carried out on Silufol (Kavalier), detection was made with a Fluotest Universal (Quazlampen, Hanau) or I2 vapors. All reagents and chemicals were obtained from Sigma-Aldrich Chemicals (Czech Republic) and were used as received unless otherwise noted.
Preparation of ketazines
Ketazines were prepared by condensation of ketones with hydrazine hydrate. The reaction water was separated by azeotropic distillation. The ketone (0.2 mol) was mixed with benzene (50 mL) in a flask. Then 100 % hydrazine hydrate (5.0 g, 0.1 mol) was slowly added. The reaction mixture spontaneously heated up and became opalescent. The opalescent solution was refluxed under a Dean - Stark apparatus for about 5 hours in order to separate water. Finally, the benzene was evaporated and the crude product was fractionally distilled under reduced pressure (~ 1 mm Hg).
Spectral Data
N,N'-dicyclopentylidenehydrazine (
1a) [
15]: Yield 48 %;
1H-NMR (CDCl
3) δ: 1.66-1.71 (8H, m, -CH
2-), 2.24 (4H, t,
3J = 6.9 Hz, -CH
2-), 2.31 (4H, t,
3J = 6.8 Hz, -CH
2-);
13C-NMR (CDCl
3) δ: 24.0, 24.1, 28.6, 32.4, 172.8 (>C=N-); MS m/z (%): 165 (11), 164 (89), 163 (47), 136 (13), 135 (29), 123 (6), 122 (63), 121 (23), 110 (26), 109 (9), 108 (12), 107 (12), 98 (13), 97 (10), 96 (78), 95 (16), 94 (10), 93 (9), 84 (21), 83 (19), 82 (90), 81 (14), 80 (24), 79 (15).
N,N'-dicyclohexylidenehydrazine (
1b) [
15]: Yield 45 %;
1H-NMR (CDCl
3) δ: 1.62-1.63 (8H, m, -CH
2-), 1.71-1.76 (4H, m, -CH
2-), 2.34 (4H, t,
3J = 6.3 Hz, -CH
2-), 2.40 (4H, t,
3J = 5.8 Hz, -CH
2-);
13C- NMR (CDCl
3) δ: 26.1, 26.6, 27.7, 28.0, 35.9, 165.4 (>C=N-); MS m/z (%): 192 [M
+], 177, 163, 149, 136, 124, 110, 96, 82, 69.
N,N'-dicycloheptylidenehydrazine (
1c) [
16]: Yield 42 %;
1H NMR (CDCl
3) δ: 1.55-1.62 (16H, m, -CH
2-), 2.33 (4H, d,
3J = 5.7 Hz, -CH
2-), 2.46 (4H, d,
3J = 5.7 Hz, -CH
2-);
13C NMR (CDCl
3) δ: 25.1, 27.7, 30.6, 31.6, 165.8 (>C=N-); MS m/z (%): 223 (2), 222 (6), 221 (8), [M]
+ 220 (29), 177 (18), 163 (38), 110 (72), 96 (21), 82 (42), 41 (100).
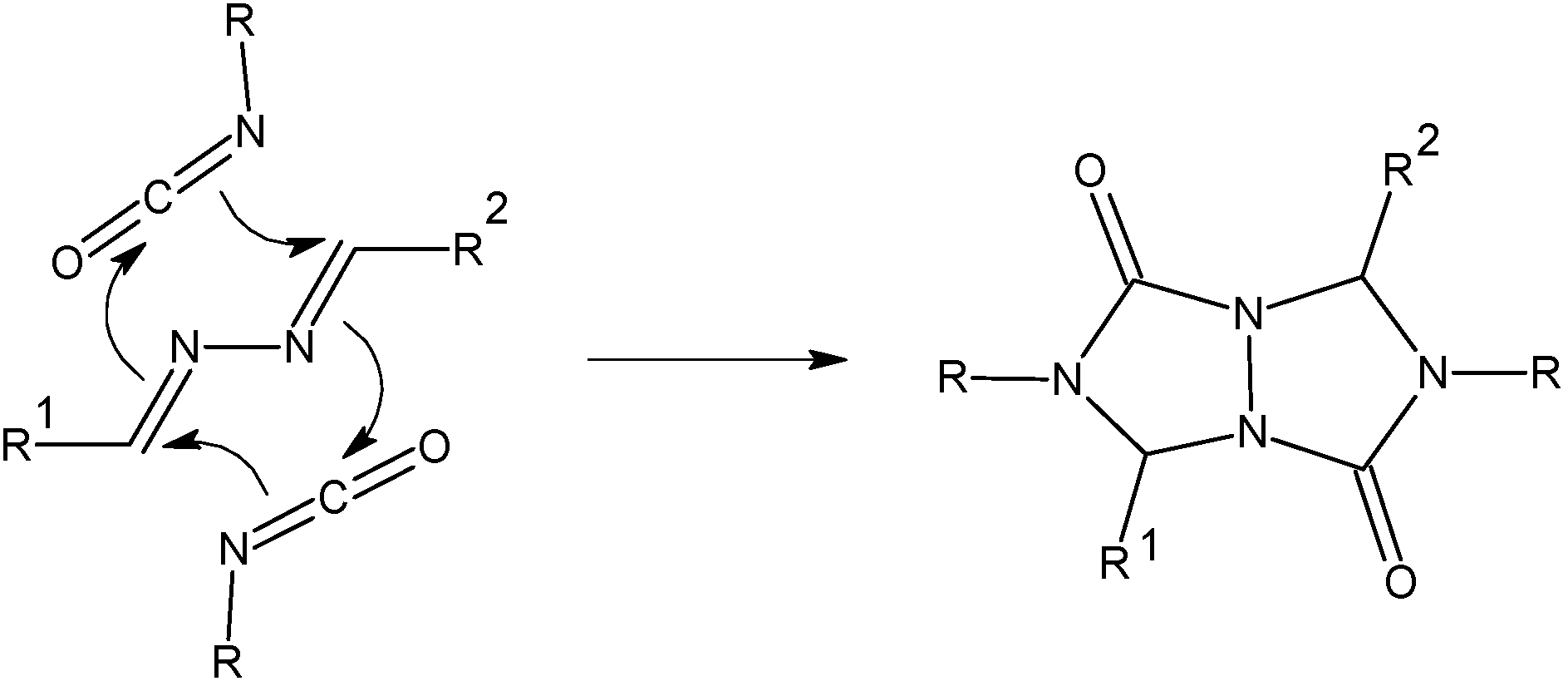
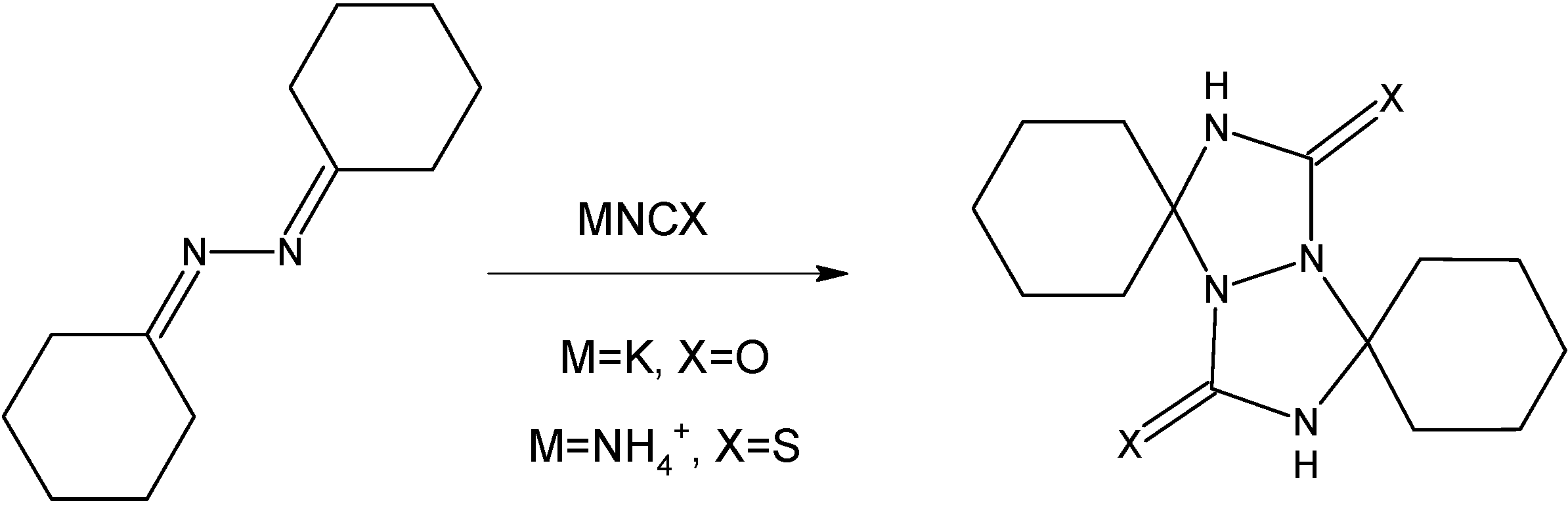
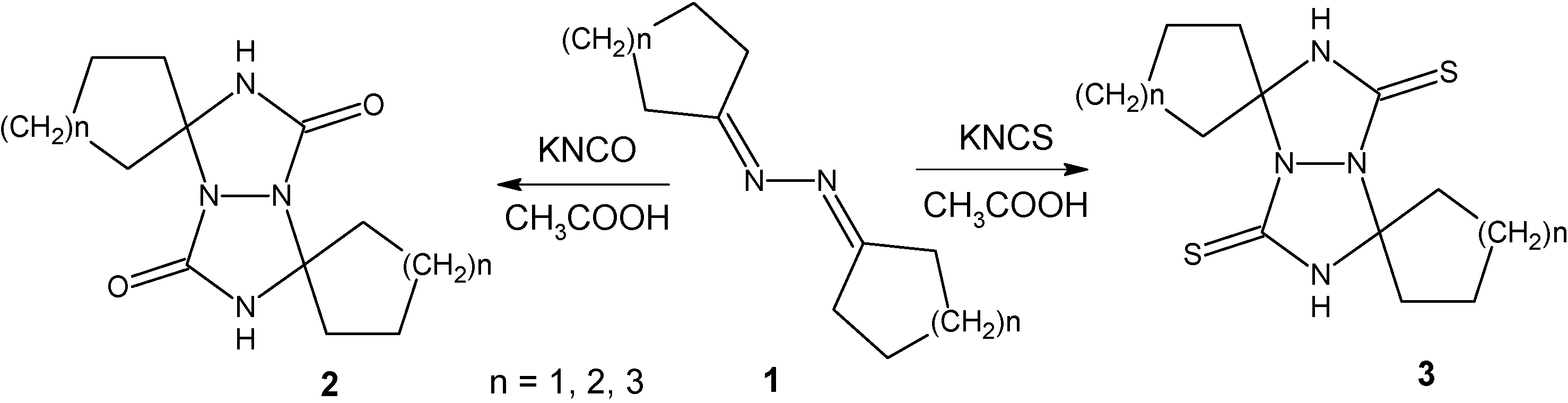
Criss-cross cycloaddition of KNCO in CH3COOH – General procedure
Reactions were carried out according to Schantl’s procedure [
17]. The solution of azine (0.01 mol) in CH
3COOH (0.024 mol, 1.44 g) was dropwise added to the solution of KNCO (0.024 mol, 1.94 g) in H
2O (3.5 mL) at 0 °C within 15 minutes. The crude product was filtered off, washed with water, acetone and finally with ether.
3,7-Di-(butane-1,4-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dione (2a): From azine 1a (1.64 g, 0.01 mol). Yield 58 %; M.p. 221-226 °C; 1H-NMR (DMSO) δ: 1.58-1.67 (12H, m, -CH2-), 2.37-2.42 (4H, m, -CH2-), 8.17 (2H, s, NH); 13C-NMR (DMSO) δ: 21.6, 34.9, 82.1 (>C<), 161.3 (>C=O); MS m/z (%): 252 (1), 251 (6), [M]+ 250 (15), 208 (3), 207 (18), 179 (16), 178 (72), 165 (9), 164 (22), 163 (17), 141 (100), 135 (9), 125 (6), 122 (19), 113 (21), 112 (75), 110 (45), 108 (7).
3,7-Di-(pentane-1,5-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dione (
2b): From azine
1b (1.92 g, 0.01 mol). Yield 66 %; M.p. 229-232 °C (Lit. 206-208 °C [
5], 210 °C [
4]);
1H-NMR (DMSO) δ: 1.37-1.66 (16H, m, -CH
2-), 2.03-2.09 (4H, m, -CH
2-), 8.20 (2H, s, NH);
13C-NMR (DMSO) δ: 22.4, 24.5, 34.6, 74.8 (>C<), 160.8 (>C=O); MS m/z (%): 280 (1), 279 (8), [M]
+ 278 (11), 235 (22), 193 (18), 192 (55), 155 (100), 149 (22), 124 (41), 112 (39), 81 (46).
3,7-Di-(hexane-1,6-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dione (2c): From azine 1c (2.20 g, 0.01 mol).Yield 33 %; M.p. 220-222 °C; 1H-NMR (DMSO) δ: 1.37-1.75 (20H, m, -CH2-), 2.26-2.30 (4H, m, -CH2-), 8.18 (2H, s, NH); 13C-NMR (DMSO) δ: 21.4, 28.5, 37.9, 78.5 (>C<), 159.3 (>C=O); MS m/z (%): 308 (2), 307 (9), [M]+ 306 (10), 263 (6), 221 (68), 220 (46), 177 (19), 169 (43), 163 (34), 150 (21), 138 (37), 112 (53), 110 (37), 95 (42), 43 (100).
Criss-cross cycloaddition of KNCS in CH3COOH – General procedure
KSCN (2.5 g, 0.0257 mol) was dissolved in CH3COOH (20 mL) at room temperature. Then ketazine (0.005 mmol) was added and the reaction mixture was stirred for 1 hour. The suspension was poured in H2O (200 ml) and the precipitated product was filtered off.
3,7-Di-(butane-1,4-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3a): From azine 1a (0.82 g, 0.005 mol). Yield 65 %; M.p. 202-205 °C; 1H-NMR (DMSO) δ: 1.73-1.78 (12H, m, -CH2-), 1.68 (4H, s, -CH2-), 9.78 (2H, s, NH); 13C-NMR (DMSO) δ: 23.5, 24.0, 36.4, 87.4 (>C<), 167.7 (>C=S); MS m/z (%): 284 (1), 283 (3), [M]+ 282 (8), 230 (9), 223 (7), 194 (12), 165 (96), 164 (100), 163 (72), 141 (66), 122 (60), 110 (26), 96 (59), 59 (62).
3,7-Di-(pentane-1,5-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3b): From azine 1b (0.96 g, 0.005 mol). Yield 90 %; M.p. 234-237 °C; 1H-NMR (DMSO) δ: 1.56-1.79 (16H, m, -CH2-), 2.55-2.65 (4H, m, -CH2-), 9.89 (2H, s, NH); 13C-NMR (DMSO) δ: 21.0, 24.2, 33.3, 81.4 (>C<), 168.2 (>C=S); MS m/z (%): 312 (2), 311 (3), [M]+ 310 (22), 193 (12), 192 (23), 174 (12), 163 (14), 155 (100), 149 (40), 147 (34), 136 (21), 110 (22), 98 (49), 96 (39), 81 (62), 59 (56).
3,7-Di-(hexane-1,6-diyl)-perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3c): From azine 1c (1.10 g, 0.005 mol). Yield 3 %; M.p. > 300 °C; 1H-NMR (DMSO) δ: 1.52-1.66 (16H, m, -CH2-), 1.75-1.91 (4H, m, -CH2-), 2.58-2.71 (4H, d, -CH2-), 9.67 (2H, s, NH); 13C-NMR (DMSO) δ: 26.1, 29.3, 35.8, 37.7, 84.2 (>C<), 167.4 (>C=S); MS m/z (%): [M]+ 338 (1), 269 (3), 268 (22), 267 (11), 221 (8), 220 (56), 163 (52), 150 (33), 112 (27), 110 (59), 96 (26), 58 (100).
Preparation of double azine: Diethoxyphosphinylhydrazine
Diethoxyphosphinylhydrazine was prepared according to the literature [
18] with slight modifications. Finely pulverised water-free K
2CO
3 (13.8 g, 0.1 mol), triethylbenzylammonium chloride (0.15 g, 0.66 mol), CH
2Cl
2 (70 mL) and CCl
4 (40 mL) were placed to a flask equipped with a magnetic stirrer and cooled in water-ice bath to 0-5 °C. Then hydrazine hydrate (6.15 g, 0.13 mol) was in one portion added and stirred for 15-20 minutes. Finally diethyl phosphonate (9.11 g, 0.066 mol) in CH
2Cl
2 (10 mL) was added within 15 minutes under continuous cooling. The reaction mixture was cooled for another 30 minutes. Then the bath was removed and the reaction mixture was stirred overnight. The next day, the organic layer was separated, the solid was washed with CH
2Cl
2 (20 ml) and the united organic layers were concentrated in high vacuum. The resulting diethoxyphosphinyl-hydrazine (11 g, 99 %) was of sufficient purity to be used for the next steps.
1H-NMR (CDCl
3) δ: 1.33 (6H, t,
J=6.93 Hz,-CH
3), 3.36 (2H, s, PONHN
H2), 4.06 (4H, dq,
J=6.93 Hz,
JPH=7.18 Hz, OC
H2CH
3), 4.61 (1H, d,
2JP,H=29.7 Hz, PON
HNH
2);
13C-NMR (CDCl
3) δ: 16.3 (d,
4JC,P=24.1 Hz, CH
2CH
3), 62.9 (d,
3JC,P=20.1 Hz,
CH
2CH
3);
31P-NMR (CDCl
3): 9.49 Hz; MS m/z (%): 169 (10), [M
+] 168 (53), 141 (6), 140 (100), 139 (7), 138 (7), 126 (6), 125 (27), 123 (5), 114 (5), 113 (72), 112 (100), 111 (32), 110 (11), 109 (23), 108 (3), 107 (5), 98 (24), 97 (5), 96 (11), 95 (42), 94 (32), 93 (9), 91 (25), 83 (95), 82 (100), 81 (100), 80 (25).
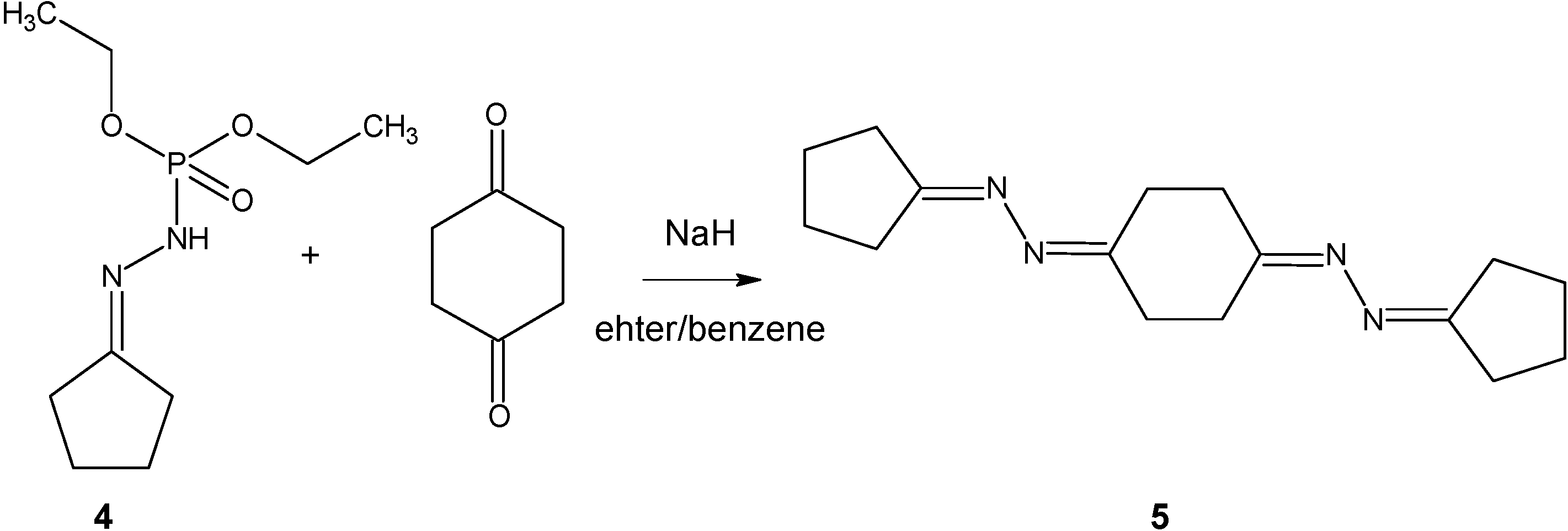
Cyclopentanone diethoxyphosphinylhydrazone (4).
Diethoxyphosphinylhydrazine (5.04 g, 0.03 mol) was added to a cooled solution of cyclopentanone (3.02 g, 0.036 mol) in benzene (10 mL). Then the reaction mixture was refluxed under a Dean - Stark apparatus for 3 hours and then concentrated in high vacuum to give a crude product (7.26 g, 97%). An analytical sample was prepared by crystallization from 1:2 benzene-hexane. M.p. 53-54.5 °C (benzene/hexane). 1H-NMR (CDCl3) δ: 1.31 (6H, t, -CH3), 1.69-1.84 (4H, m, -CH2-), 2.16 (2H, t, 3JH,H=7.25 Hz), 2.37 (2H, t, 3JH,H=7.25 Hz), 4.06-4.21 (4H, m, -CH2-), 6.24 (1H, d, 2JP,H=25.1 Hz, NH); 13C-NMR (CDCl3) δ: 16.3 (d, 3JC,P=24.1 Hz, CH2CH3), 24.9, 26.8, 33.3, 63.3 (d, 3JC,P=24.1 Hz, CH2CH3), 163.0 (d, 3JC,P=63.3 Hz, C=N-N-PO); 31P-NMR (CDCl3): 4.10 Hz; MS m/z (%): 235 (4), [M]+ 234 (25), 233 (5), 205 (8), 180 (10), 178 (5), 177 (16), 161 (8), 154 (12), 152 (21), 126 (23), 124 (28), 110 (8), 109 (21), 108 (8), 98 (96), 97 (48), 95 (7), 91 (6), 83 (18), 82 (100).
Cyclohexane-1,4-dione bis(cyclopentylidenehydrazone) (5)
A solution of cyclopentanone diethoxyphosphinylhydrazone (2.15 g, 0.009 mol) and cyclohexane-1,4-dione (0.50 g, 0.0046 mol) in benzene (28 mL) was added to a stirred suspension of NaH (0.34 g, 0.014 mol) in ether (28 mL) in a cooling bath. Immediately evolution of hydrogen occurred and the reaction mixture was stirred for 3 hours. Bright-yellow organic layer was separated and tarry residue washed with ether. After evaporation of solvent, crude product (1.17 g, 93%) was obtained. M.p. 105-108 °C; 1H-NMR (CDCl3) δ: 1.72-1.80 (8H, m, -CH2-), 2.28-2.46 (8H, m, -CH2-), 2.55-2.68 (8H, m, -CH2-); 13C-NMR (CDCl3) δ: 24.97, 26.88, 29.41, 31.64, 33.35, 163.76 (C=N), 174.84 (C=N); MS m/z (%): 273 (3), [M]+ 272 (15), 191 (5), 190 (10), 189 (18), 188 (7), 162 (9), 161 (63), 160 (6), 147 (7), 135 (5), 134 (11), 110 (9), 109 (23), 108 (8), 107 (14), 94 (6), 84 (31), 83 (100), 82 (40), 81 (22), 80 (22).