Eco-friendly Oxidative Iodination of Various Arenes with Sodium Percarbonate as the Oxidant†
Abstract
:Introduction
Results and discussion
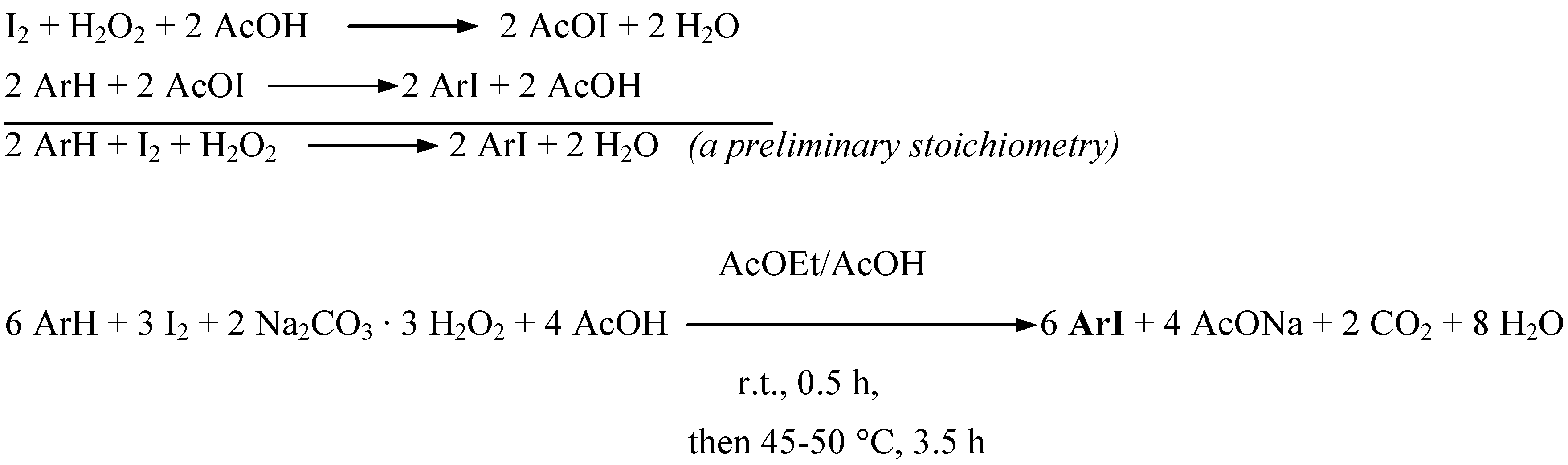
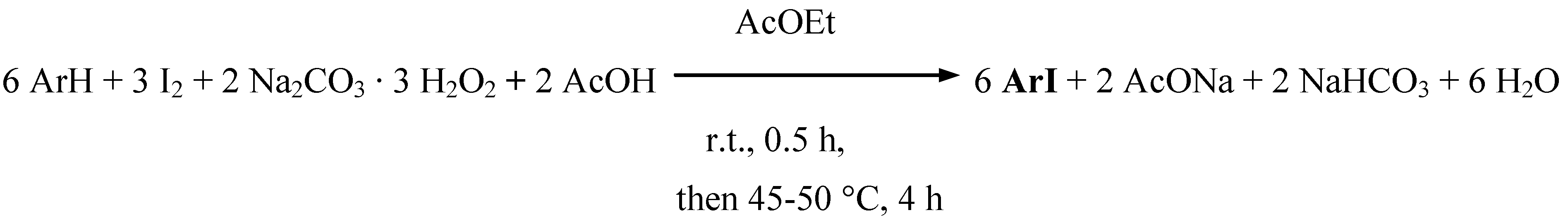
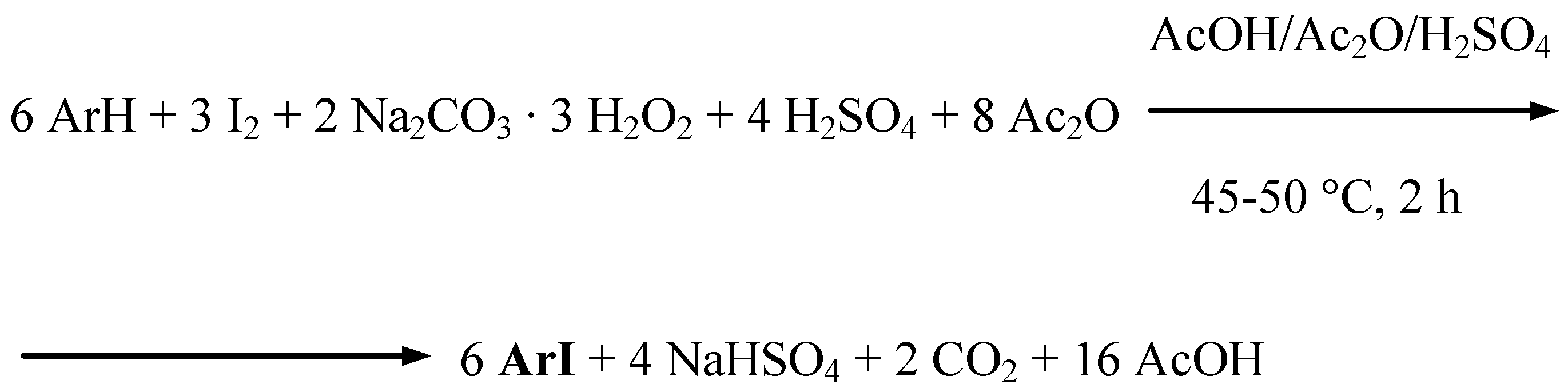
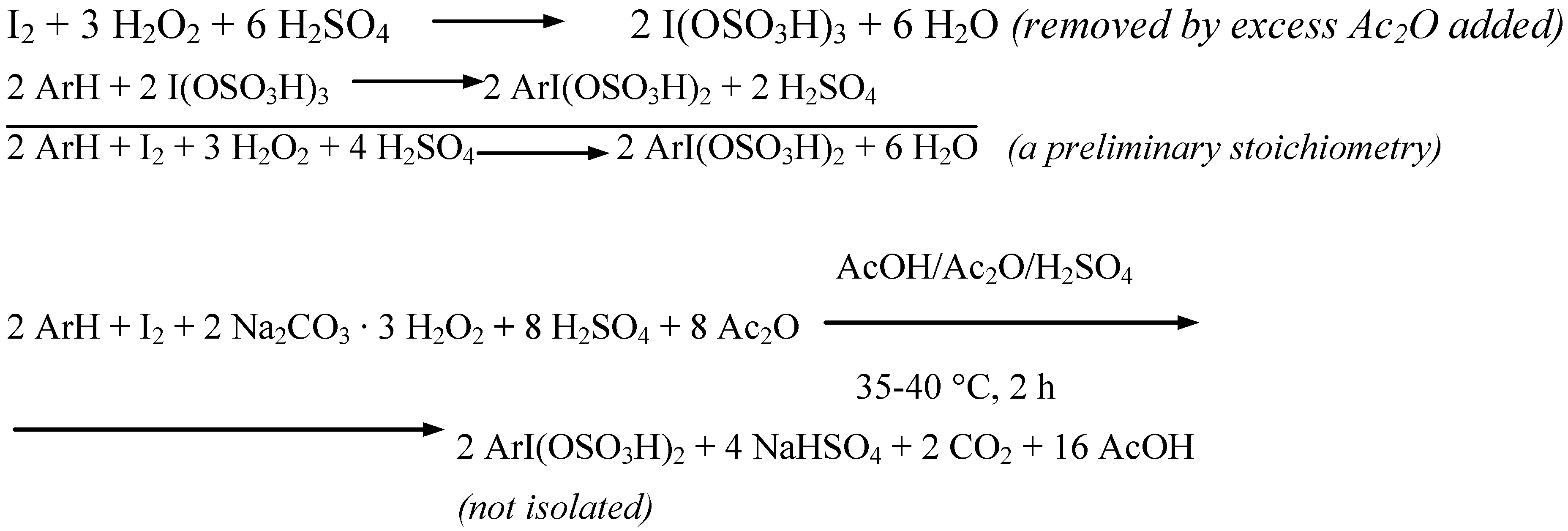
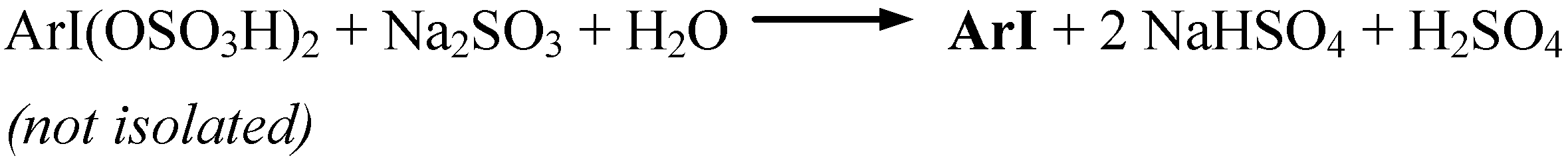
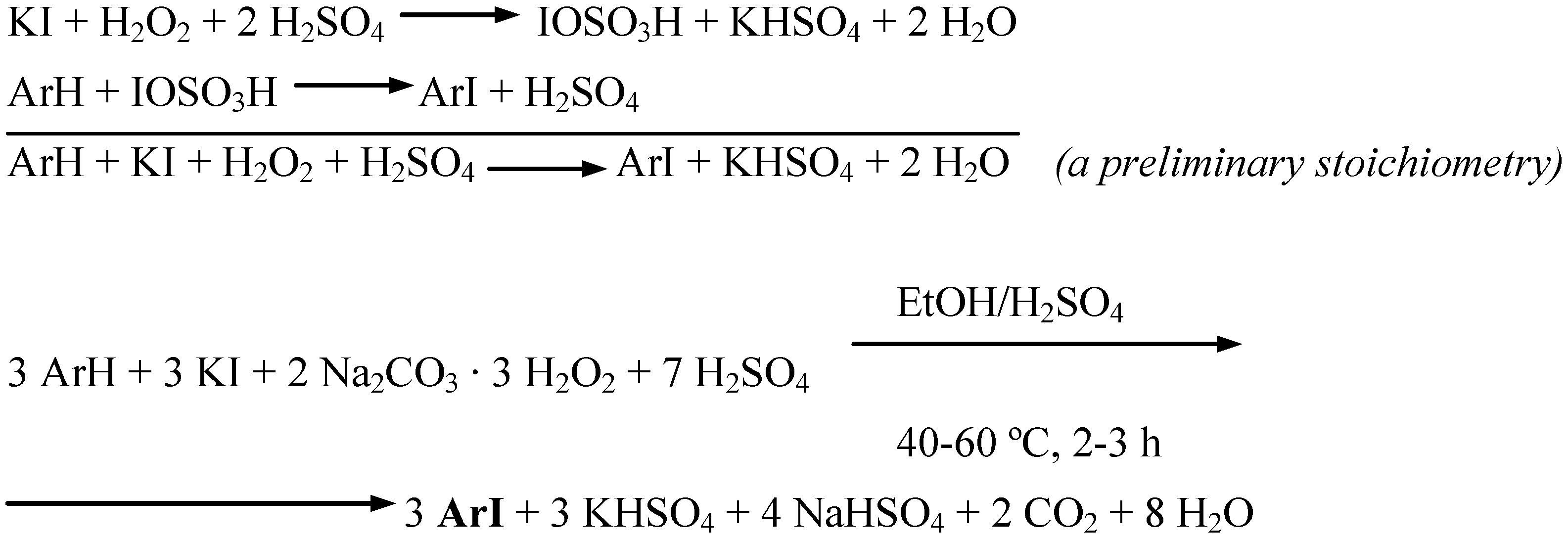
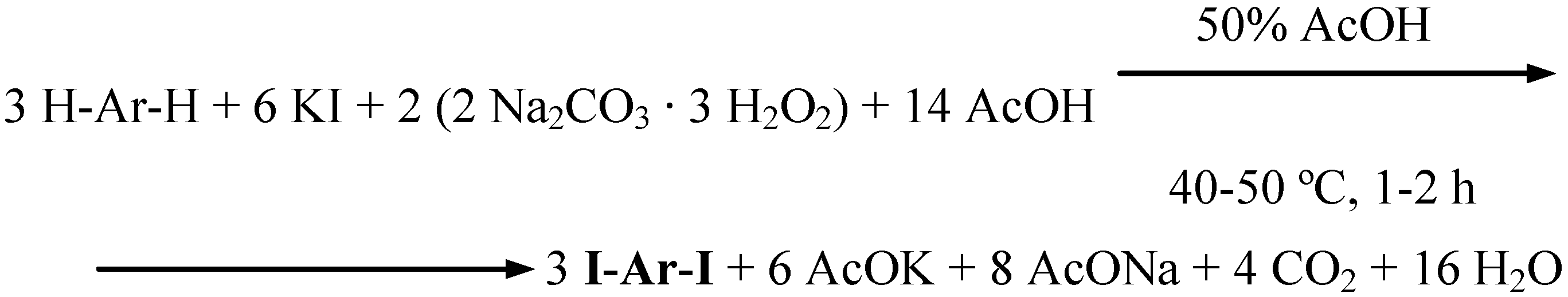
Conclusions
Experimental
General
Optimized iodinating procedures, with using sodium percarbonate (SPC) as the oxidant
- (a)
- 4.26 mL H2SO4 (7.84 g; 80 mmol) for the iodination of C6H5NHAc or uracil;
- (b)
- 4.80 mL H2SO4 (8.83 g; 90 mmol) for the iodination of C6H6, C6H5Br or C6H5Cl;
- (c)
- 5.33 mL H2SO4 (9.80 g; 100 mmol) for the iodination of 4-MeC6H4COOH, 4-MeC6H4COOMe, 4-O2NC6H4Me or 4-O2NC6H4OMe;
- (d)
- 7.50 mL H2SO4 (13.8 g; 140 mmol) for the diiodination of PhH.
- (a)
- 3.60 mL H2SO4 (6.60 g; 67.5 mmol) for the iodination of PhCOOH;
- (b)
- 4.80 mL H2SO4 (8.83 g; 90.0 mmol) for the iodination of PhI, PhCOOMe, 4-MeC6H4COOH, 4-O2NC6H4Me, and for the diiodination of PhH;
- (c)
- 6.80 mL H2SO4 (12.5 g; 127 mmol) for the diiodination of PhCOPh.
- (a)
- for 2 h at 40 °C for the iodination of PhOMe;
- (b)
- for 2 h at 50−60 °C for the iodination of 1- or 2-MeOC10H7;
- (c)
- for 3 h at 40 °C for the iodination of PhNH2.
| Substrate | Procedure | Product | Yield (%)a | Mp (°C) (S),b or bp (°C/mmHg); |
|---|---|---|---|---|
| Lit. [12] mp (°C), or bp (°C/mmHg) | ||||
| PhNH2 | 1 | 4-IC6H4NH2 | 68 | 63−65 (H); 63−65 |
| PhNH2 | 1 | 2,4-I2C6H3NH2 | 85 | 93−94 (H); 95−96 |
| 4-IC6H4NH2 | 1 | 2,4-I2C6H3NH2 | 78 | 96−97 (H); 95−96 |
| 2-BrC6H4NH2 | 1 | 2-Br-4-IC6H3NH2 | 67 | 71−74 (Hp); 71−72 |
| 2-MeC6H4NH2 | 1 | 4-I-2-MeC6H3NH2 | 86 | 86−87 (H); 88 |
| PhNMe2 | 2 | 4-IC6H4NMe2 | 60 | 81−83 (E); 82 |
| 2-ClC6H4NH2 | 2 | 2-Cl-4-IC6H3NH2 | 73 | 60−61 (H); 62−63 |
| 2-BrC6H4NH2 | 2 | 2-Br-4-IC6H3NH2 | 62 | 70−73 (Hp); 71−72 |
| 2-MeC6H4NH2 | 2 | 4-I-2-MeC6H3NH2 | 48 | 87−88 (H); 86−88 |
| PhH | 3 | PhI | 40 | bp 76−78/20; bp 78-80/25 [5] |
| PhH | 3 | 1,4-I2C6H4 | 83 | 128−130 (L); 129 |
| 4-O2NC6H4Me | 3 | 2-I-4-O2NC6H3Me | 75 | 51−52 (N); 53−54 |
| 4-O2NC6H4OMe | 3 | 2-I-4-O2NC6H3OMe | 92 | 95−96 (L); 97 |
| 4-MeOC6H4CO2Me | 3 | 3-I-4-MeOC6H4CO2Me | 85 | 93−95 (N); 95−97 |
| PhNHCOMe | 3 | 4-IC6H4NHCOMe | 62 | 183−185 (E); 184 |
| PhCl | 3 | 4-ClC6H4I | 80 | 55−56 (E); 57 |
| PhBr | 3 | 4-BrC6H4I | 68 | 91−92 (L); 91−92 |
| uracil | 3 | 5-iodouracil | 84 | 276−276.5 (E); 276−278 |
| 4-RC6H4CO2Hc | 3 | 3-I-4-RC6H3CO2Hc | 92 | 238−239 (W); 230 |
| PhH | 4 | 1,4-I2C6H4 | 83 | 128−130 (L); 129 |
| PhI | 4 | 1,4-I2C6H4 | 94 | 128−129 (L); 129 |
| PhCO2H | 4 | 3-IC6H4CO2H | 93 | 185−187 (C); 187−188 |
| 4-MeC6H4CO2H | 4 | 3-I-4-MeC6H3CO2H | 79 | 208−209 (C); 210−212 |
| PhCO2Me | 4 | 3-IC6H4CO2Me | 60 | 52−53 (L); 54−55 |
| 4-O2NC6H4Me | 4 | 2-I-4-O2NC6H3Me | 87 | 51−53 (N); 53−54 |
| PhCOPh | 4 | 3-IC6H4COC6H4I-3’ | 51 | 140−142 (A); 141−143 [5] |
| C6H5OMe | 5 | 4-IC6H4OMe | 64 | 50−51 (H); 51−52 |
| 1-MeOC10H7 | 5 | 4-I-1-MeOC10H6 | 68 | 50−51 (E); 52−53 [11] |
| 2-MeOC10H7 | 5 | 1-I-2-MeOC10H6 | 62 | 82−84 (E); 82−84 [11] |
| PhNH2 | 5 | 4-IC6H4NH2 | 53 | 60−62 (H); 63−65 |
| 2-O2NC6H4OH | 6 | 2,4-I2-6-O2NC6H2OH | 80 | 93−94 (E); 94 [13] |
| 4-O2NC6H4OH | 6 | 2,6-I2-4-O2NC6H2OH | 72 | 149−150 (E); 150−151 [11] |
| 4-CH3C6H4OH | 6 | 2,6-I2-4-CH3C6H2OH | 83 | 58−59 (E); 55−58 [14] |
| 8-hydroxyquinoline | 6 | 5,7-diiodo-8-hydroxyquinoline | 85 | 216−217 dec. (E); ca. 214 dec. |
| PhNH2 | 6 | 4-IC6H4NH2 | 77 | 60−62 (H); 63−65 |
| 4-IC6H4NH2 | 6 | 2,4-I2C6H3NH2 | 59 | 95−96 (H); 95−96 |
- a
- Optimized yield of pure isolated product. Satisfactory microanalyses obtained for the purified products: I% ± 0.4; their purities and homogeneities were checked by TLC and 1H and 13C NMR solution spectra (not shown here).
- b
- S = Solvent used for recrystallization. A: acetone; C: CCl4; E: EtOH; H: hexane; Hp: heptane; L: EtOH−H2O (4:1); N: EtOH−H2O (3:2); W: H2O−EtOH (5:1).
- c
- R = 4-MeCONH.
References and Notes
- Reviews on aromatic iodination: Roedig, A. Houben-Weyl, Methoden der organischen Chemie, 4th ed.; Vol. 5/4, Mueller, E., Ed.; Thieme: Stuttgart, 1960; pp. 517–678. [Google Scholar] Merkushev, E. B. Usp. Khim. 1984, 53, 583–594, Russ. Chem. Rev. 1984, 53, 343-353. Merkushev, E. B. Synthesis 1988, 923–937. Sasson, Y. The Chemistry of Halides, Pseudohalides and Azides, Suppl. D1; Patai, S., Rappoport, Z., Eds.; Wiley−Interscience: Chichester, 1995; pp. 535–620. [Google Scholar] Steel, P. G. Rodd’s Chemistry of Carbon Compounds, 2nd ed.; Part 1; Vol. 3, Sainsbury, M., Ed.; Elsevier: Amsterdam, 1996; pp. 178–224. [Google Scholar]
- The latest reviews on organic hypervalent iodine compounds: Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; VCH: Weinheim, 1992. [Google Scholar] Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: San Diego, 1997. [Google Scholar] Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. Hypervalent Iodine Chemistry; Wirth, T. (Ed.) Topics in Current Chemistry; Vol. 224, Springer: Berlin, 2003. Stang, P. J. J. Org. Chem. 2003, 68, 2997–3008. Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903.
- Skulski, L. Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland (1990-2000). Molecules 2000, 5, 1331–1371. http://www.mdpi.org/molecules/papers/51201331.pdf. [Google Scholar]
- Skulski, L. Novel Easy Preparations of Some Aromatic Iodine(I, III, and V) Reagents, Widely Applied in Modern Organic Synthesis. Molecules 2003, 8, 45–52. http://www.mdpi.org/molecules/papers/80100045.pdf. [Google Scholar]
- Lulinski, P.; Kryska, A.; Sosnowski, M.; Skulski, L. Eco-friendly Oxidative Iodination of Various Arenes with a Urea – Hydrogen Peroxide Adduct (UHP) as the Oxidant. Synthesis 2004, 441–445, We presented there three eco-friendly procedures for the oxidative iodination of both activated and deactivated arenes, using UHP as the oxidant. [Google Scholar]
- Reviews on the uses of a urea-hydrogen peroxide adduct (UHP) in organic synthesis: Heaney, H. Aldrichim. Acta 1993, 26, 35–45. Heaney, H. Organic Peroxygen Chemistry; Herrmann, W. A., Ed.; Topics in Current Chemistry; Vol. 164, Springer: Berlin, 1993; pp. 1–19. [Google Scholar]
- Reviews on the uses of sodium perborate (SPB) and sodium percarbonate (SPC) in organic synthesis: Muzart, J. Synthesis 1995, 1325–1347. McKillop, A.; Sanderson, W. R. Tetrahedron 1995, 51, 6145–6166. McKillop, A.; Sanderson, W. R. J. Chem. Soc., Perkin Trans. 1 2000, 471–476. SPB in the presence of a sodium tungstate catalyst was shown to be a cheap oxidant for the iodination of aromatic amides using KI as the source of iodine(I) species; see: Beinker, P.; Hanson, J. R.; Meindl, N.; Rodriguez Medina, I. C. J. Chem. Res. (S) 1998, 204–205.
- Aldrich Advancing Science; Aldrich: Milwaukee, 2005–2006; The following prices are given therein: (a) Sodium percarbonate (25% H2O2), 13.10 €/500 g. (b) Urea-hydrogen peroxide addition compound (98%), 161.60 €/500 g. (c) Sodium perborate monohydrate, 83.60 €/500 g. (d) Sodium perborate tetrahydrate, 26.80 €/500 g.
- The varied amounts of conc. H2SO4 added to the reaction mixtures (established experimentally and given in the experimental section for each of the substrates iodinated) clearly depended on the relative reactivities of the arenes investigated. The more deactivated the arene, the more conc. H2SO4 had to be added to catalyze better the oxidative iodination reaction. For more details see Ref. 3, pp. 1334−1337.
- Such strongly hygroscopic compounds as I2(SO4)3, I(OSO3H)3, ArISO4, ArI(OSO3H)2 are stable under strongly acidic and anhydrous conditions. For more details see the following review: Kasumov, T. M.; Koz’min, A. S.; Zefirov, N. S. The Chemistry of Inorganic Sulfates and Sulfonates of Polyvalent Iodine. Usp. Khim. 1997, 66, 936–952, Russ. Chem. Rev. 1997, 66, 843-857. [Google Scholar]
- Iskra, J.; Stavber, S.; Zupan, M. Nonmetal-catalyzed Iodination of Arenes with Iodide and Hydrogen Peroxide. Synthesis 2004, 1869–1873. [Google Scholar] [CrossRef] [Green Version]
- Dictionary of Organic Compounds, 6th ed.; Chapman & Hall: London, 1996.
- Hodgson, H. H.; Smith, E. W. J. Chem. Soc. 1932, 503–505.
- Bell, N. V.; Bowman, W. R.; Coe, P. F.; Turner, A. T.; Whybrow, D. Can. J. Chem. 1997, 75, 873–889.
- Sample Availability: Contact the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zielinska, A.; Skulski, L. Eco-friendly Oxidative Iodination of Various Arenes with Sodium Percarbonate as the Oxidant†. Molecules 2005, 10, 1307-1317. https://doi.org/10.3390/10101307
Zielinska A, Skulski L. Eco-friendly Oxidative Iodination of Various Arenes with Sodium Percarbonate as the Oxidant†. Molecules. 2005; 10(10):1307-1317. https://doi.org/10.3390/10101307
Chicago/Turabian StyleZielinska, Agnieszka, and Lech Skulski. 2005. "Eco-friendly Oxidative Iodination of Various Arenes with Sodium Percarbonate as the Oxidant†" Molecules 10, no. 10: 1307-1317. https://doi.org/10.3390/10101307
APA StyleZielinska, A., & Skulski, L. (2005). Eco-friendly Oxidative Iodination of Various Arenes with Sodium Percarbonate as the Oxidant†. Molecules, 10(10), 1307-1317. https://doi.org/10.3390/10101307




