Optimization of Protein Therapies by Polymer-Conjugation as an Effective DDS
Abstract
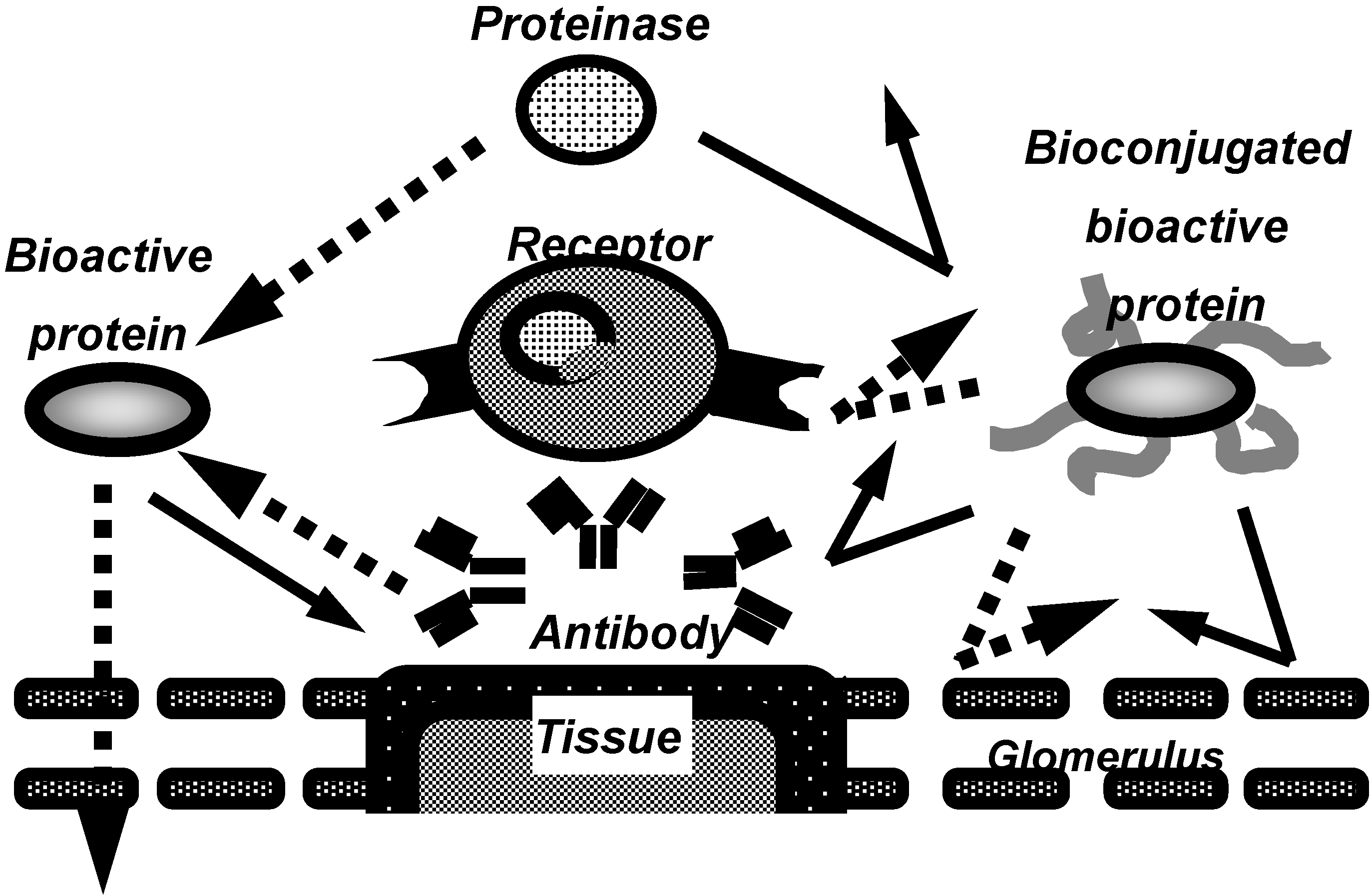
:Introduction
PEGylation of bioactive proteins
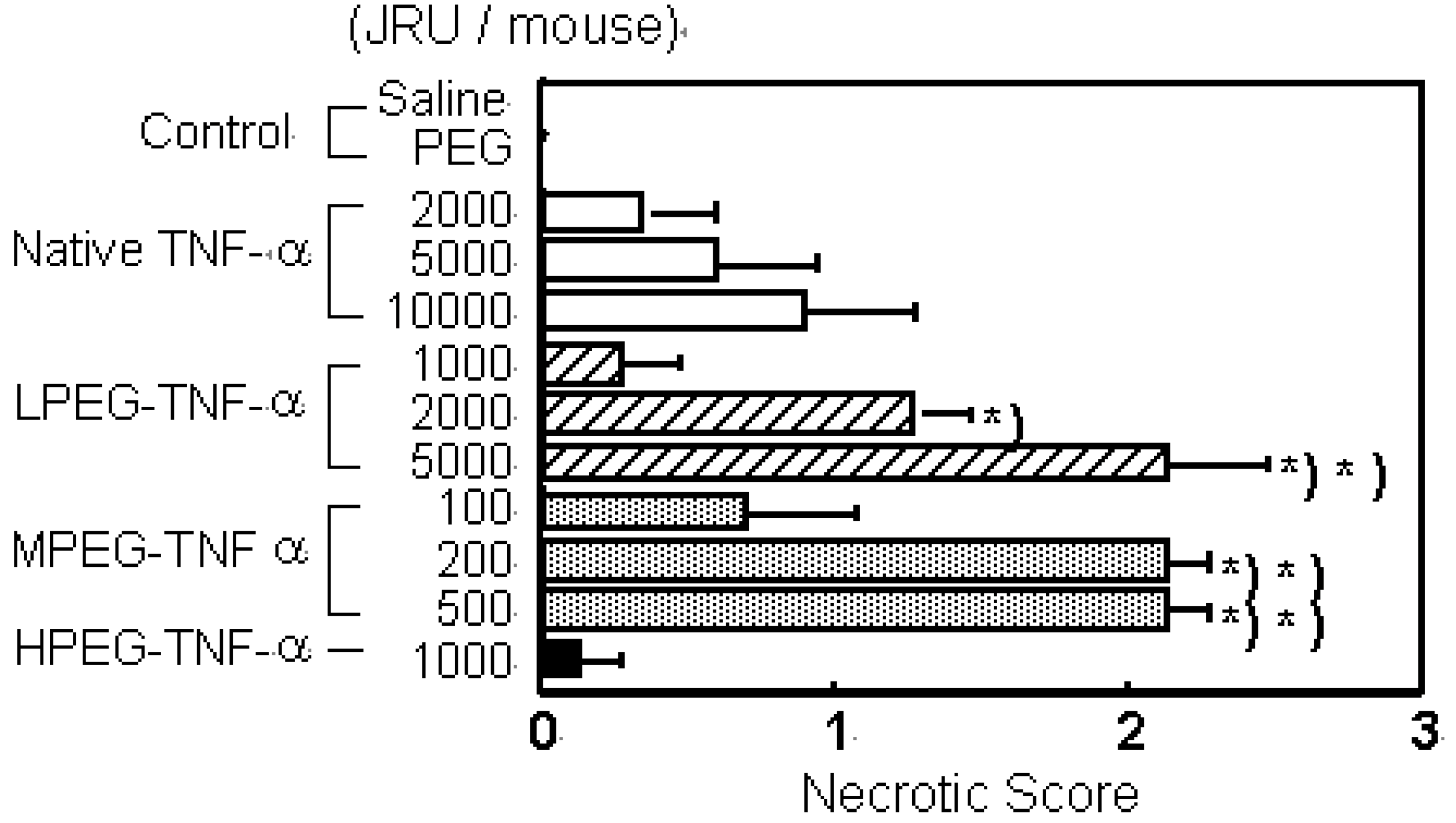
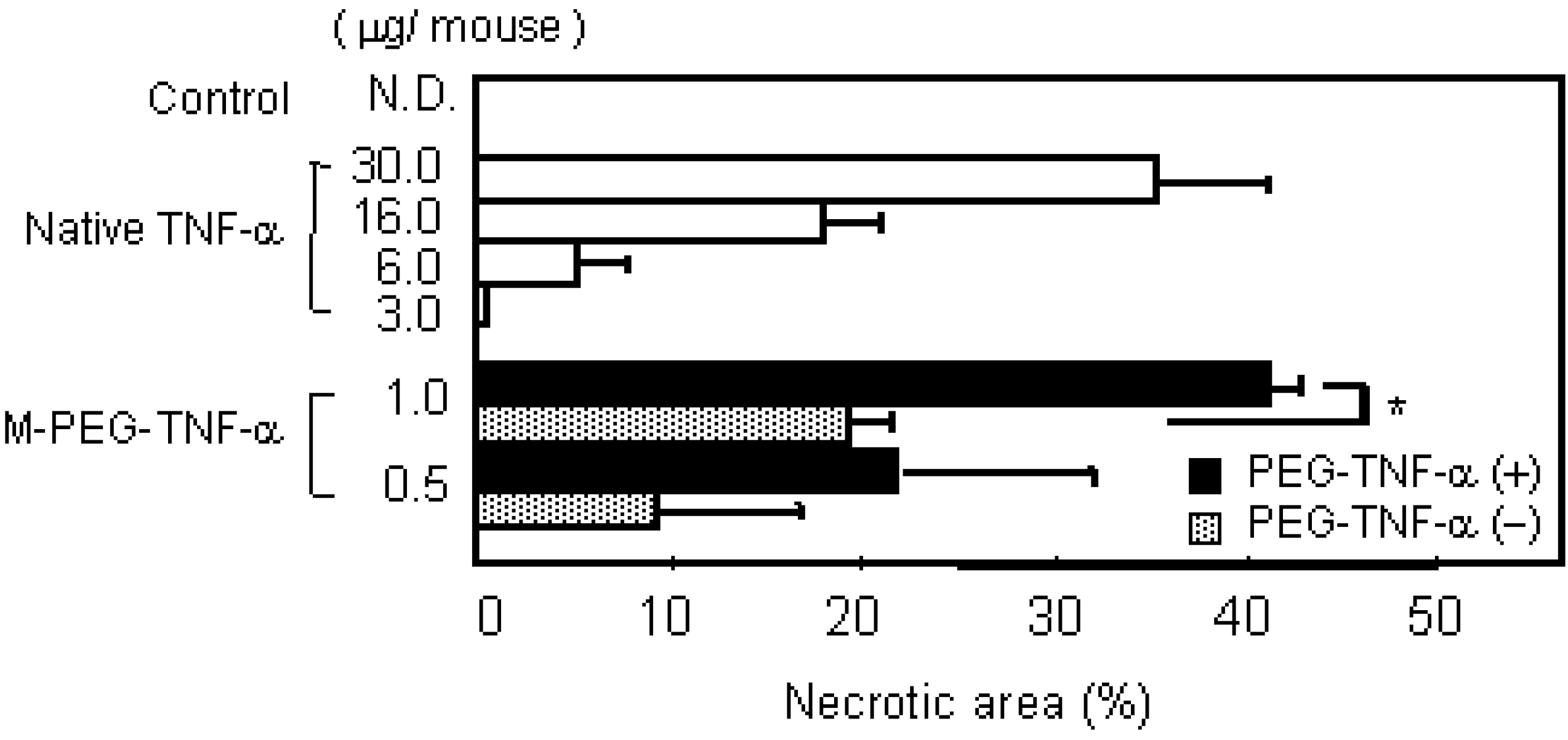
a) PEG-TNF-alpha

b) PEG-IL-6
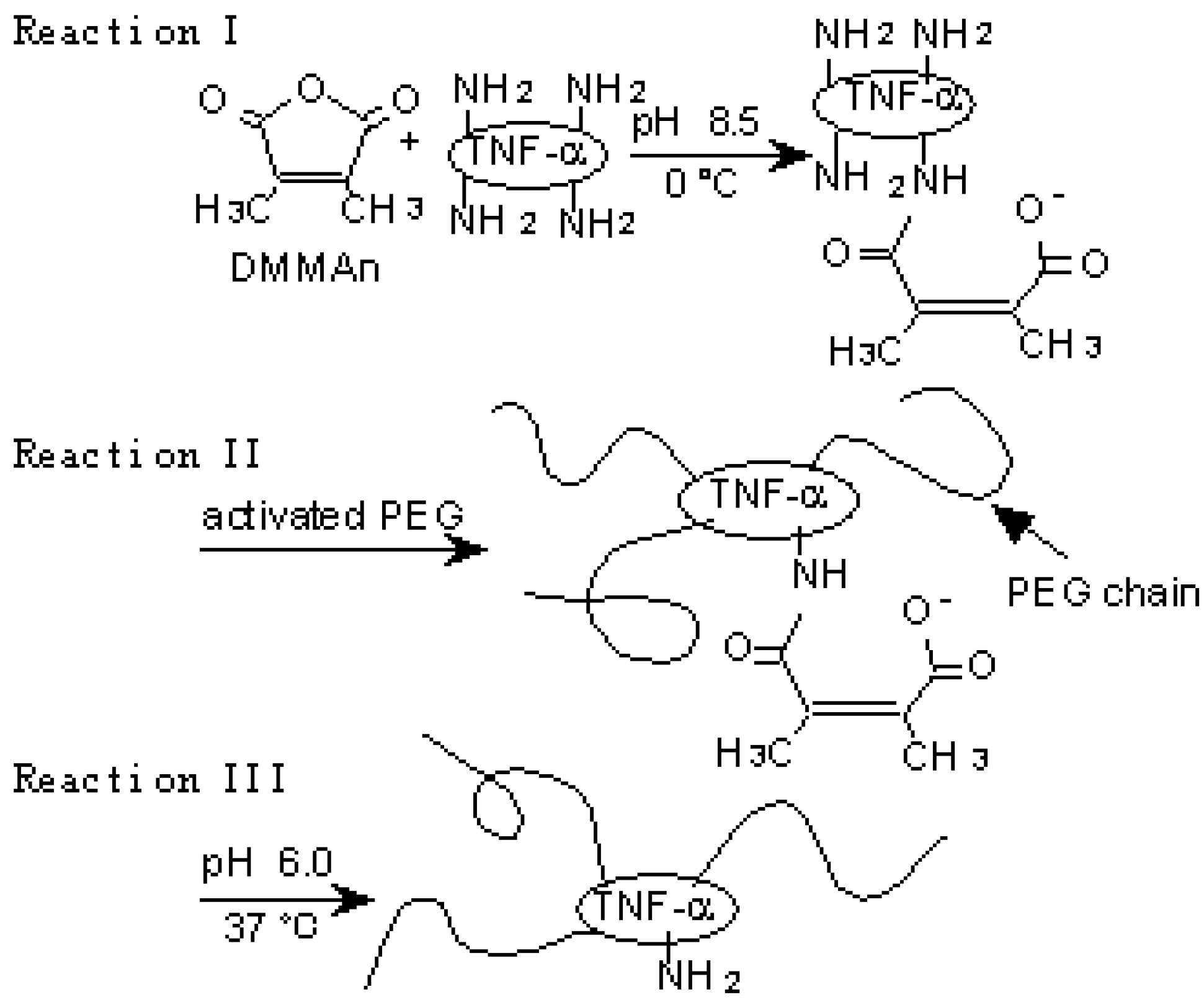
A novel polymer-conjugation technique
Screening of polymeric drug carriers
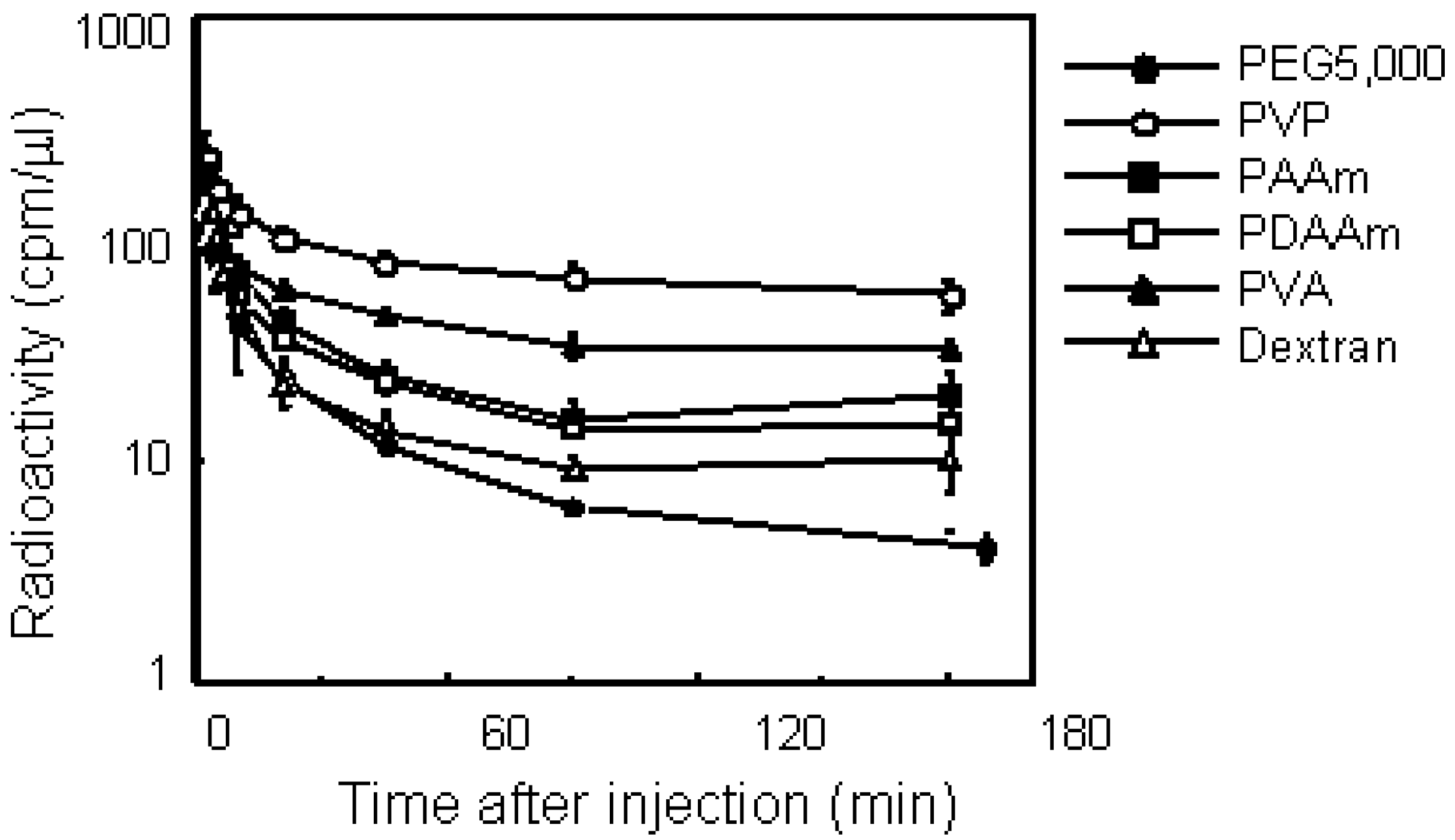
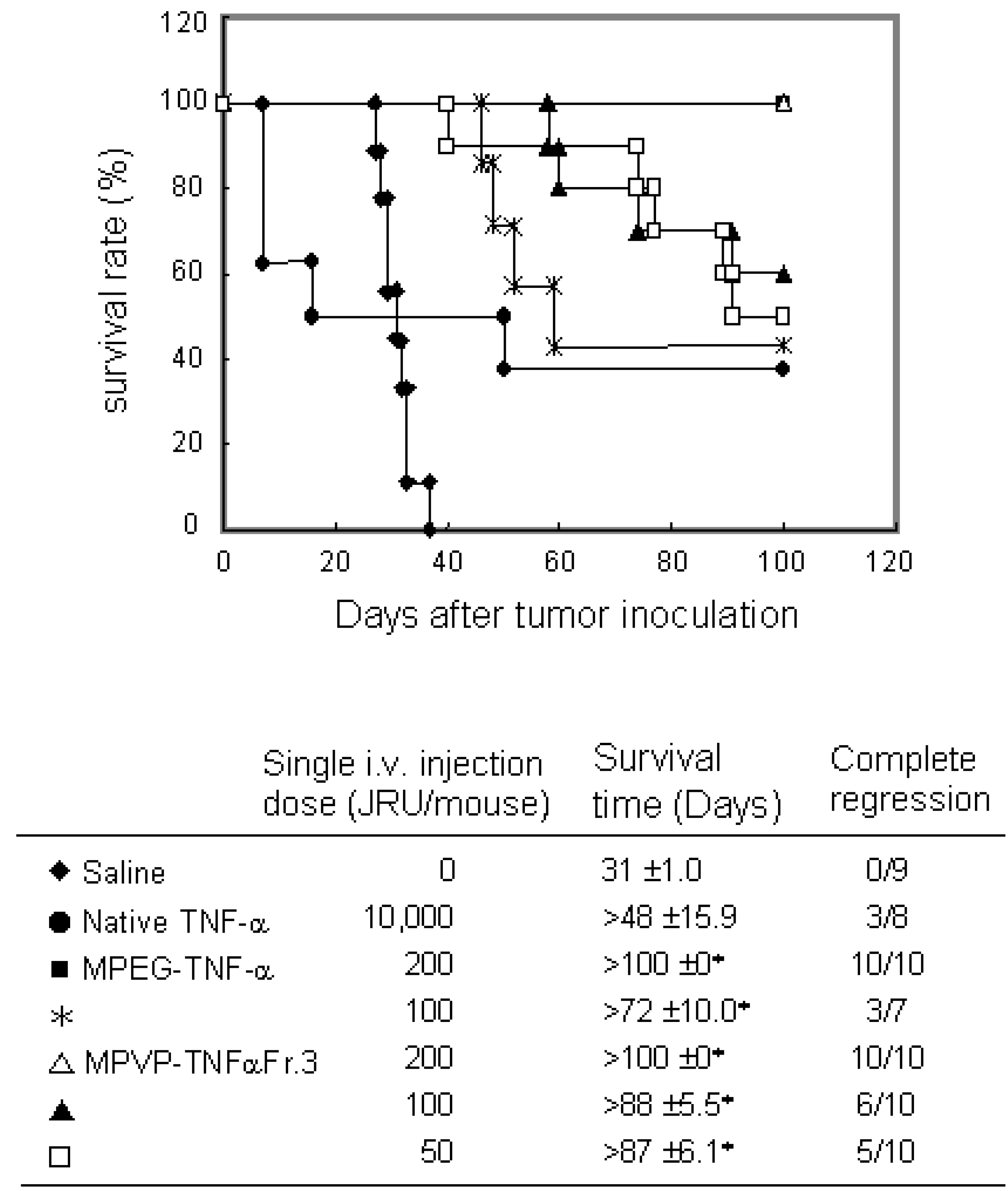
Conjugation with PVP
a) PVP- TNF-alpha
b) PVP-IL-6
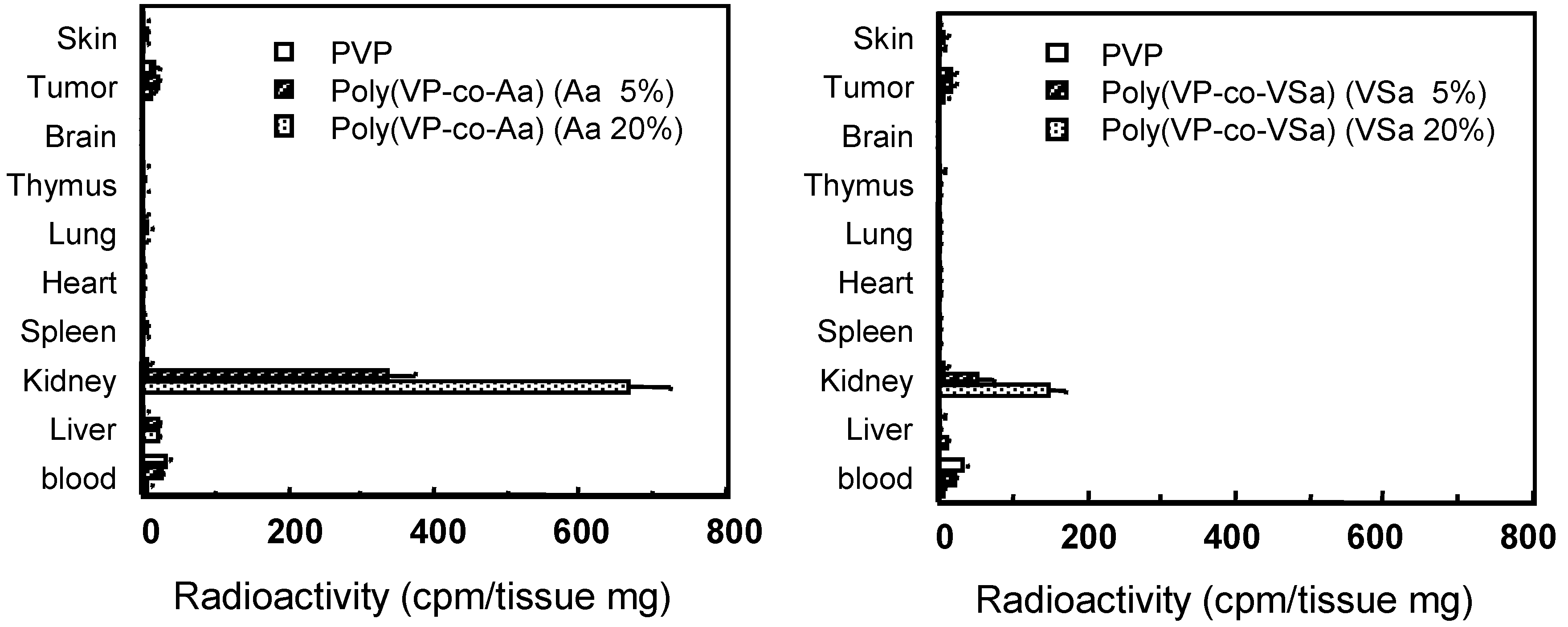
PVP-derivates
Conclusions
Acknowledgments
References
- Glue, P.; Rouzier-Panis, R.; Raffanel, C.; Sabo, R.; Gupta, S. K.; Salfi, M.; Jacobs, S.; Clement, R. P. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. The Hepatitis C Intervention Therapy Group. Hepatology 2000, 32, 647–53. [Google Scholar] [PubMed]
- Furman, W. L.; Strother, D.; McClain, K.; Bell, B.; Leventhal, B.; Pratt, C. B. Phase I clinical trial of recombinant human tumor necrosis factor in children with refractory solid tumors: a Pediatric Oncology Group study. J. Clin. Oncol. 1993, 11, 2205–10. [Google Scholar] [PubMed]
- Barnard, D. L. Pegasys (Hoffmann-La Roche). Curr. Opin. Investig. Drugs 2001, 2, 1530–8. [Google Scholar] [PubMed]
- Borden, E. C.; Sondel, P. M. Lymphokines and cytokines as cancer treatment. Immunotherapy realized. Cancer 1990, 65, 800–14. [Google Scholar] [PubMed]
- Waters, C. A.; Schimke, P. A.; Snider, C. E.; Itoh, K.; Smith, K. A.; Nichols, J. C.; Strom, T. B.; Murphy, J. R. Interleukin 2 receptor-targeted cytotoxicity. Receptor binding requirements for entry of a diphtheria toxin-related interleukin 2 fusion protein into cells. Eur. J. Immunol. 1990, 20, 785–91. [Google Scholar]
- Mohler, K. M.; Torrance, D. S.; Smith, C. A.; Goodwin, R. G.; Stremler, K. E.; Fung, V. P.; Madani, H.; Widmer, M. B. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J. Immunol. 1993, 151, 1548–61. [Google Scholar] [PubMed]
- Rosenberg, S. A.; Lotze, M. T.; Muul, L. M.; Chang, A. E.; Avis, F. P.; Leitman, S.; Linehan, W. M.; Robertson, C. N.; Lee, R. E.; Rubin, J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987, 316, 889–97. [Google Scholar] [PubMed]
- Gordon, M. S.; Nemunaitis, J.; Hoffman, R.; Paquette, R. L.; Rosenfeld, C.; Manfreda, S.; Isaacs, R.; Nimer, S. D. A phase I trial of recombinant human interleukin-6 in patients with myelodysplastic syndromes and thrombocytopenia. Blood 1995, 85, 3066–76. [Google Scholar] [PubMed]
- Kreitman, R. J.; Wilson, W. H.; Bergeron, K.; Raggio, M.; Stetler-Stevenson, M.; FitzGerald, D. J.; Pastan, I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 2001, 345, 241–7. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Taguchi, T.; Urushizaki, I.; Ohno, R.; Abe, O.; Furue, H.; Hattori, T.; Ichihashi, H.; Inoguchi, K.; Majima, H. Phase I study of recombinant human tumor necrosis factor. Cancer Chemother. Pharmacol. 1987, 20, 223–9. [Google Scholar] [PubMed]
- Nagata, S. Steering anti-cancer drugs away from the TRAIL. Nat. Med. 2000, 6, 502–3. [Google Scholar] [PubMed]
- Talpaz, M.; O'Brien, S.; Rose, E.; Gupta, S.; Shan, J.; Cortes, J.; Giles, F. J.; Faderl, S.; Kantarjian, H. M. Phase 1 study of polyethylene glycol formulation of interferon alpha-2B (Schering 54031) in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 2001, 98, 1708–13. [Google Scholar] [PubMed]
- Hershfield, M. S.; Buckley, R. H.; Greenberg, M. L.; Melton, A. L.; Schiff, R.; Hatem, C.; Kurtzberg, J.; Markert, M. L.; Kobayashi, R. H.; Kobayashi, A. L. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N. Engl. J. Med. 1987, 316, 589–96. [Google Scholar] [PubMed]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv. Drug. Deliv. Rev. 2001, 46, 169–85. [Google Scholar] [CrossRef] [PubMed]
- Goodson, R. J.; Katre, N. V. Site-directed pegylation of recombinant interleukin-2 at its glycosylation site. Biotechnology (N Y) 1990, 8, 343–6. [Google Scholar]
- Chapes, S. K.; Simske, S. J.; Sonnenfeld, G.; Miller, E. S.; Zimmerman, R. J. Effects of spaceflight and PEG-IL-2 on rat physiological and immunological responses. J. Appl. Physiol. 1999, 86, 2065–76. [Google Scholar] [PubMed]
- Tsutsumi, Y.; Tsunoda, S.; Kamada, H.; Kihira, T.; Nakagawa, S.; Kaneda, Y.; Kanamori, T.; Mayumi, T. Molecular design of hybrid tumour necrosis factor-alpha. II: The molecular size of polyethylene glycol-modified tumour necrosis factor-alpha affects its anti-tumour potency. Br. J. Cancer 1996, 74, 1090–5. [Google Scholar]
- Tsutsumi, Y.; Kihira, T.; Tsunoda, S.; Kanamori, T.; Nakagawa, S.; Mayumi, T. Molecular design of hybrid tumour necrosis factor alpha with polyethylene glycol increases its anti-tumour potency. Br. J. Cancer 1995, 71, 963–8. [Google Scholar] [PubMed]
- Kaneda, Y.; Yamamoto, Y.; Kamada, H.; Tsunoda, S.; Tsutsumi, Y.; Hirano, T.; Mayumi, T. Antitumor activity of tumor necrosis factor alpha conjugated with divinyl ether and maleic anhydride copolymer on solid tumors in mice. Cancer Res. 1998, 58, 290–5. [Google Scholar]
- Tsunoda, S.; Ishikawa, T.; Yamamoto, Y.; Kamada, H.; Koizumi, K.; Matsui, J.; Tsutsumi, Y.; Hirano, T.; Mayumi, T. Enhanced antitumor potency of polyethylene glycolylated tumor necrosis factor-alpha: a novel polymer-conjugation technique with a reversible amino-protective reagent. J. Pharmacol. Exp. Ther. 1999, 290, 368–72. [Google Scholar] [PubMed]
- Kamada, H.; Tsutsumi, Y.; Tsunoda, S.; Kihira, T.; Kaneda, Y.; Yamamoto, Y.; Nakagawa, S.; Horisawa, Y.; Mayumi, T. Molecular design of conjugated tumor necrosis factor-alpha: synthesis and characteristics of polyvinyl pyrrolidone modified tumor necrosis factor-alpha. Biochem. Biophys. Res. Commun. 1999, 257, 448–53. [Google Scholar] [PubMed]
- Kamada, H.; Tsutsumi, Y.; Yamamoto, Y.; Kihira, T.; Kaneda, Y.; Mu, Y.; Kodaira, H.; Tsunoda, S. I.; Nakagawa, S.; Mayumi, T. Antitumor activity of tumor necrosis factor-alpha conjugated with polyvinylpyrrolidone on solid tumors in mice. Cancer Res. 2000, 60, 6416–20. [Google Scholar] [PubMed]
- Kodaira, H.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Kaneda, Y.; Yamamoto, Y.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; Nakagawa, S.; Mayumi, T. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials 2004, 25, 4309–15. [Google Scholar] [CrossRef]
- Debs, R. J.; Fuchs, H. J.; Philip, R.; Brunette, E. N.; Duzgunes, N.; Shellito, J. E.; Liggitt, D.; Patton, J. R. Immunomodulatory and toxic effects of free and liposome-encapsulated tumor necrosis factor alpha in rats. Cancer Res. 1990, 50, 375–80. [Google Scholar] [PubMed]
- Nobuhara, M.; Kanamori, T.; Ashida, Y.; Ogino, H.; Horisawa, Y.; Nakayama, K.; Asami, T.; Iketani, M.; Noda, K.; Andoh, S. The inhibition of neoplastic cell proliferation with human natural tumor necrosis factor. Jpn. J. Cancer Res. 1987, 78, 193–201. [Google Scholar] [PubMed]
- Moritz, T.; Niederle, N.; Baumann, J.; May, D.; Kurschel, E.; Osieka, R.; Kempeni, J.; Schlick, E.; Schmidt, C. G. Phase I study of recombinant human tumor necrosis factor alpha in advanced malignant disease. Cancer Immunol. Immunother. 1989, 29, 144–50. [Google Scholar]
- Noguchi, K.; Inagawa, H.; Tsuji, Y.; Morikawa, A.; Mizuno, D.; Soma, G. Antitumor activity of a novel chimera tumor necrosis factor (TNF-STH) constructed by connecting rTNF-S with thymosin beta 4 against murine syngeneic tumors. J. Immunother. 1991, 10, 105–11. [Google Scholar] [PubMed]
- Blick, M.; Sherwin, S. A.; Rosenblum, M.; Gutterman, J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987, 47, 2986–9. [Google Scholar] [PubMed]
- Hill, R. J.; Warren, M. K.; Stenberg, P.; Levin, J.; Corash, L.; Drummond, R.; Baker, G.; Levin, F.; Mok, Y. Stimulation of megakaryocytopoiesis in mice by human recombinant interleukin-6. Blood 1991, 77, 42–8. [Google Scholar] [PubMed]
- Ishibashi, T.; Kimura, H.; Shikama, Y.; Uchida, T.; Kariyone, S.; Hirano, T.; Kishimoto, T.; Takatsuki, F.; Akiyama, Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989, 74, 1241–4. [Google Scholar] [PubMed]
- Zeidler, C.; Kanz, L.; Hurkuck, F.; Rittmann, K. L.; Wildfang, I.; Kadoya, T.; Mikayama, T.; Souza, L.; Welte, K. In vivo effects of interleukin-6 on thrombopoiesis in healthy and irradiated primates. Blood 1992, 80, 2740–5. [Google Scholar] [PubMed]
- Navarro, S.; Debili, N.; Le Couedic, J. P.; Klein, B.; Breton-Gorius, J.; Doly, J.; Vainchenker, W. Interleukin-6 and its receptor are expressed by human megakaryocytes: in vitro effects on proliferation and endoreplication. Blood 1991, 77, 461–71. [Google Scholar] [PubMed]
- Taga, T.; Kishimoto, T. Role of a two-chain IL-6 receptor system in immune and hematopoietic cell regulation. Crit. Rev. Immunol. 1992, 11, 265–80. [Google Scholar]
- Castell, J. V.; Geiger, T.; Gross, V.; Andus, T.; Walter, E.; Hirano, T.; Kishimoto, T.; Heinrich, P. C. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur. J. Biochem. 1988, 177, 357–61. [Google Scholar] [PubMed]
- Suematsu, S.; Matsuda, T.; Aozasa, K.; Akira, S.; Nakano, N.; Ohno, S.; Miyazaki, J.; Yamamura, K.; Hirano, T.; Kishimoto, T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 7547–51. [Google Scholar] [PubMed]
- Weber, J.; Yang, J. C.; Topalian, S. L.; Parkinson, D. R.; Schwartzentruber, D. S.; Ettinghausen, S. E.; Gunn, H.; Mixon, A.; Kim, H.; Cole, D. Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J. Clin. Oncol. 1993, 11, 499–506. [Google Scholar] [PubMed]
- Delgado, C.; Francis, G. E.; Fisher, D. The uses and properties of PEG-linked proteins. Crit. Rev. Ther. Drug. Carrier. Syst. 1992, 9, 249–304. [Google Scholar]
- Inoue, M.; Ebashi, I.; Watanabe, N.; Morino, Y. Synthesis of a superoxide dismutase derivative that circulates bound to albumin and accumulates in tissues whose pH is decreased. Biochemistry. 1989, 28, 6619–24. [Google Scholar] [PubMed]
- Nagasaki, Y.; Iijima, M.; Kato, M.; Kataoka, K. Primary amino-terminal heterobifunctional poly(ethylene oxide). Facile synthesis of poly(ethylene oxide) with a primary amino group at one end and a hydroxyl group at the other end. Bioconjug. Chem. 1995, 6, 702–4. [Google Scholar] [PubMed]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; Nakagawa, S.; Mayumi, T. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25, 3259–66. [Google Scholar] [PubMed]
- Tsunoda, S.; Kamada, H.; Yamamoto, Y.; Ishikawa, T.; Matsui, J.; Koizumi, K.; Kaneda, Y.; Tsutsumi, Y.; Ohsugi, Y.; Hirano, T.; Mayumi, T. Molecular design of polyvinyl- pyrrolidone-conjugated interleukin-6 for enhancement of in vivo thrombopoietic activity in mice. J. Control. Release 2000, 68, 335–41. [Google Scholar] [PubMed]
- Simionescu, N. Cellular aspects of transcapillary exchange. Physiol. Rev. 1983, 63, 1536–79. [Google Scholar] [PubMed]
- Chang, R. L.; Deen, W. M.; Robertson, C. R.; Brenner, B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975, 8, 212–8. [Google Scholar] [PubMed]
- Takakura, Y.; Fujita, T.; Hashida, M.; Sezaki, H. Disposition characteristics of macromolecules in tumor-bearing mice. Pharm. Res. 1990, 7, 339–46. [Google Scholar] [PubMed]
- Yamamoto, Y.; Tsutsumi, Y.; Yoshioka, Y.; Nishibata, T.; Kobayashi, K.; Okamoto, T.; Mukai, Y.; Shimizu, T.; Nakagawa, S.; Nagata, S.; Mayumi, T. Site-specific PEGylation of a lysine-deficient TNF-alpha with full bioactivity. Nat. Biotechnol. 2003, 21, 546–52. [Google Scholar] [PubMed]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Shibata, H.; Nakagawa, S.; Tsutsumi, Y. Optimization of Protein Therapies by Polymer-Conjugation as an Effective DDS. Molecules 2005, 10, 162-180. https://doi.org/10.3390/10010162
Shibata H, Nakagawa S, Tsutsumi Y. Optimization of Protein Therapies by Polymer-Conjugation as an Effective DDS. Molecules. 2005; 10(1):162-180. https://doi.org/10.3390/10010162
Chicago/Turabian StyleShibata, Hiroko, Shinsaku Nakagawa, and Yasuo Tsutsumi. 2005. "Optimization of Protein Therapies by Polymer-Conjugation as an Effective DDS" Molecules 10, no. 1: 162-180. https://doi.org/10.3390/10010162
APA StyleShibata, H., Nakagawa, S., & Tsutsumi, Y. (2005). Optimization of Protein Therapies by Polymer-Conjugation as an Effective DDS. Molecules, 10(1), 162-180. https://doi.org/10.3390/10010162



