Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery
Abstract
:Introduction
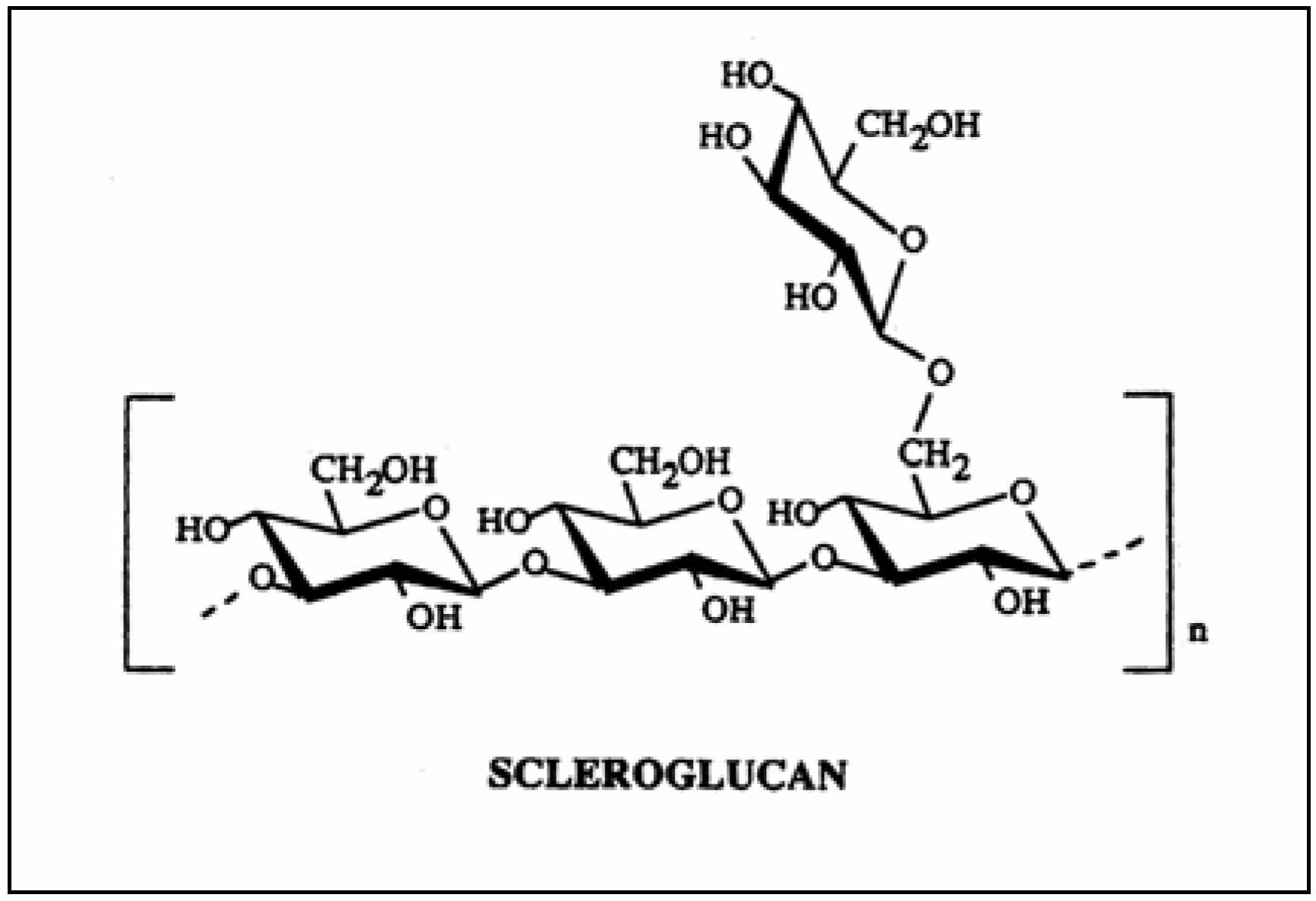
Modified drug delivery
Native Scleroglucan
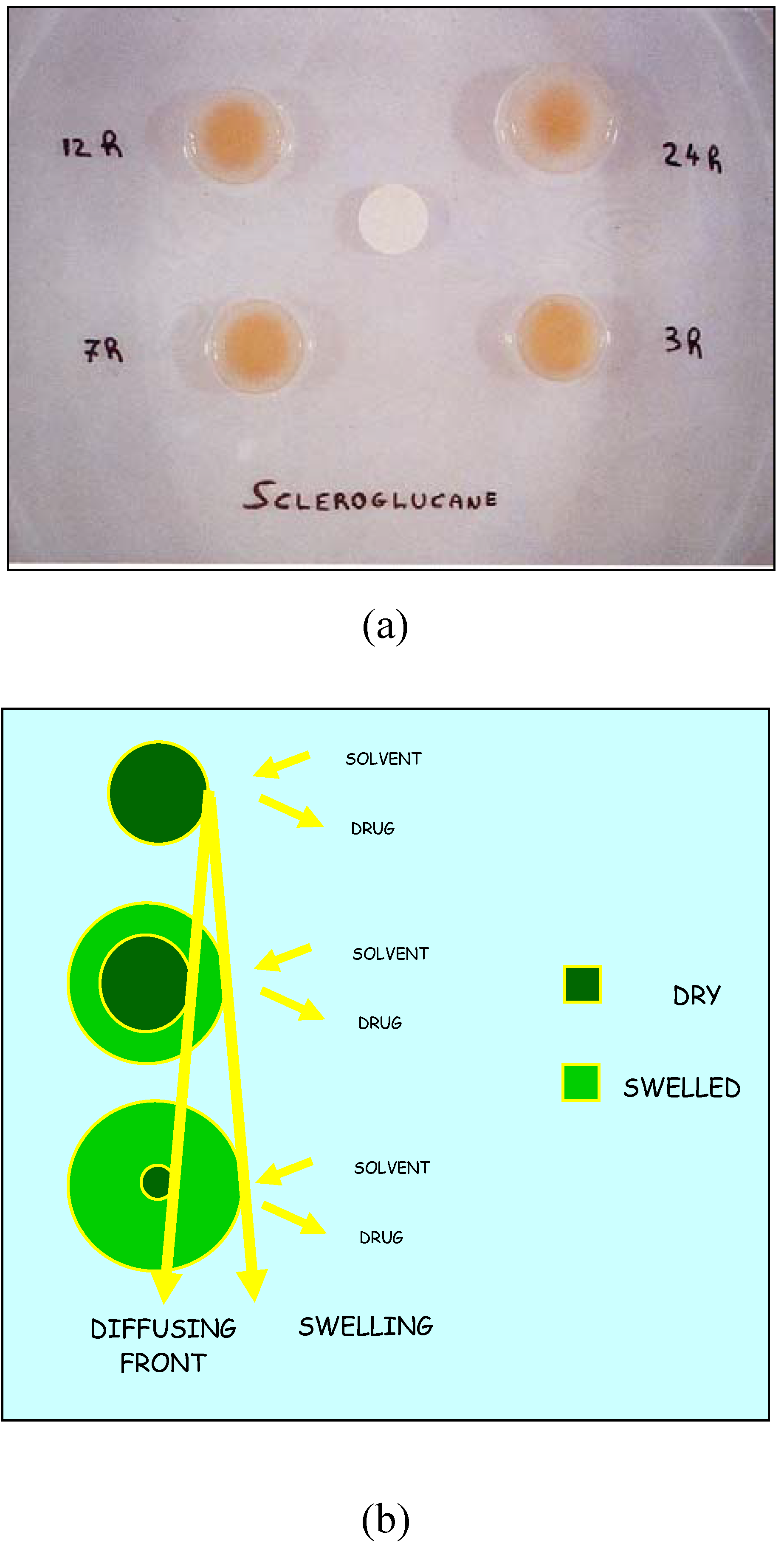
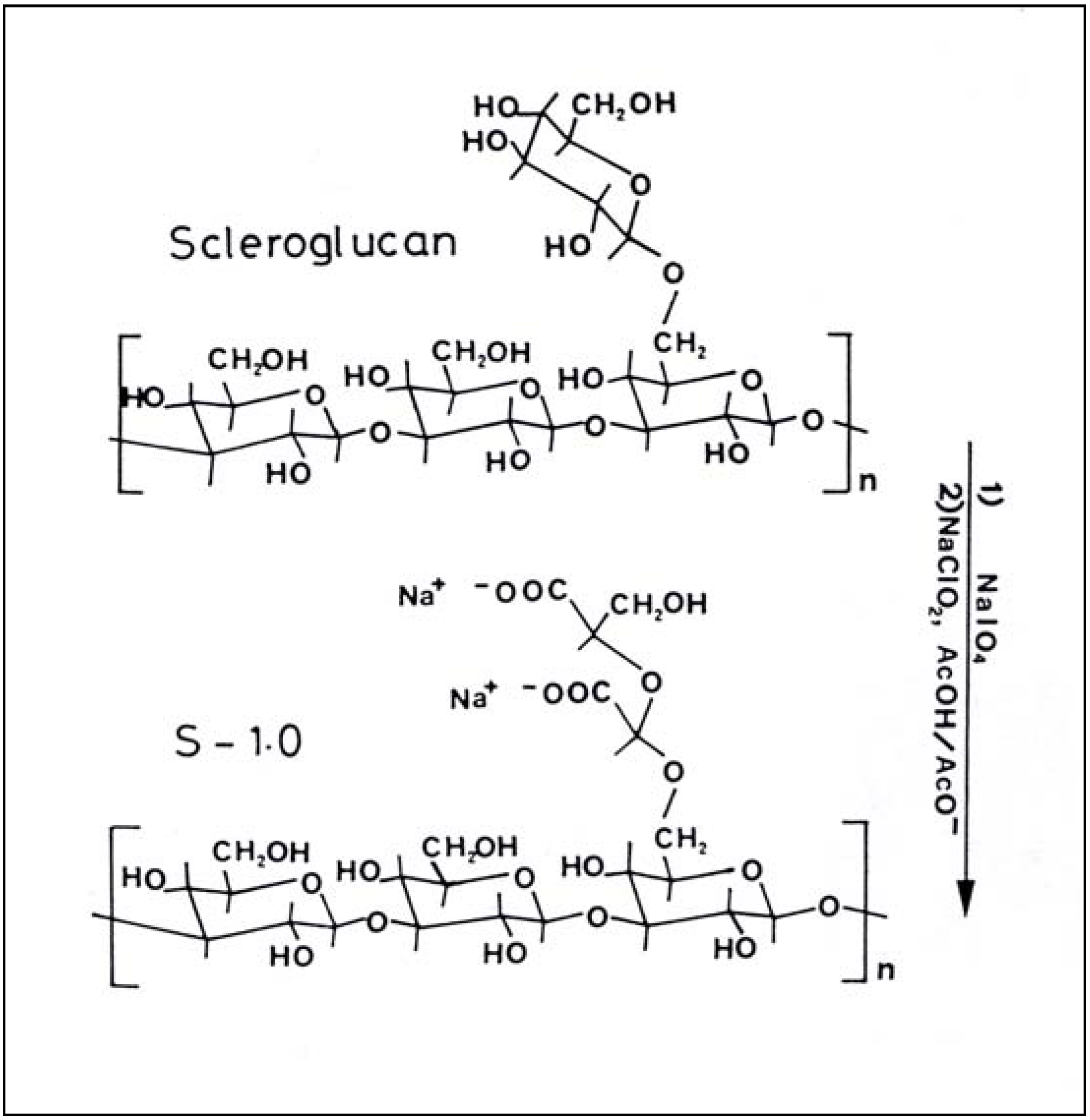
Oxidized Scleroglucan
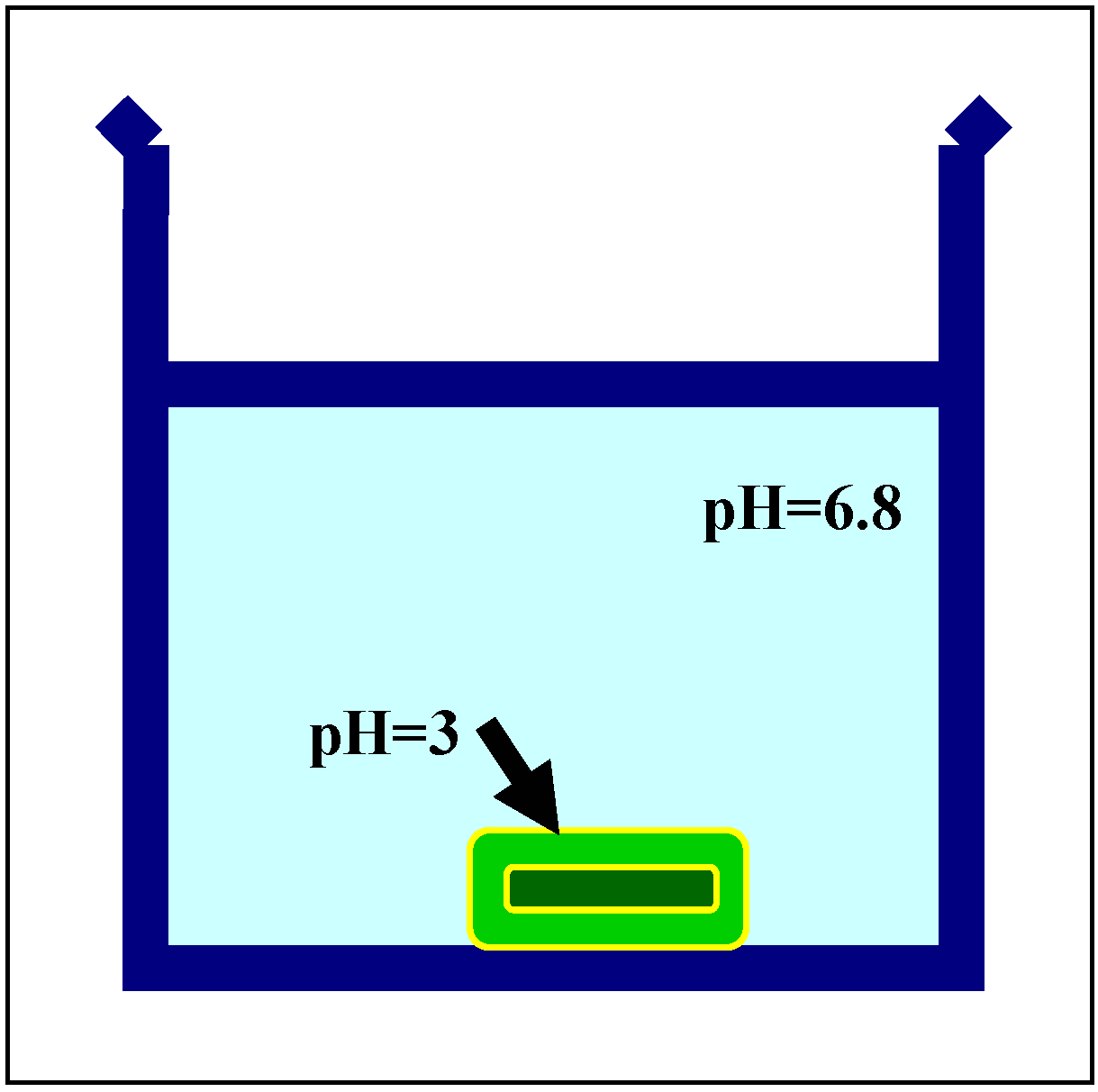
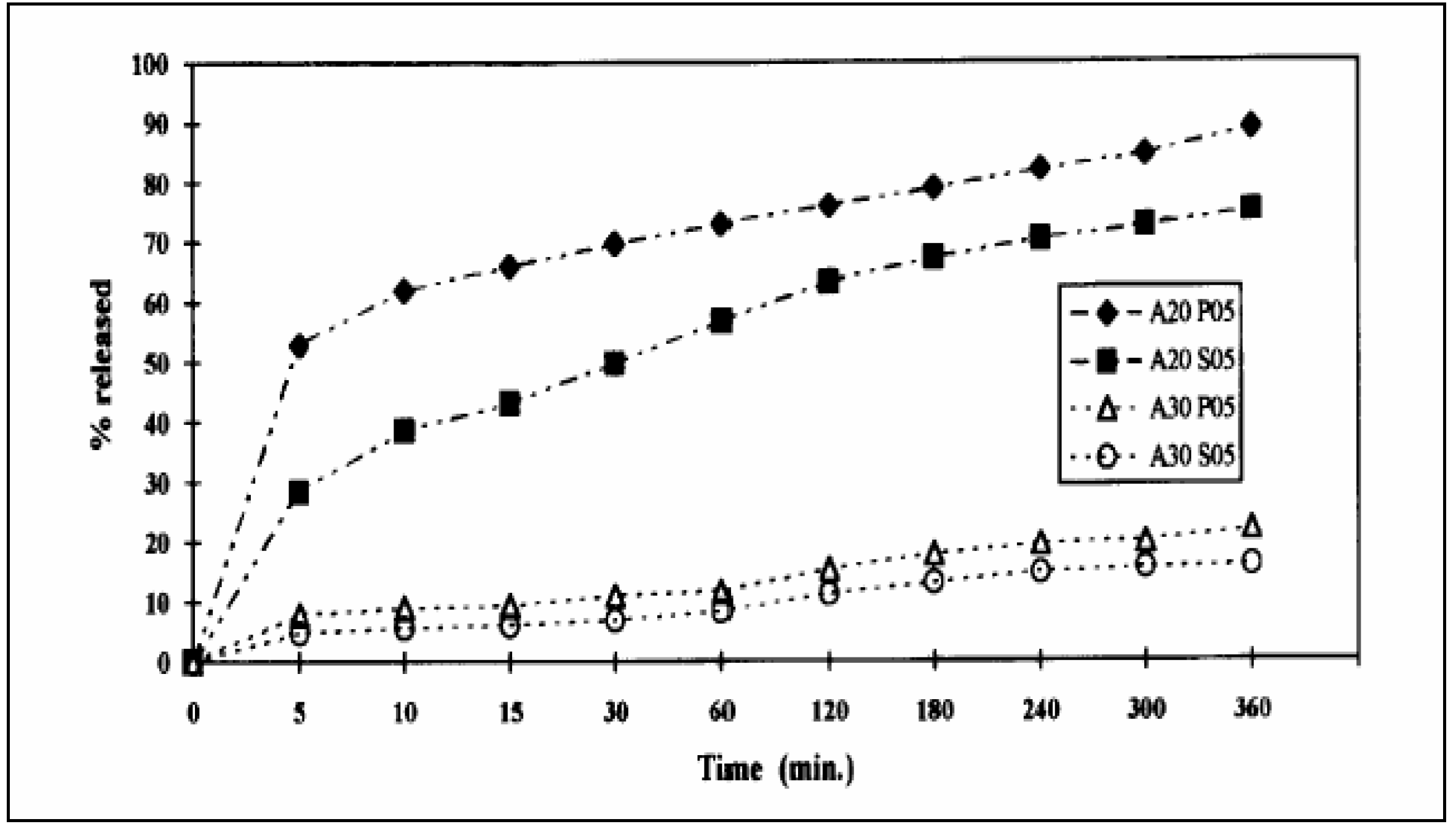
Crosslinked Scleroglucan
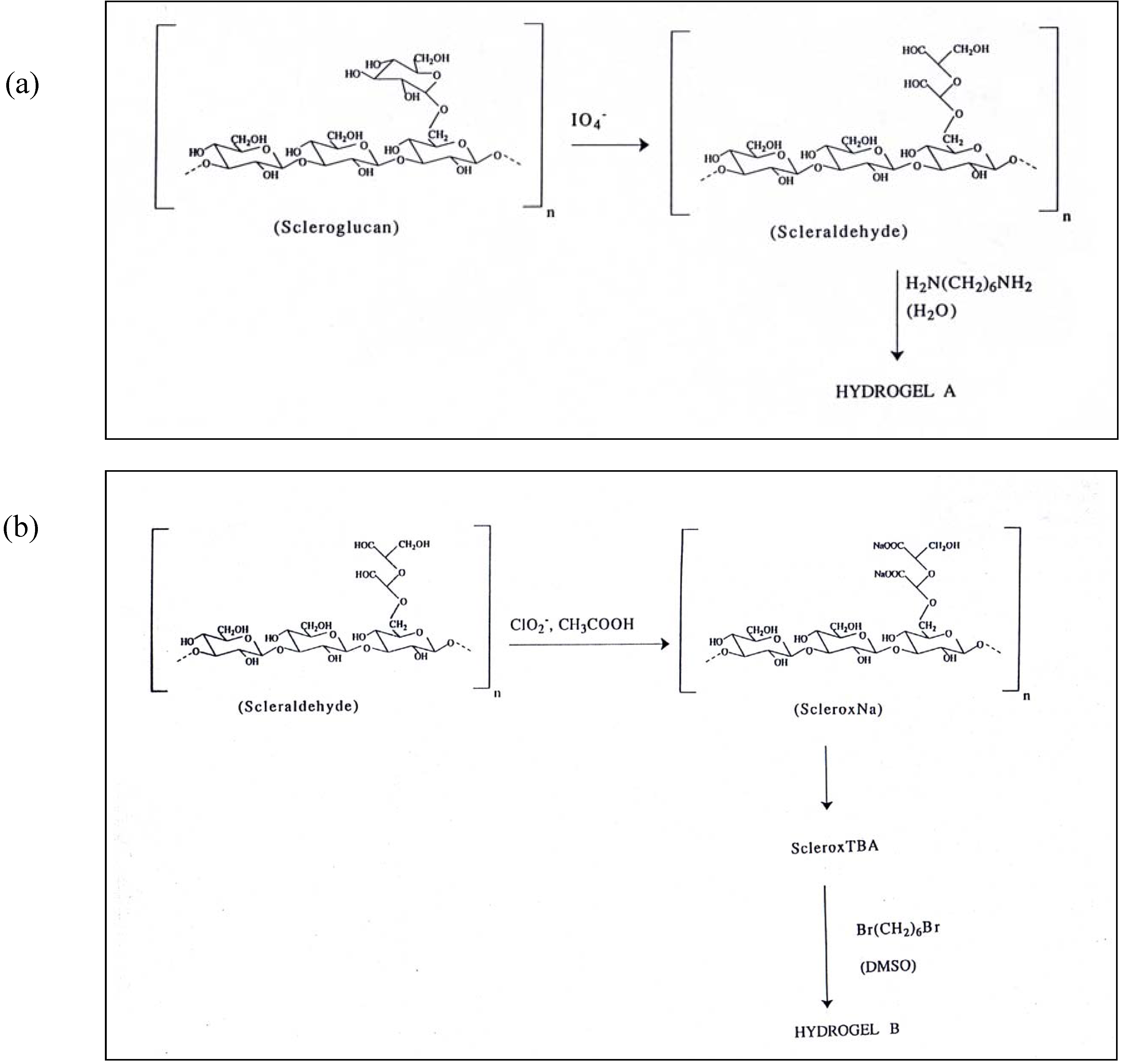

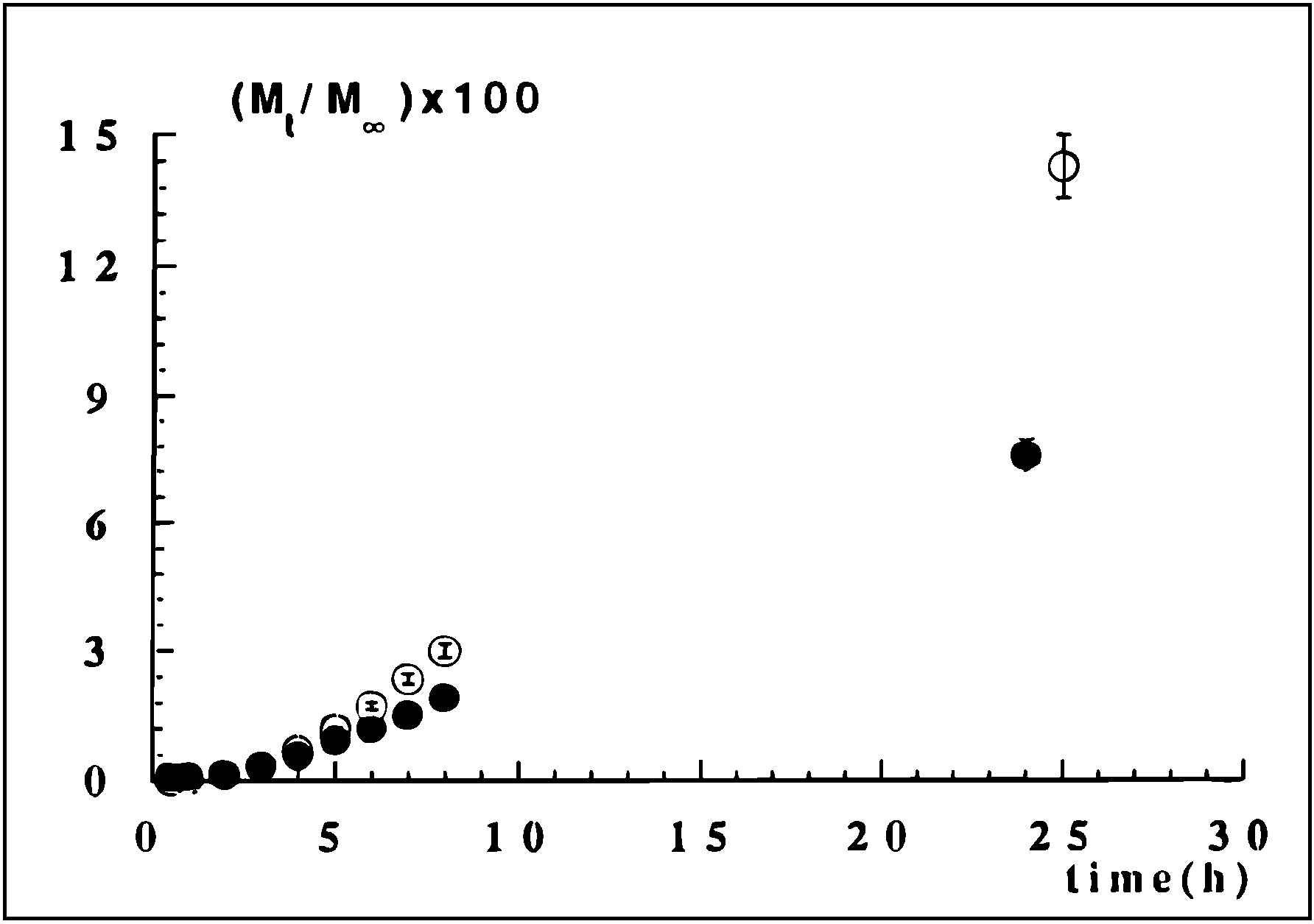
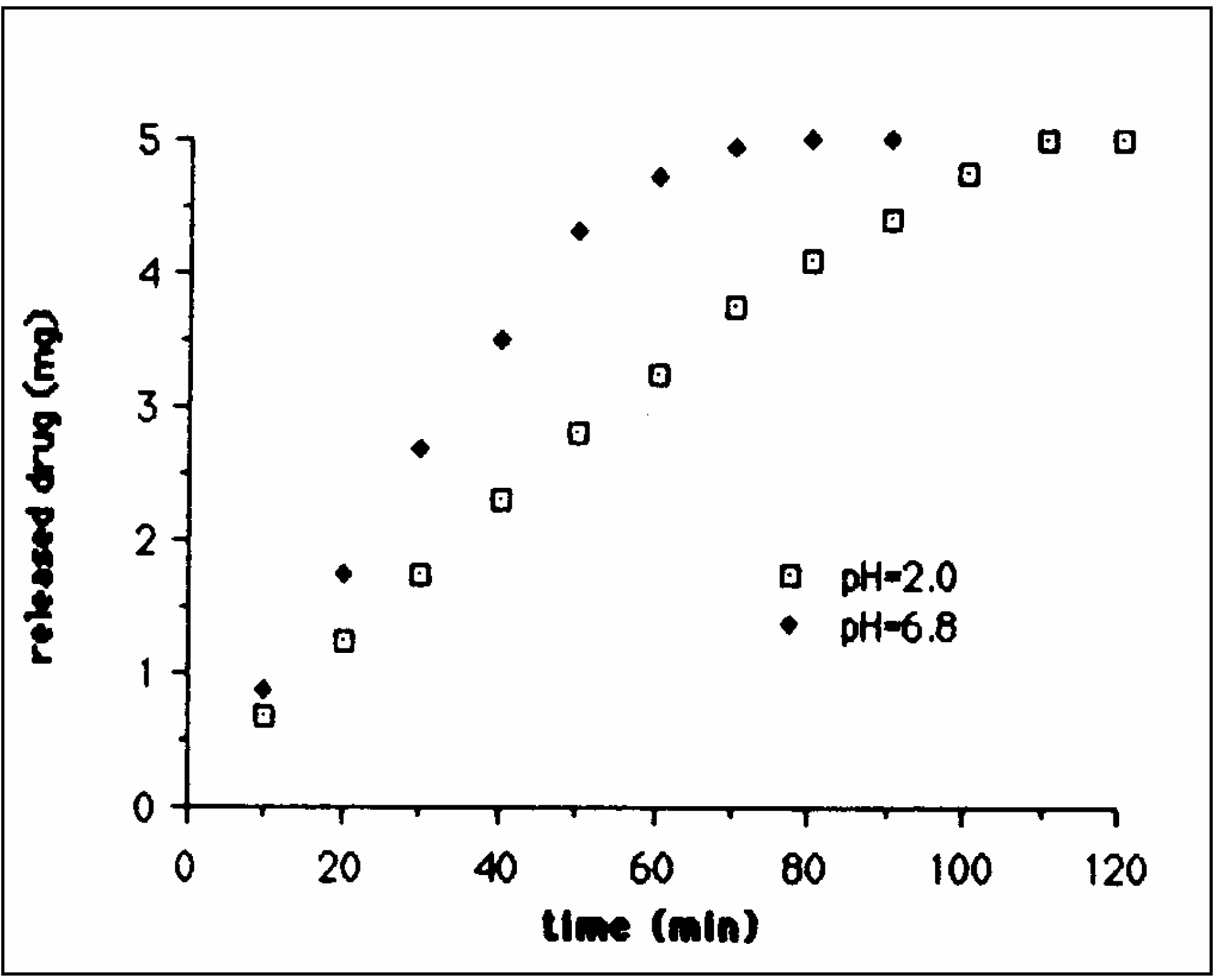
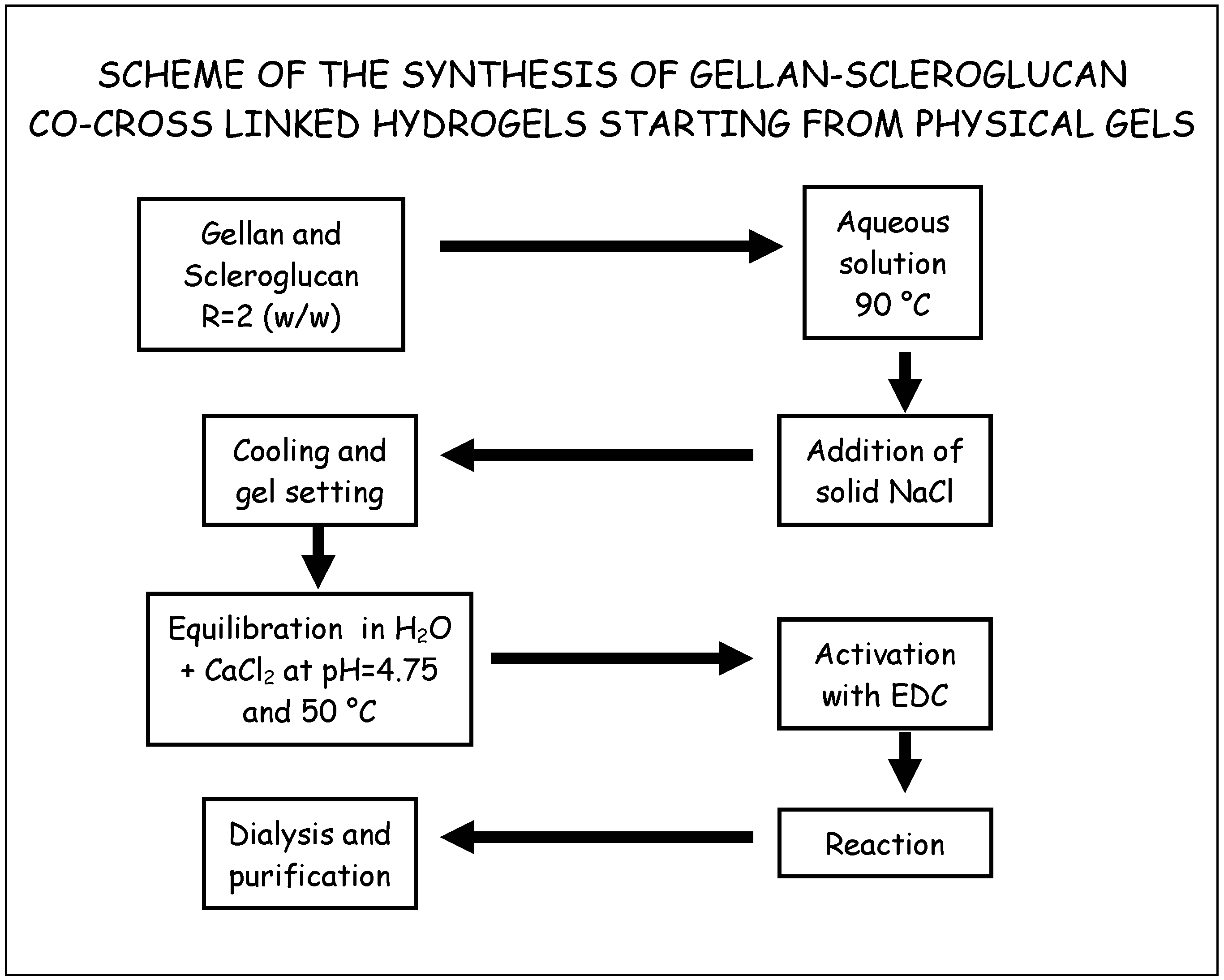
Co-crosslinked Scleroglucan


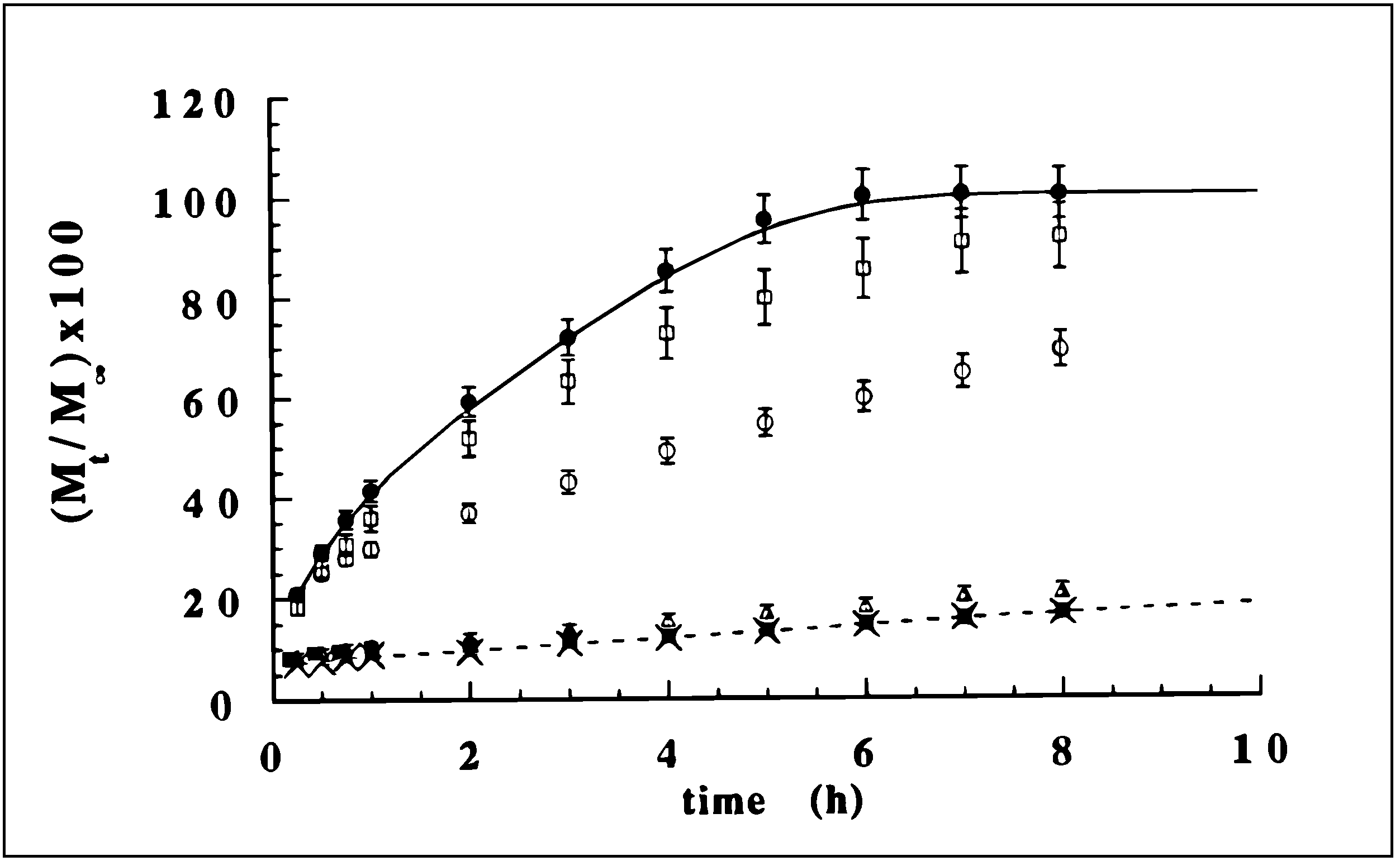
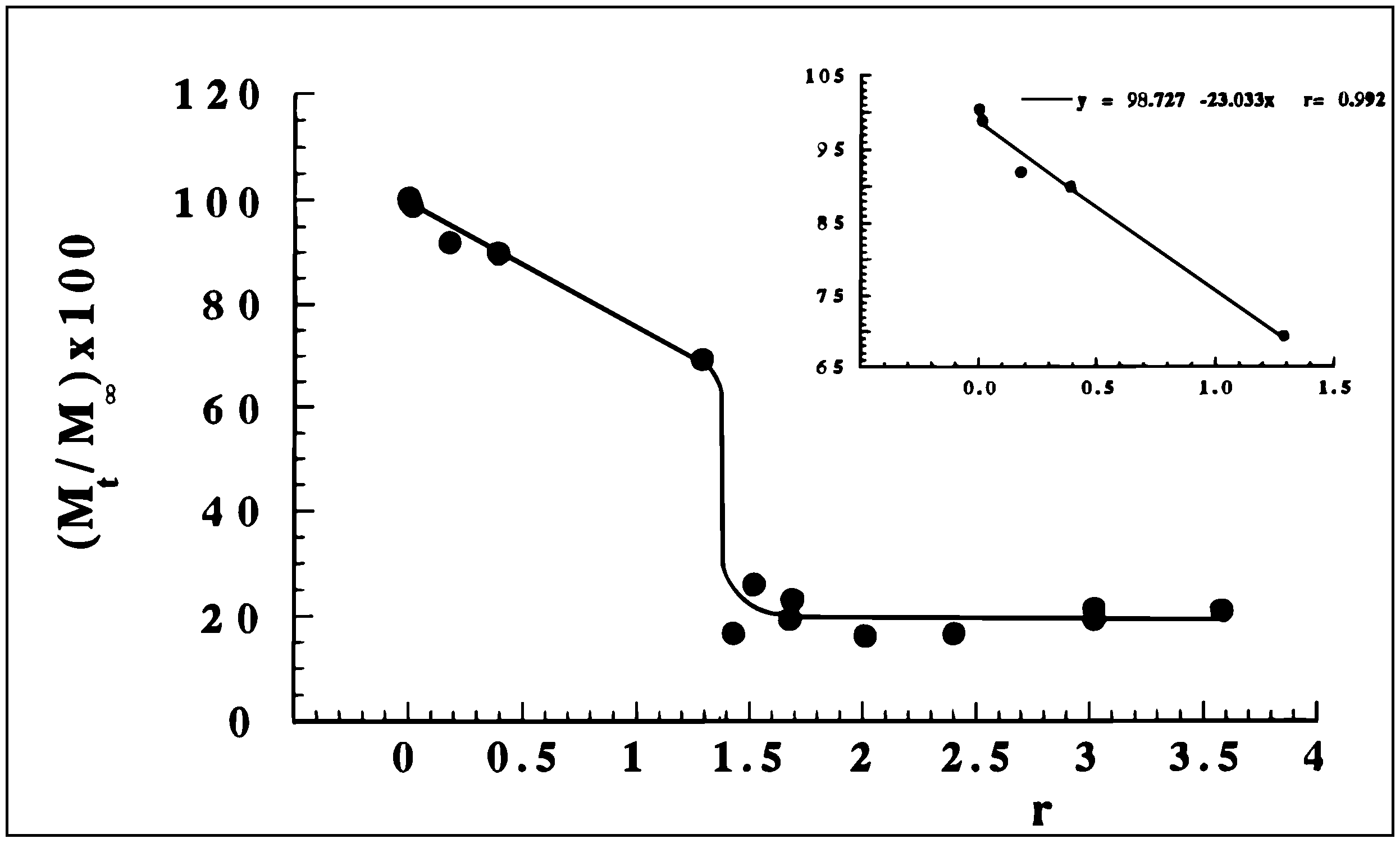
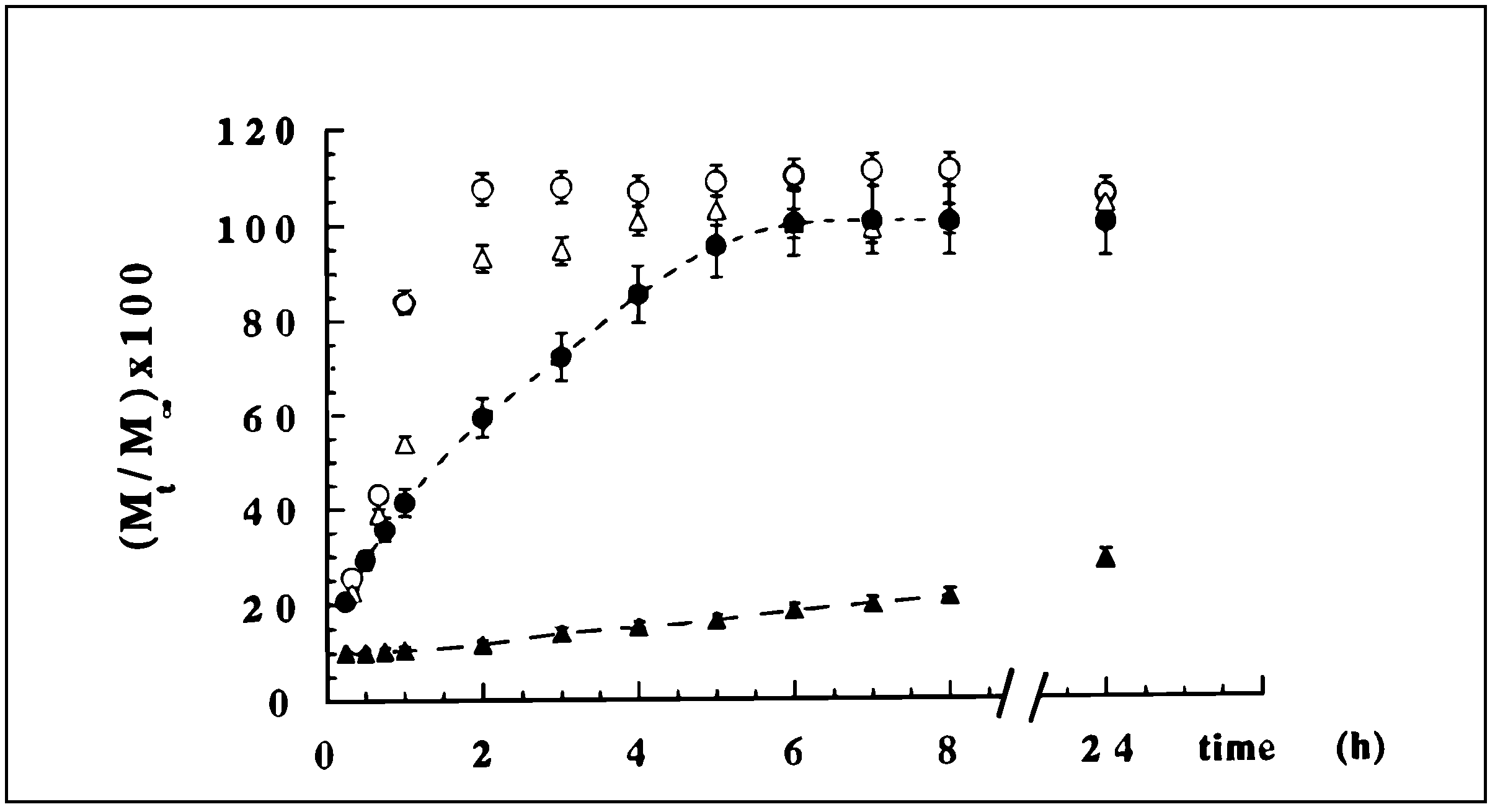
Scleroglucan/borax Hydrogel
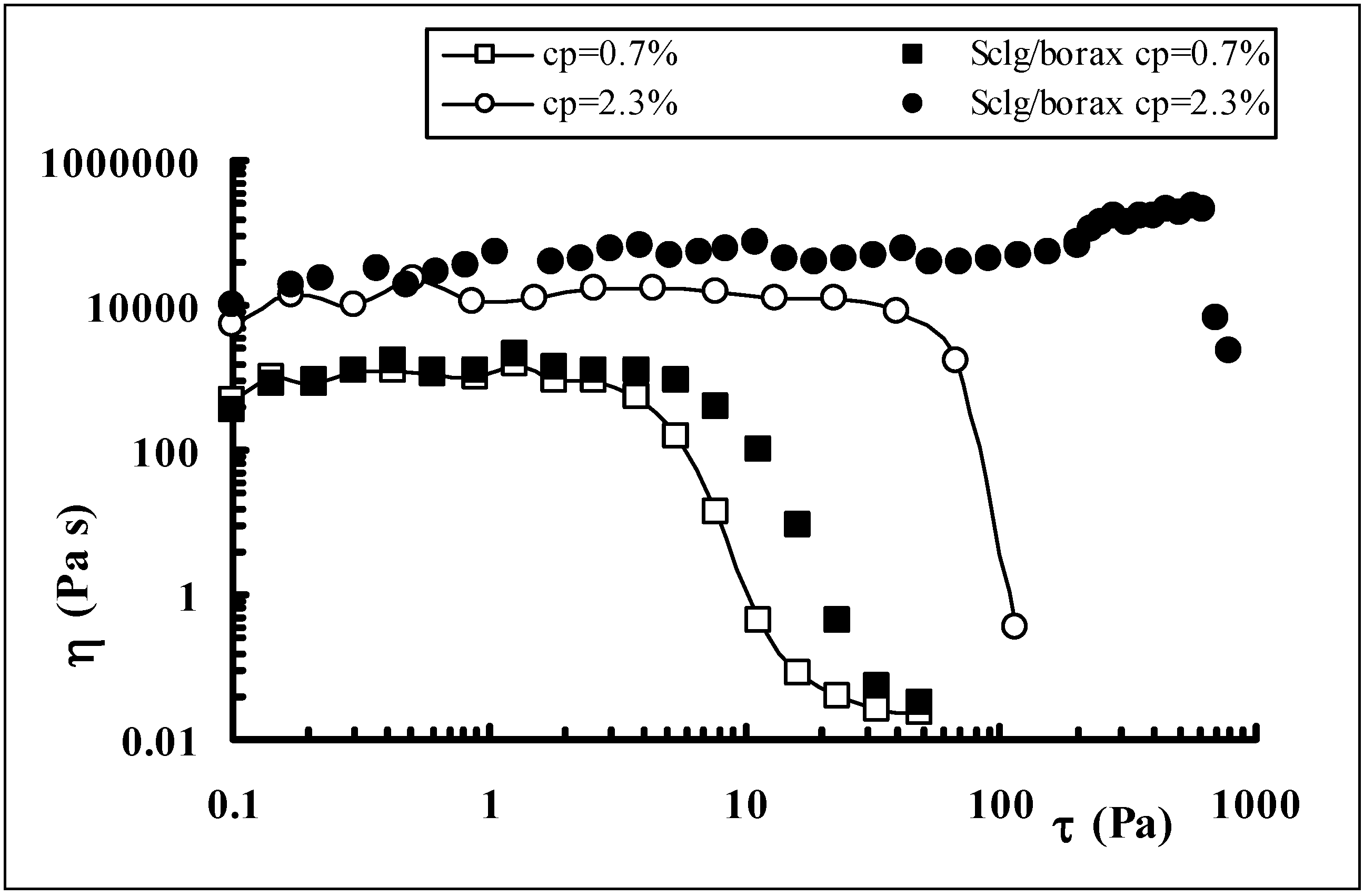
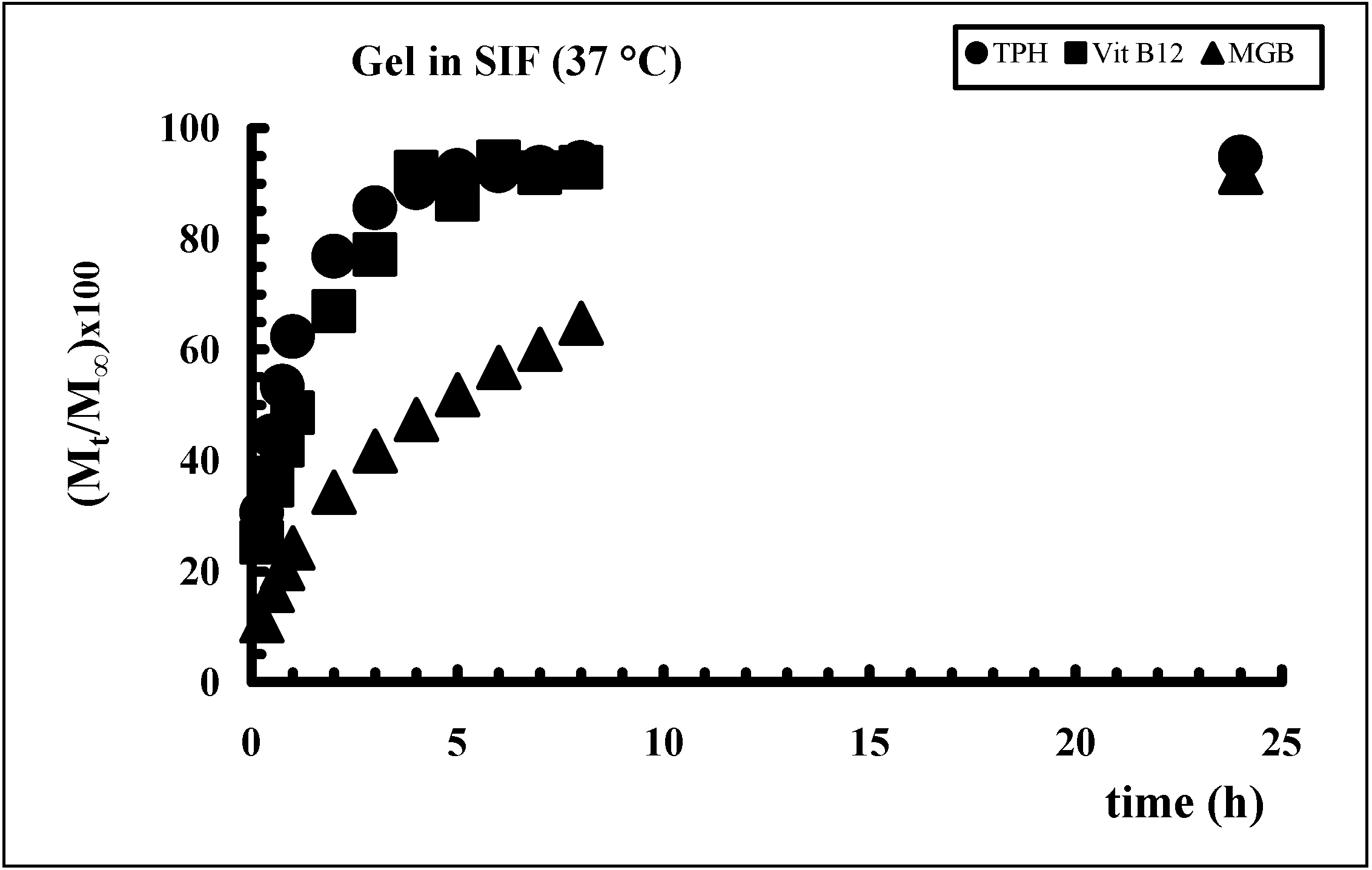
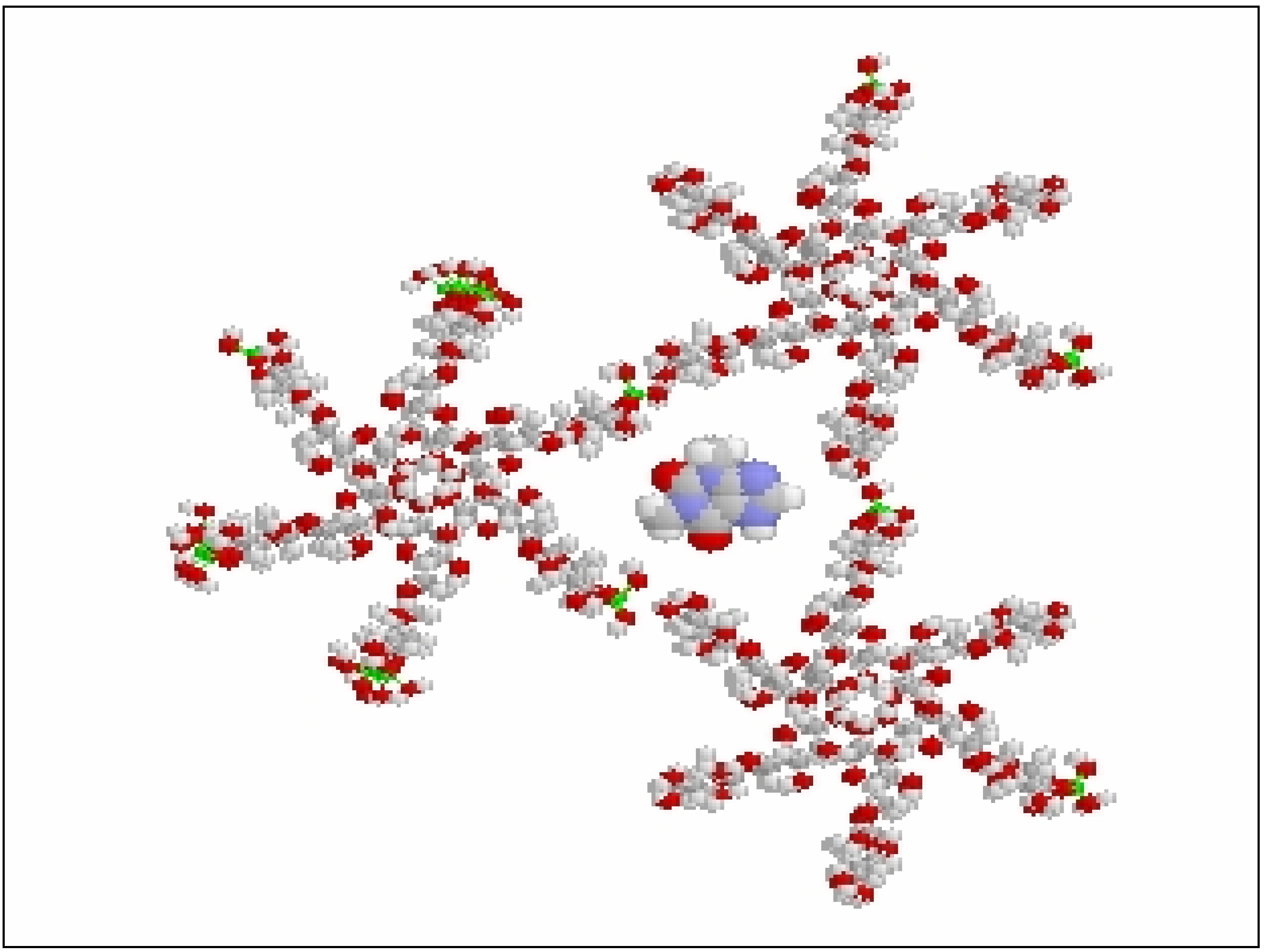
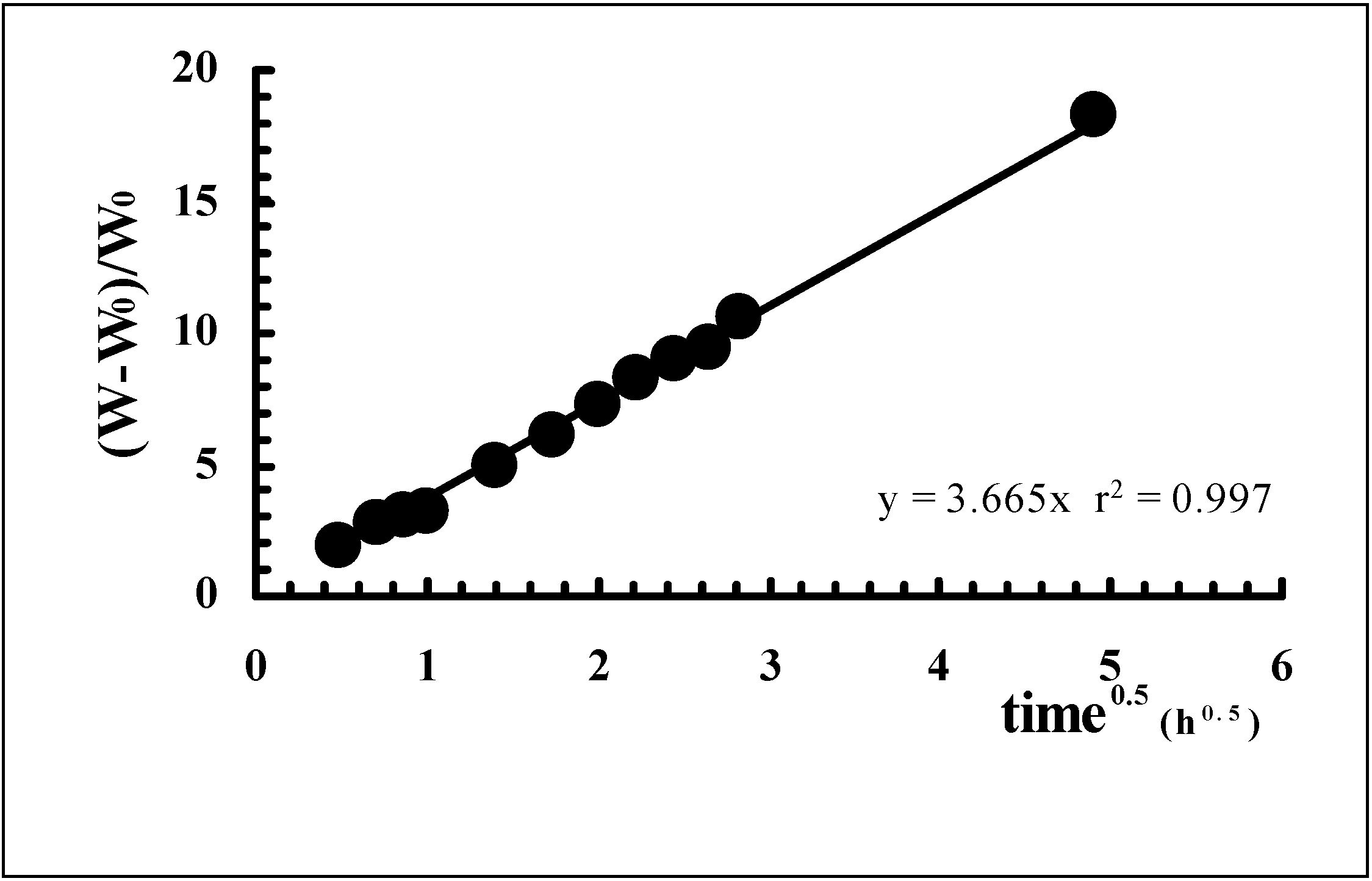
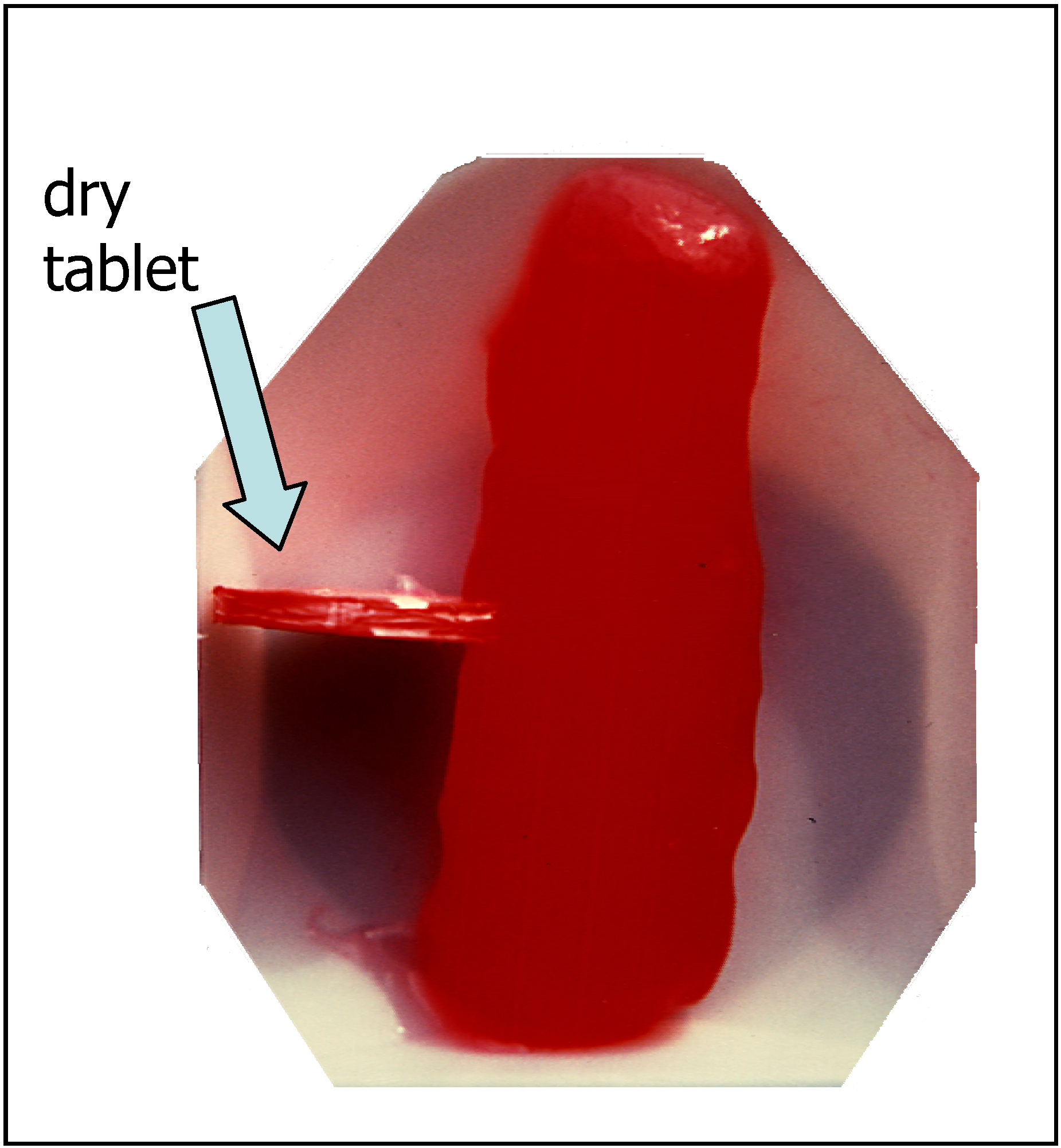
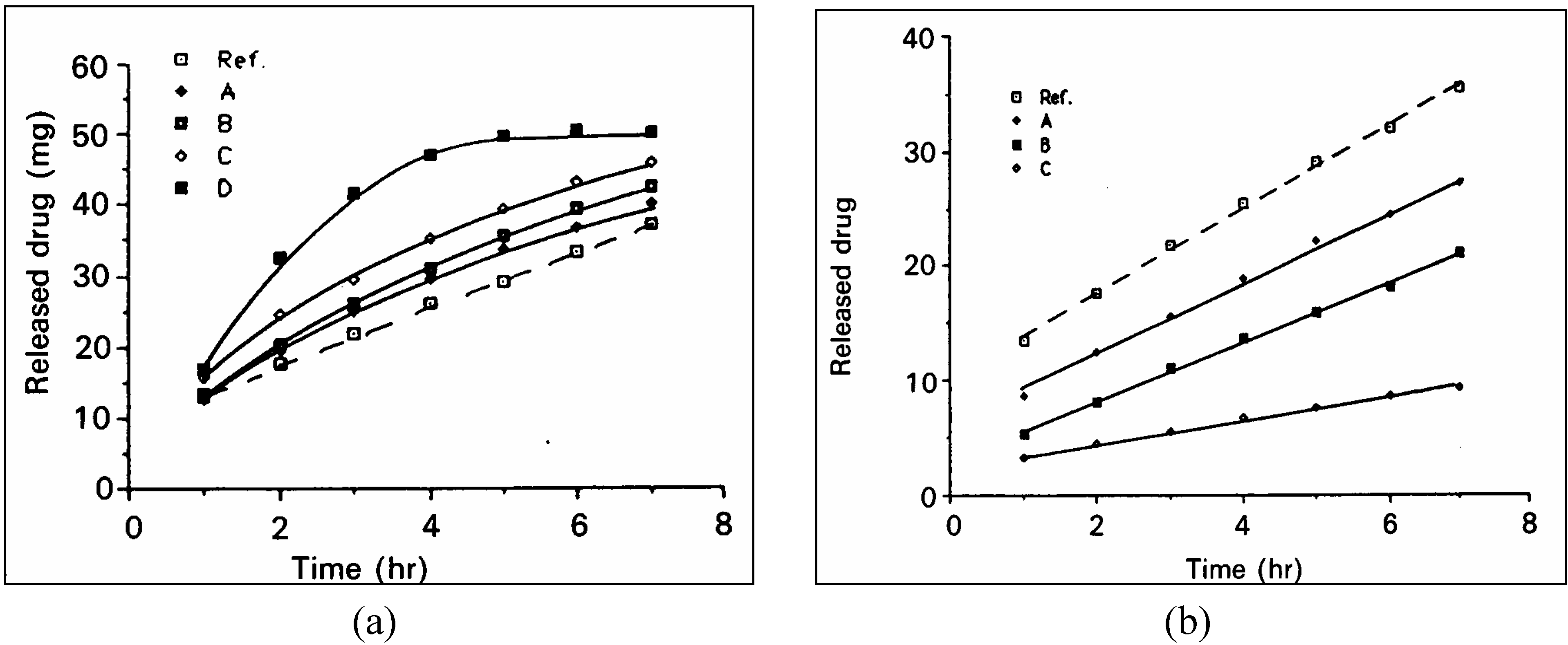
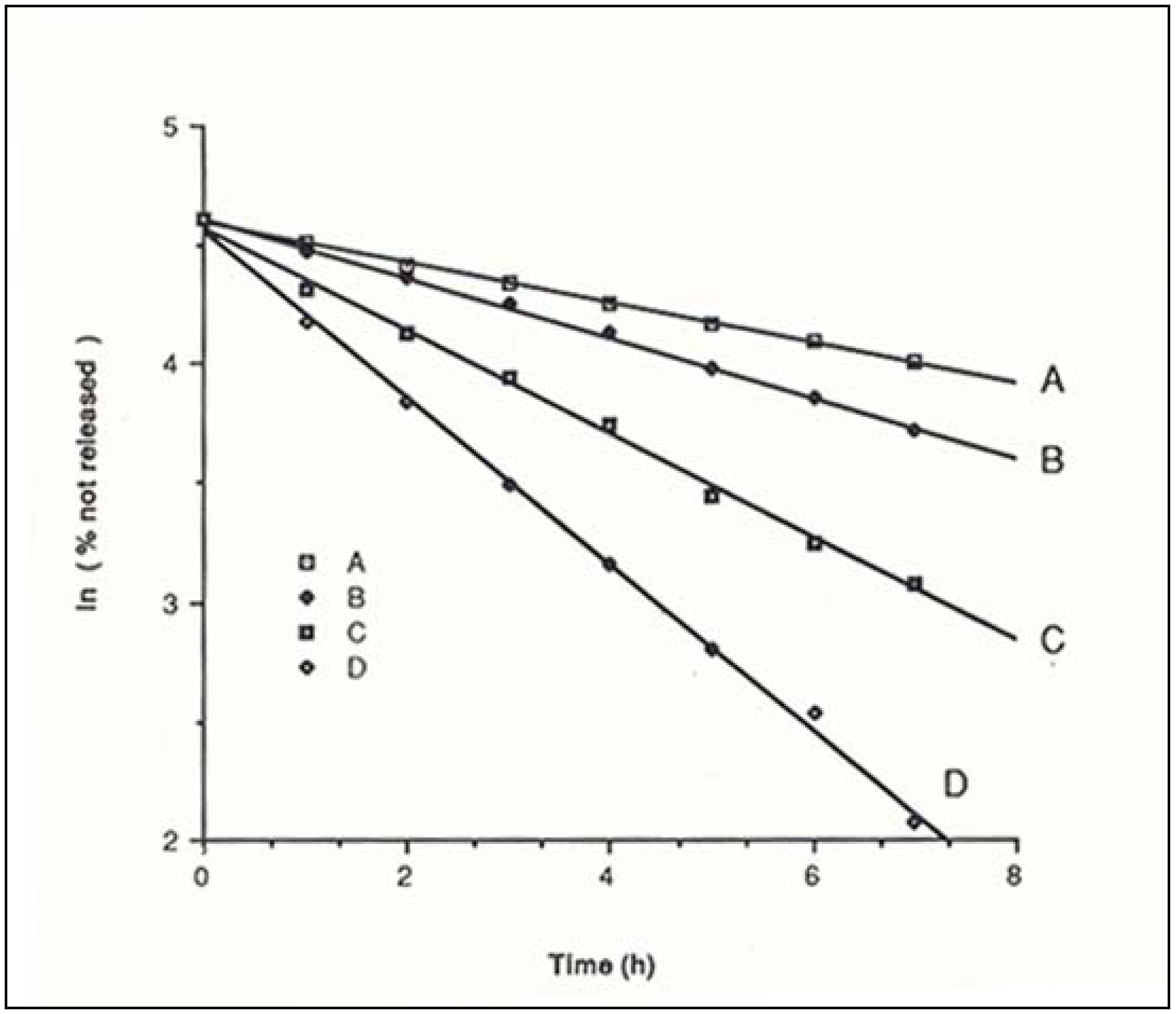
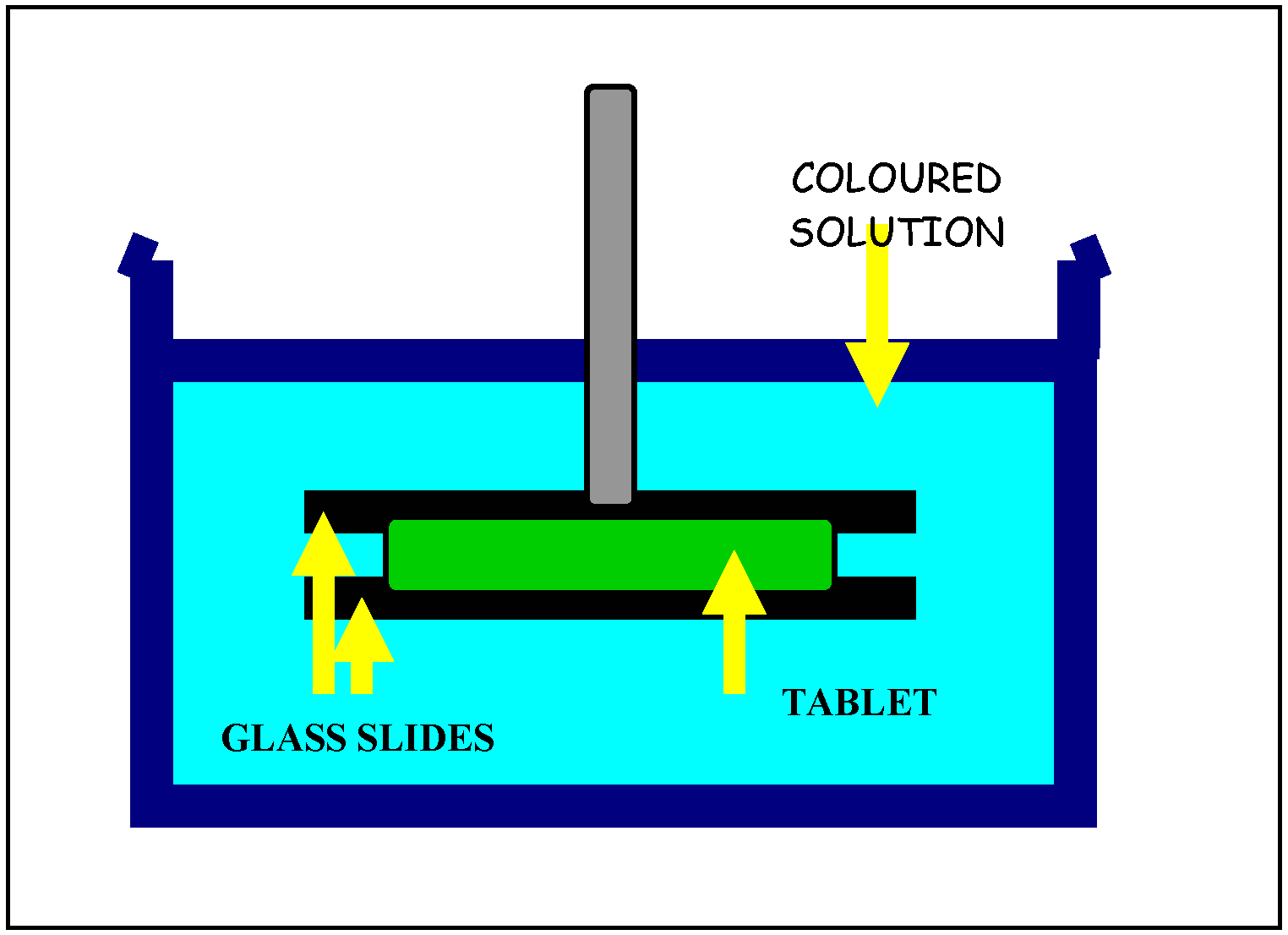
Drug release from polymeric matrices

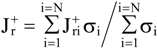
Conclusions
References
- Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug. Deliv. Rev. 2002, 43, 3. [Google Scholar]
- Peppas, N. A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 43, 53. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L. M.; McNeil, B. Biopolymers; De Baets, S., Vandamme, E. J., Steinbüchel, A., Eds.; Wiley-VCH: Weinheim, 2002; Vol. 6, Chapter 2; p. 37. [Google Scholar]
- Halleck, F. E. Polysaccharides and methods for production thereof. U.S. Patent 3,301,848, 1967. [Chem. Abstr. 1967, 66, 84772b]. [Google Scholar]
- Doster, M. S.; Nute, A. J.; Christopher, C. A. Recovering petroleum from underground formations. U.S. Patent 4,457,372; Chem. Abstr. 1984, 101, 113713g, 1984. [Google Scholar]
- Pirri, R.; Gadioux, J.; Riveno, R. Scleroglucan gel applied to the oil industry. Societé Nationale Elf Aquitaine (Courbevoie, France) 5,555,936, 1996. [Google Scholar]
- Halleck, F. E. Paint composition containing polysaccharides. U.S. Patent 3,447,940, 1969. [Chem. Abstr. 1969, 100, 51304a; Fed. Reg, 1969, 34, 13162]. [Google Scholar]
- Halleck, F. E. Wave set composition containing a polysaccharides. U.S. Patent 3,507,290, 1970. [Chem. Abstr. 1970, 73, 28775n]. [Google Scholar]
- Halleck, F. E. Cosmetic composition employing water-soluble polysaccharide. U.S. Patent 3,659,025, 1972. [Chem. Abstr. 1972, 77, 52227p]. [Google Scholar]
- Dubief, C. Composition for washing keratinous materials, in particular hair and/or skin. L’Oréal (Paris, France) 1996. 5,536,493 patent? country?. [Google Scholar]
- Duc, A. N. C. Glucosylglucans and their use in gastroenterology, especially in the treatment of colon disorders. Eur. Patent 45,338, 1982. [Chem. Abstr. 1982, 96, 168769p]. [Google Scholar]
- Sheth, P.; Lachman, L. The coating of tablets. Fr. Patent 1,480,874, 1967. [Chem. Abstr. 1967, 67, 111442y]. [Google Scholar]
- San-Ei Chemical Industries, Ltd. Improvement of frozen food quality with sclerogum. Japan Kokai Tokkyo Koho 57,163,451, 1982. [Chem. Abstr. 1983, 98, 15728r]. [Google Scholar]
- San-Ei Chemical Industries, Ltd. Improvement of quality of Japanese cake with sclerogum. Japan Kokai Tokkyo Koho 57,163,442, 1982. [Chem. Abstr. 1983, 98, 15729s]. [Google Scholar]
- San-Ei Chemical Industries, Ltd. Improvement of steamed food quality with sclerogum. Japan Kokai Tokkyo Koho 163,163,441, 1982. [Chem. Abstr. 1983, 98, 15730k]. [Google Scholar]
- San-Ei Chemical Industries, Ltd. Rice cracker quality improvement with sclerogum. Japan Kokai Tokkyo Koho 57,163,440, 1982. [Chem. Abstr. 1983, 98, 15731m]. [Google Scholar]
- San-Ei Chemical Industries, Ltd. Quality improvement of bakery products with sclerogum. Japan Kokai Tokkyo Koho 57,163,432, 1982. [Chem. Abstr. 1983, 98, 15732n]. [Google Scholar]
- Singh, P.; Wisler, R.; Tokuzen, R.; Nakahara, W. Scleroglucan, an antitumor polysaccharide from Sclerotium glucanicum. Carbohyd. Res. 1974, 37, 245. [Google Scholar] [CrossRef]
- Jong, S.; Donovick, R. Antitumor and antiviral substances from fungi. Adv. Appl. Microbiol. 1989, 34, 183. [Google Scholar] [PubMed]
- Prets, H.; Eusley, H.; McNamee, R.; Jones, E.; Browder, I.; Williams, D. Isolation, physicochemical characterisation and pre-clinical efficiacy evaluation of a soluble Scleroglucan. J. Pharmacol. Exp. Ther. 1991, 257, 500. [Google Scholar] [PubMed]
- Mastromarino, P.; Petruzziello, R.; Macchia, S.; Rieti, S.; Nicoletti, R.; Orsi, N. Antiviral activity of natural and semisynthetic polysaccharides on early steps of rubella virus infection. J. Antimicrob. Chemother. 1997, 39, 339. [Google Scholar] [CrossRef]
- Patchen, L.; Bleicher, P. Mobilisation of peripheral blood precursor cells by beta (1,3)-glucan. The Collaborative Group, Ltd. (New York, USA) 2000. 6,117,850. US patent?. [Google Scholar]
- Bluhm, T. L.; Deslandes, Y.; Marchessault, R. H.; Perez, S.; Rinaudo, M. Solid-state and solution conformations of Scleroglucan. Carbohyd. Res. 1982, 100, 117. [Google Scholar] [CrossRef]
- Yanaki, T.; Kojima, T.; Norisuye, T. Triple helices in dilute aqueous sodium hydroxide. Polymer J. 1981, 13, 1135. [Google Scholar] [CrossRef]
- Yanaki, T.; Norisuye, T. Triple helix and random coil Scleroglucan in dilute solution. Polymer J. 1983, 15, 389. [Google Scholar] [CrossRef]
- Lovreicich, M.; Riccioni, G. Pharmaceutical tablets and capsule granulates of Scleroglucan and active substance. Vectorpharma International S.P.A. (Trieste, Italy). 1991. 5,068,111. US patent?. [Google Scholar]
- Lovreicich, M.; Riccioni, G. Pharmaceutical tablets and capsule granulates of Scleroglucan and active substance. Vectorpharma International S.P.A. (Trieste, Italy). 1993. 5,215,752. US patent?. [Google Scholar]
- Touitou, E.; Alhaique, F.; Riccieri, F. M.; Riccioni, G.; Santucci, E. Scleroglucan sustained release oral preparations. Part I. In vitro experiments. Drug Des. Deliv. 1989, 5, 141. [Google Scholar]
- Rizk, S.; Duru, C.; Gaudy, D.; Jacob, M.; Ferrari, F.; Bertoni, M.; Caramella, C. Physico-chemical characterization and tabletting properties of Scleroglucan. Int. J. Pharmac. 1994, 112, 125. [Google Scholar] [CrossRef]
- Alhaique, F.; Beltrami, E.; Riccieri, F. M.; Santucci, E. Scleroglucan sustained release oral preparations. Part II. Effects of additives. Drug Des. Deliv. 1990, 5, 249. [Google Scholar] [CrossRef]
- Bamba, M.; Puisieux, F.; Marty, J. P.; Carstensen, J. T. Release mechanisms in gel forming sustained release preparations. Int. J. Pharm. 1979, 2, 307. [Google Scholar] [CrossRef]
- Alhaique, F.; Carafa, M.; Riccieri, F. M.; Santucci, E.; Touitou, E. Studies on the release behavior of a polysaccharide matrix. Pharmazie 1993, 48, 432. [Google Scholar]
- Rizk, S.; Guyot, J.C.; Duru, C.; Gaudy, D. Influence of lubricant properties on compression behavior and drug dissolution rate of Scleroglucan hydrophilic matrix. Int. J. Pharmac. 1995, 126, 57. [Google Scholar] [CrossRef]
- Hofreiter, B. T.; Wolff, I. A.; Mehltretter, C. L. Chlorus acid oxidation of periodate oxidized cornstarch. J. Am. Chem. Soc. 1957, 79, 6457. [Google Scholar] [CrossRef]
- Alhaique, F.; Riccieri, F. M.; Santucci, E.; Carafa, M. Environmental effects on the delivery of drugs from a pH-sensitive matrix. Acta Technol. Legis Med. 1990, 1, 1. [Google Scholar]
- Maeda, H.; Coviello, T.; Yuguchi, Y.; Urakawa, H.; Rambone, G.; Alhaique, F.; Kajiwara, K. Structural characteristics of oxidized Scleroglucan and its network. Polym. Gels Netw. 1998, 6, 355. [Google Scholar] [CrossRef]
- Maeda, H.; Rambone, G.; Coviello, T.; Yuguchi, Y.; Urakawa, H.; Alhaique, F.; Kajiwara, K. Low-Degree Oxidized Scleroglucan and Its Hydrogel. Int. J. Biol. Macromol. 2001, 28, 351. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Maeda, H.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K.; Dentini, M.; Crescenzi, V. Conformational Characteristics of Oxidized Scleroglucan. Macromolecules 1998, 31, 1602. [Google Scholar] [CrossRef]
- Ichikawa, H.; Fukumori, Y. A novel positively thermosensitive controlled-release microcapsule with membrane of nano-sized poly(N-isopropylacrylamide) gel dispersed in ethylcellulose matrix. J. Control. Release 2000, 63, 107. [Google Scholar]
- Jones, D. S. Dynamic mechanical analysis of polymeric systems of pharmaceutical and biomedical significance. Int. J. Pharm. 1999, 179, 167. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lugo, M.; Peppas, N. A. Molecular Design and in Vitro Studies of Novel pH-Sensitive Hydrogels for the Oral Delivery of Calcitonin. Macromolecules 1999, 32, 6646. [Google Scholar] [CrossRef]
- Siegel, R. A.; Falamarzian, M.; Firestone, B. A.; Moxley, B. C. pH-Controlled release from hydrophobic/polyelectrolyte copolymer hydrogels. J. Control. Release, 1988, 8, 179. [Google Scholar]
- Eichenbaum, G. M.; Kiser, P. F.; Simon, S. A.; Needham, D. pH and Ion-Triggered Volume Response of Anionic Hydrogel Microspheres. Macromolecules 1998, 31, 5084. [Google Scholar] [CrossRef]
- Coviello, T.; Grassi, M.; Rambone, G.; Santucci, E.; Carafa, M.; Murtas, E.; Riccieri, F. M.; Alhaique, F. Novel hydrogel system from scleroglucan: synthesis and characterization. J. Controlled Rel. 1999, 60, 367. [Google Scholar]
- Coviello, T.; Grassi, M.; Rambone, G.; Alhaique, F. A crosslinked system from Scleroglucan derivative: preparation and characterization. Biomaterials 2001, 22, 1998. [Google Scholar] [CrossRef]
- Grasdalen, H.; Smidsrød, O. Gelation of Gellan gum. Carbohyd. Polym. 1987, 7, 371. [Google Scholar] [CrossRef]
- Crescenzi, V.; Dentini, M.; Coviello, T. Solution and gelling properties of microbial polysaccharides of industrial interest: the case of Gellan. In Novel Biodegradable Microbial Polymers; Dawes, E.A., Ed.; NATO ASI Series E: Applied Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; vol. 186, p. 277. [Google Scholar]
- Morikata, H.; Fukuba, H.; Kumeno, K.; Nakahama, N.; Nishinari, K. Effect of monovalent and divalent cations on the rheological properties of gellan gels. Food Hydrocolloid. 1991, 4, 495. [Google Scholar] [CrossRef]
- Coviello, T.; Dentini, M.; Rambone, G.; Desideri, P.; Carafa, M.; Murtas, E.; Riccieri, F. M.; Alhaique, F. A novel co-crosslinked polysaccharide: studies for a controlled delivery matrix. J. Controlled Rel. 1998, 55, 57. [Google Scholar] [CrossRef]
- Deuel, H.; Neukom, H.; Weber, F. Reaction of boric acid with polysaccharides. Nature 1948, 161, 96. [Google Scholar] [CrossRef]
- Shibayama, M.; Yoshizawa, H.; Kurokawa, H.; Fujiwara, H.; Nomura, S. Sol-gel transition of poly(vinyl alcohol)-borate complex. Polymer 1988, 29, 2066. [Google Scholar] [CrossRef]
- Davis, H. B.; Mott, C. J. B. Interaction of boric acid and borates with carbohydrates and related substances. J. Chem. Soc. Faraday I 1980, 76, 1991. [Google Scholar] [CrossRef]
- Verchere, J. F.; Hlaibi, M. Stability constants of borate complexes of oligosaccharides. Polyhedron 1987, 6, 1415. [Google Scholar] [CrossRef]
- Sandford, P. Exocellular, microbial polysaccharides. Adv. Carbohydr. Chem. Biochem. 1979, 36, 265. [Google Scholar] [PubMed]
- Brigand, G. Industrial Gums; Whistler, R. L., BeMiller, J. N., Eds.; Academic Press: New York, 1993; Chapter 17. [Google Scholar]
- Coviello, T.; Grassi, M.; Lapasin, R.; Marino, A.; Alhaique, F. Scleroglucan/borax: characterization of a novel hydrogel system suitable for drug delivery. Biomaterials 2003, 24, 2789. [Google Scholar] [PubMed]
- Coviello, T.; Coluzzi, G.; Palleschi, A.; Grassi, M.; Santucci, E.; Alhaique, F. Structural and rheological characterization of Scleroglucan/borax hydrogel for drug delivery. Int. J. Biol. Macromol. 2003, 32, 38. [Google Scholar] [CrossRef]
- Colombo, I.; Grassi, M.; Fermeglia, M.; Lapasin, R.; Pricl, S. Modeling phase transitions and sorption-desorption kinetics in thermo-sensitive gels for controlled drug delivery systems. Fluid Phase Equilibr. 1996, 116, 148. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; De Ascentiis, A.; Peppas, N. A. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug and water transport. J. Control. Release 1996, 39, 231. [Google Scholar] [CrossRef]
- Peppas, N.A.; Korsmeyer, R.W. In Controlled Release Applications, Hydrogels in Medicine and Pharmacy: Volume III Properties and Applications; Peppas, N. A., Ed.; CRC Press, Inc.: Boca Raton, FL, 1987; p. 110. [Google Scholar]
- Lee, P. I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277. [Google Scholar] [CrossRef]
- Vrentas, J. S.; Jarzebski, C. M.; Duda, J. L. A Deborah Number for Diffusion in Polymer-Penetrant Systems. AIChE J. 1975, 21, 1. [Google Scholar] [CrossRef]
- Vrentas, J. S.; Duda, J. L. Molecular Diffusion in Polymer Solutions. AIChE J. 1979, 25, 1. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, second edition; Clarendon Press: Oxford, 1975; p. 254. [Google Scholar]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccharides. Theory and Applications; Chapman & Hall: London, 1995. [Google Scholar]
- Ritger, P.L.; Peppas, N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N. A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Astarita, G. Diffusion-relaxation coupling in polymers which show two-stage sorption phenomena. Polymer 1979, 20, 455. [Google Scholar]
- Adib, F.; Neogi, P. Sorption with oscillations in solid polymers. AIChE J. 1987, 33, 164. [Google Scholar] [CrossRef]
- Cohen, D. S. Sharp Fronts Due to Diffusion and Stress at the Glass Transition in Polymers. J. Polym. Science: Part B: Polymer Physics. 1989, 27, 1731. [Google Scholar] [CrossRef]
- Lustig, S. R.; Caruthers, J. M.; Peppas, N. A. Continuum Thermodynamics and Transport Theory for Polymer-Fluid Mixtures. Chem. Engineering Sci. 1992, 47, 3037. [Google Scholar] [CrossRef]
- Hariharan, D.; Peppas, N. A. Modelling of water transport and solute release in physiologically sensitive gels. J. Control. Release 1993, 23, 123. [Google Scholar] [CrossRef]
- Edwards, D. A.; Cohen, D. S. A Mathematical Model for a Dissolving Polymer. AIChE J. 1995, 41, 2345. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Sarti, G. C. Mass Transport with Relaxation in Polymers. AIChE J. 1990, 36, 851. [Google Scholar]
- Grassi, M.; Lapasin, R.; Pricl, S. Modeling of drug release from a swellable matrix. (1998). Chem. Eng. Comm. 169, 79–109. [Google Scholar]
- Grassi, M. Modeling of penetrant uptake in viscoelastic polymeric matrices. In Proceedings of the Joint Conference of Italian, Austrian, Slovenian Rheologists, Trieste (Italy), 1998, 20 – 23 May; pp. 91–96.
- Grassi, M. Diffusione di principi attivi in matrici gel polimeriche di impiego farmaceutico. PhD. Thesis, Dept. of Chem. Eng. (DICAMP), University of Trieste (Italy), 1996. [Google Scholar]
- Grassi, M.; Lapasin, R.; Pricl, S.; Colombo, I. Apparent non-fickian release from a scleroglucan gel matrix. Chem. Eng. Comm. 1996, 155, 89. [Google Scholar] [CrossRef]
- Adrover, A.; Giona, M.; Grassi, M.; Lapasin, R.; Pricl, S. Controlled release of theophylline from water-swollen scleroglucan matrices. J. Memb. Sci. 1996, 113, 7. [Google Scholar] [CrossRef]
- Adrover, A.; Giona, M.; Grassi, M. Analysis of controlled release in disordered structures: a percolation model. J. Memb. Sci. 1996, 113, 21. [Google Scholar] [CrossRef]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Coviello, T.; Palleschi, A.; Grassi, M.; Matricardi, P.; Bocchinfuso, G.; Alhaique, F. Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery. Molecules 2005, 10, 6-33. https://doi.org/10.3390/10010006
Coviello T, Palleschi A, Grassi M, Matricardi P, Bocchinfuso G, Alhaique F. Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery. Molecules. 2005; 10(1):6-33. https://doi.org/10.3390/10010006
Chicago/Turabian StyleCoviello, Tommasina, Antonio Palleschi, Mario Grassi, Pietro Matricardi, Gianfranco Bocchinfuso, and Franco Alhaique. 2005. "Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery" Molecules 10, no. 1: 6-33. https://doi.org/10.3390/10010006
APA StyleCoviello, T., Palleschi, A., Grassi, M., Matricardi, P., Bocchinfuso, G., & Alhaique, F. (2005). Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery. Molecules, 10(1), 6-33. https://doi.org/10.3390/10010006



