Abstract
Benzene, halobenzenes, and a number of more or less deactivated arenes, including nitrobenzene, readily reacted in anhydrous HIO3/AcOH/Ac2O/conc. H2SO4 mixtures to probably give ArIO2 intermediates or other hypervalent species (not isolated). The final reaction mixtures were poured into excess aq. Na2SO3 solution (a reductant) to give the purified iodinated products in 39-83% yields.
Introduction
In 1892 Meyer and Wachter [1], in describing iodosylbenzoic acid, the first iodosylarene, included there the following passage: “Ferner ist zu prüfen, ob man durch Behandlung von aromatischen Substanzen mir Schwefelsäure und Jodsaüre, - JO2OH, entsprechend der Reaction der Salpetersäure, NO2OH, - Verbindungen mit der Gruppe -JO2 erhalten kann. Wir können vorläufig nur mittheilen, dass Benzol beim Zusammenbringen mit Jodsäure und Schwefelsäure äusserst heftig reagirt und auf Zusatz von Wasser einen festen Körper abscheidet, welchen wir not näher untersuchen werden“. It means [1] that they had suggested the following reaction to occur, but this idea was not exploited by them further on:
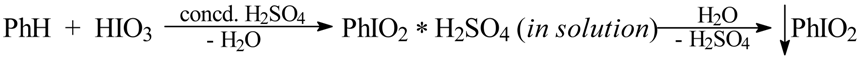
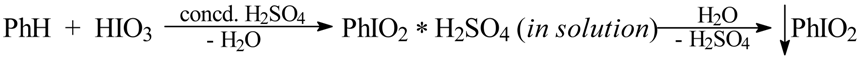
Iodylbenzene sulfate [2]
In 1935 Masson and co-workers [2] had however established that benzene derivatives and HIO3 in sulfuric acid readily formed diaryliodonium salts in high yield; later on, their initial discovery was extended considerably [3]:
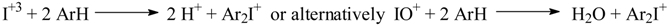
Some experimental evidence was given that a primary deoxidation of the iodic acid to the iodous stage by a fraction (ca. 15%) of the aromatic reagent, ArH (which is thereby converted into an open-chain unsaturated substance), was followed by the direct interaction of the iodous acid and the aromatic reagent to give the diaryliodonium salts; aryl iodides were also formed there as side products, and the production of CO2 was observed. Hence, the above reactions had a very complex mechanism, the details of which are still not clear.
Beringer and co-workers [4a, 5] coupled some reactive aromatic compounds (benzene, toluene, mesitylene, t-butylbenzene, n-dodecylbenzene, cyclohexylbenzene, anisole, acetanilide, and succinanil) with KIO3 in AcOH/Ac2O/H2SO4 mixtures to obtain the respective symmetrical diaryliodonium bisulfates:


If the temperature of reaction mixtures was not kept below 15 °C during the addition of the sulfuric acid and for some hours thereafter, a vigorous, exothermic reaction was observed. They suggested that in such instances the iodate was effecting a deep-seated oxidation of the aromatic compounds. By anion metatheses of the diaryliodonium bisulfates, they isolated the corresponding chlorides, bromides, iodides, and one nitrate in 9-88% yields. Diaryliodonium salts are fairly resistant towards the action of cold aq. Na2SO3 solutions; only their prolonged treatment with excess sodium sulfite in boiling water can effectively split them up as follows [4]: Ar2I+X- + Na2SO3 → ArI + ArSO3Na + NaX.
In 1969 Ciustea and Panescu [6] briefly reported that the treatment of halobenzenes, benzoic acid, nitrobenzene or benzamide, dissolved in H2SO4, H3PO4, (CH3CO)2O, CH3COOH, H2O or in sulfuric acid with an amount of water added, with HIO3, possibly resulted in the formation of the respective iodylarenes, ArIO2, or 3,5-diiodylbenzamide; these compounds can next be reduced to the corresponding aryl iodides or 3,5-diiodobenzamide. This short communication, with no experimental details, analytical data or physico-chemical support, was never followed up either then or after 1969 in a more detailed experimental paper [7, p. 1355].
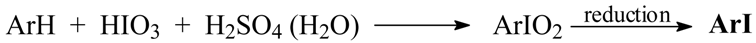
Taking into account all the aforementioned literature information, in fact all very controversial and potentially misleading, in 2003 we endeavored in our laboratory to carry out some preliminary experiments on the possibility of preparing iodylarenes, ArIO2, from the corresponding arenes. Rudzki [8] on reacting chlorobenzene with HIO3 in anhydrous Ac2O/concd H2SO4 mixtures, under widely varied reaction conditions, obtained in the best possible manner 4-chloroiodylbenzene, 4-ClC6H4IO2, albeit in only 18% crude yield. In contrast, when he reacted chlorobenzene for 10 hours with HIO3 in hot (80 °C) 75% (v/v) H2SO4, he isolated bis(4-chlorophenyl)iodonium sulfate in 63% yield; 1-chloro-4-iodobenzene (30%) was isolated there as a side product. Simultaneously, we have established [9] that benzene, halobenzenes and some deactivated arenes (excluding however nitrobenzene) readily reacted in anhydrous NaIO4/AcOH/Ac2O/concd H2SO4 mixtures. Next, the final reaction mixtures were poured into excess aq. Na2SO3 solution (a reductant) to give the purified iodinated products in 27-88% yields. This is a quite novel approach to aromatic iodination:
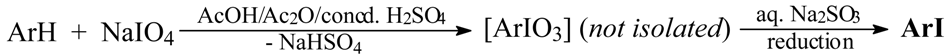 where [ArIO3] are hypothetical transient periodylarenes, not isolable in spite of many attempts.
where [ArIO3] are hypothetical transient periodylarenes, not isolable in spite of many attempts.
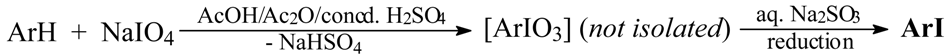
So, we have decided to carry out the iodinating reactions as follows:

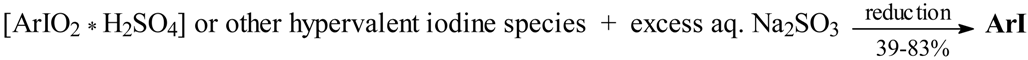

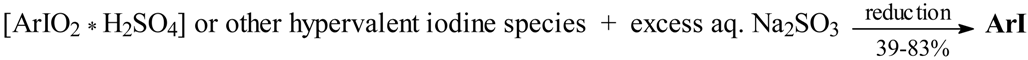
Our final results presented in the Table 1 do confirm that this approach to aromatic iodination is quite successful and promising.

Table 1.
Iodinated pure products obtained from some arenes, their yields, elemental analyses, and melting or boiling points (uncorrected).
| Substrate | Product | Yield, (%) | Analysis, % I calcd (found) | Mp (°C)/Sa) | Lit, mp (°C) [10] |
|---|---|---|---|---|---|
| C6H6 | 1,4-I2C6H4 | 60 | 76.95 (76.5) | 127-128/E | 129 |
| PhI | 1,4-I2C6H4 | 58 | 76.95 (77.2) | 127.5-129/E | 129 |
| PhBr | 4-BrC6H4I | 71 | 44.86 (44.8) | 95-97/E | 92 |
| PhCl | 4-ClC6H4I | 39 | 53.22 (52.7) | 54-56/E | 57 |
| PhNO2 | 3-IC6H4NO2 | 82 | 50.96 (50.4) | 35-37/P | 35-36 |
| PhCOOH | 3-IC6H4COOH | 67 | 51.17 (51.2) | 189-190/C | 188-189 |
| PhCOOMe | 3-IC6H4COOMe | 59 | 48.43 (48.5) | 55-56/EW | 54-55 |
| PhCOOEt | 3-IC6H4COOEt | 51 | 45.97 (45.8) | bp 170-172/29 | bp 150/15 |
| 4-MeC6H4COOH | 3-I-4-MeC6H3COOH | 78 | 48.43 (48.2) | 211-212/C | 210-212 |
| 4-MeC6H4NO2 | 3-I-4-MeC6H3NO2 | 40 | 48.25 (47.9) | 52-54/E | 54-56 |
| 4-MeOC6H4NO2 | 3-I-4-MeOC6H3NO2 | 83 | 45.51 (45.1) | 94-96/E | 97 |
| 2-O2NC6H4NHCOCH3 | 4-I-2-O2NC6H3NHCOCH3 | 52 | 41.50 (41.5) | 110-112/EW | 112 |
a) Solvents used for crystallization: C – CCl4; E – EtOH; P – petroleum ether; EW – EtOH/H2O
Results and Discussion
For benzene (which was only diiodinated), three halobenzenes and eight deactivated arenes, including nitrobenzene (Table 1), we carried out the iodinating reactions according to the stoichiometry shown in Eq. 6, where: [ArIO2 * H2SO4] are the assumed iodine(V) intermediates, possibly strongly admixed with ArI(OSO3H)2; it is known that amphoteric iodylarenes freely form salts with strong mineral acids, and the hygroscopic sulfate PhIO2·H2SO4 (mp 127 °C) was analyzed and examined [2].
Thus, iodic acid and chosen arenes, ArH, were suspended in cooled (5 °C) anhydrous AcOH/Ac2O mixtures, and then a large excess of concd. (95%) sulfuric acid was slowly added dropwise, with vigorous stirring, to the reaction mixtures, while keeping the temperature below 10 °C. The strongly acidified reaction mixtures were then stirred for one hour in an ice-water bath, next for one hour at room temperature and then for 2 hours at 45-50 °C. We have established that somewhat better yields are achieved when the reaction mixtures were finally left overnight at room temperature. The final reaction mixtures were quenched by pouring into stirred ice-water containing a previously dissolved excess of Na2SO3 (a reductant), Eq. 7. The oily or solid crude products that precipitated out were typically isolated and purified (see the Experimental) to give the purified monoiodinated products in 39-83% yields, except in the case of 1,4-diiodobenzene, which was obtained in 60% yield (Table 1). The chemical structures of the purified products were next confirmed by their melting or boiling points, all close to those reported in the literature [10], elemental analyses (%I), and comparison of the 1H and 13C NMR spectra (not reported here) with the corresponding spectra of authentic samples. Their homogeneities were checked by TLC.
We have also tried to isolate the purported iodine(V) intermediates, i.e. ArIO2, by pouring the final reaction mixtures into ice-water (ArIO2·H2SO4 is readily hydrolyzable [2]). However, the crude yields of ArIO2 thus obtained were usually lower than 20%, and the crude ArIO2 had to be recrystallized from boiling water at least once – which lowered the yields further. The best result was obtained with bromobenzene that afforded 1-bromo-4-iodylbenzene in 40% crude yield (see the Experimental). Hence, these attempts were deemed unworthy of further study. It should be pointed out that we previously devised in our laboratory two easy and effective methods of preparing ArIO2 from ArI using NaIO4 as the oxidant, acting either in boiling water [11] or in boiling 30% (v/v) aq. acetic acid [12]. The crude yields of ArIO2 thus obtained were good to excellent, while the snow-white crude products had a 98-99% purity, established iodometrically.
We present in this paper an easy and effective approach to the aromatic iodination, which allows one to obtain iodoarenes, in good yields, from halobenzenes and several deactivated arenes, including nitrobenzene; cf. Ref. [9]. The transient protonated iodic acid, O3I+H2, was the sole iodinating agent present, capable of forming the stable C-I bond in the starting arenes, which is preserved after the reduction with excess aq. Na2SO3 solution to afford the final mono- or diiodinated arenes (Table 1). However, activated arenes, e.g. anisole, acetanilide, N, N – dimethylaniline, etc., were easily oxidized under the given reaction conditions to form some tarry products, which could not be purified in spite of our numerous attempts.
Note added in proof. For comparison, also NaBrO3 is a powerful brominating agent for deactivated aromatics. Addition of a strong acid into a stirred aq. solution, or slurry, of the substrate and NaBrO3 at 40-100 °C, leads to the decomposition of the bromate ions and production of the active brominating species. Substrates such as nitrobenzene, benzoic acid, and benzaldehyde were specifically brominated in 85-98% yields. The reaction is especially useful for the bromination of disubstituted benzenes, such as 4-FC6H4NO2 or 4-FC6H4COOH. Dinitrobenzenes and nitrobenzoic acids did not undergo bromination at all. See Ref. 13.
Experimental
General
All the reagents and solvents were commercial (Aldrich) and were used without further purification. The melting or boiling points of pure iodinated products (Table 1) are uncorrected. Elemental microanalyses (%I) were performed at the Institute of Organic Chemistry, the Polish Academy of Sciences in Warsaw. 1H- and 13C-NMR spectra (not shown here) were recorded at room temperature, in CDCl3 solutions, with a Bruker Avance DMX 400 MHz spectrometer; sometimes, in order to get better assignments, also 1H-13C correlation spectra were recorded. The spectra confirmed the chemical structures of the purified iodinated products (Table 1).
Optimized Preparations of Iodoarenes from Arenes
Iodic acid (3.25 g, 20 mmol) was suspended in a mixture made of glacial AcOH (20 mL) and acetic anhydride (10 mL), then an appropriate arene (20 mmol) was added with stirring. The stirred mixture was cooled to ca. 5 °C, and concd. (95%) sulfuric acid (10 mL, ca. 0.2 mol) was very slowly added dropwise while keeping the temperature below 10 °C. The reaction mixtures were stirred for 1 h in an ice-water bath, then for 1 h at r.t. and next for 2 h at 45-50 °C. The stirring was continued overnight at r.t. The final reaction mixtures were poured into ice-water (100 g) containing previously dissolved Na2SO3 (a reductant, 5 g). After a few hours, the precipitated crude solid products were collected by filtration, washed well with cold water until the filtrates were neutral, dried preliminarily by suction, and next air-dried in the dark. They were recrystallized from appropriate organic solvents (Table 1) to give the purified solid iodinated products. If the crude iodinated products were oily or semisolid, they were extracted with CHCl3 (3 x 20 mL), the combined extracts were dried over anh. MgSO4, filtered, the solvent was distilled off, and the residues were fractionated under vacuum to give the purified liquid iodinated products (Table 1). The purified iodoarenes should be preserved in the dark, preferably at 0-5 °C. Note: When benzene was diiodinated as above to give pure 1,4-diiodo-benzene, only half of its amount, i.e. 10 mmol, was added to the reaction mixture.
Possibly Optimized Preparation of 1-Bromo-4-iodylbenzene from Bromobenzene
Iodic acid (5.28 g, 30 mmol) was suspended in a mixture made of glacial AcOH (10 mL) and Ac2O (5 mL), then bromobenzene (4.71 g, 30 mmol) was added with stirring. Concd. (95%) H2SO4 (2 mL) was very slowly added dropwise with vigorous stirring, keeping the temperature below 50 °C. Next, the stirring was prolonged within 2 h at ambient temperature. The final reaction mixture was poured into ice-water, and the precipitated oily product slowly solidified. The precipitate was collected by filtration, washed with a little ice water, then with acetone. The colorless final product, obtained in 40% crude yield, decomposed at 235 °C with detonation, lit. [12] mp 236 °C with detonation.
References and Notes
- Meyer, V.; Wachter, W. Ueber Jodosobenzoësäure. Ber. Dtsch. Chem. Ges. 1892, 25, 2632–2635. [Google Scholar] [CrossRef] The German passage in the text can be translated as follows: “Next, it has to be checked out whether the reaction of aromatic compounds with sulfuric acid and iodic acid, IO2OH, corresponding to the reaction with nitric acid, NO2OH, can afford the respective compounds substitued by the –IO2 group. We can only preliminarily report that benzene reacts violently with iodic acid and sulfuric acid, and after addition of water, a solid compound precipitates out, which was not closer investigated”. Mason and Race (Ref. 3, footnote in p. 1723) commented the first German attempt to prepare PhIO2 from benzene as follows: “It does not appear that the experiment was followed up either then or after 1894, when the iodoxy- and the iodonium group were discovered”.
- Masson, I.; Race, E.; Pounder, F. E. The Iodoxy-group and its Relations. J. Chem. Soc. 1935, 1669–1679. [Google Scholar]
- Masson, I.; Race, E. The Direct Conversion of Iodic Acid and Aromatic Hydrocarbons into Iodonium Compounds. J. Chem. Soc. 1937, 1718–1723. [Google Scholar] [CrossRef]
- Beringer, F. M.; Drexler, M.; Gindler, E. M.; Lumpkin, C. C. Diaryliodonium Salts. I. Synthesis. J. Am. Chem. Soc. 1953, 75, 2705–2708. [Google Scholar] Krowczyński, A.; Skulski, L. p-Iodo-N-methylacetanilide and its Polyvalent Iodine Derivatives. Polish J. Chem. 1993, 67, 67–70. [Google Scholar]
- Beringer, F. M.; Falk, R. A.; Karniol, M.; Lillien, I.; Masullo, G.; Mausner, M.; Sommer, E. Diaryliodonium Salts. IX. The Synthesis of Substituted Diphenyliodonium Salts. J. Am. Chem. Soc. 1959, 81, 342–351. [Google Scholar]
- Ciustea, G.; Panescu, A. New synthetic routes in the chemistry of the organic iodine compounds (in Roumanian). Rev. Chim. (Bucharest) 1969, 20, 216, [Chem. Abstr. 1969, 71, 123755]. [Google Scholar]
- Skulski, L. Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland (1990-2000). Molecules 2000, 5, 1331–1371, Avail. at URL: http://www.mdpi.org/molecules/papers/51201311.pdf. [Google Scholar]
- Rudzki, P. An attempt of the direct iodylation of arenes. M. Sc. Thesis, Faculty of Pharmacy, Medical University of Warsaw, Poland, 2003. [Google Scholar]
- Lulinski, P.; Sosnowski, M.; Skulski, L. A Novel Aromatic Iodination Method, with Sodium Periodate Used Alone as the Iodinating Reagent. Paper presented at the 7th Electronic Conference on Synthetic Organic Chemistry (ECSOC-7, http://www.mdpi.net/ecsoc-7), November 1-30, 2003 (paper A013). Submitted for publication in Molecules.
- Dictionary of Organic Compounds,, 6th ed.; Chapman & Hall: London, 1996.
- Kazmierczak, P.; Skulski, L.; Kraszkiewicz, L. Syntheses of (Diacetoxyiodo)arenes or Iodylarenes from Iodoarenes, with Sodium Periodate as the Oxidant. Molecules 2001, 6, 881–891, Avail. at http://www.mdpi.org/molecules/papers/61200881.pdf. [Google Scholar]
- Kraszkiewicz, L.; Skulski, L. Optimized syntheses of iodylarenes from iodoarenes, with sodium periodate as the oxidant. Part II. ARKIVOC. (Gainsville, FL, USA) [online computer file]. 2003, (vi), pp. 120–125, Avail. at http://www.arkat-usa.org/ark/journal/2003/Varvoglis/AV-657A/AV-657A.htm.
- Groweiss, A. Use of Sodium Bromate for Aromatic Bromination: Research and Development. Org. Process. Res. Dev. 2000, 4, 30–33. [Google Scholar] See also: Harrison, J. J.; Pellegrini, J. P.; Selwitz, C. M. Bromination of Deactivated Aromatics Using Potassium Bromate. J. Org. Chem. 1981, 46, 2169–2171. [Google Scholar] , and references therein; Yamaoka, Y. Preparation of 1-bromo-3-nitrobenzene. Jpn. Kokai Tokkyo Koho JP 11 228,505 [99 217,344], 10 Aug 1999; [Chem. Abstr. 1999, 131, 144408].
- Sample Availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.