A Pivotal Role of Chitosan Nanoparticles in Enhancing the Essential Oil Productivity and Antioxidant Capacity in Matricaria chamomilla L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. CSNPs Preparation and Application
2.3. Growth and Yield Measurements
2.4. Relative Water Content (RWC)
2.5. Essential Oil Determination
2.6. Photosynthetic Pigments Assessment
2.7. Total Soluble Sugars (TSS) Determination
2.8. Assessment of Total Phenol Content
2.9. Antioxidant Activity (DPPH Assay)
2.10. N, P and K Assessment
2.11. Statistical Analysis
3. Results
3.1. Growth Characters
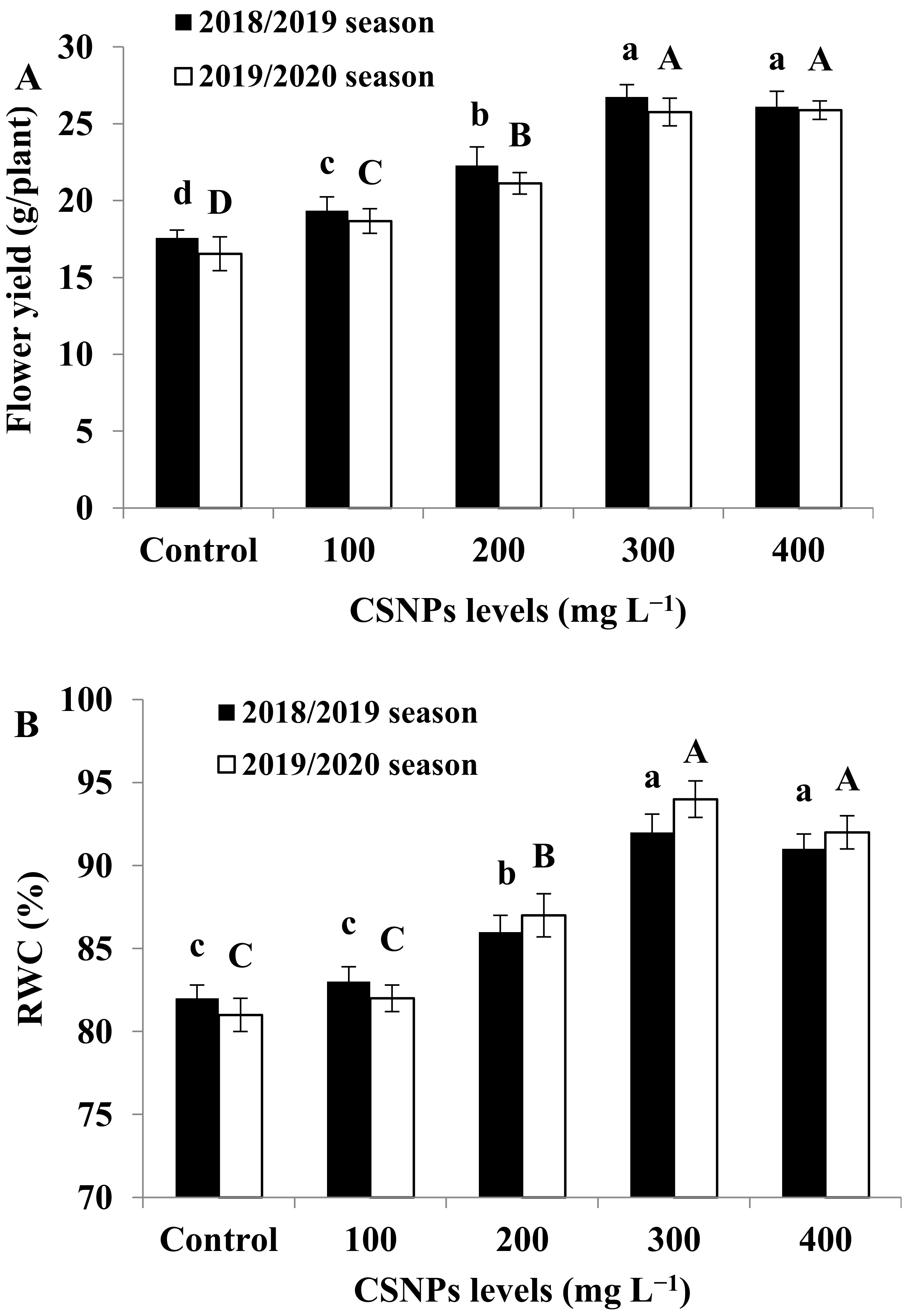
3.2. Flower Yield
3.3. Relative Water Content (RWC)
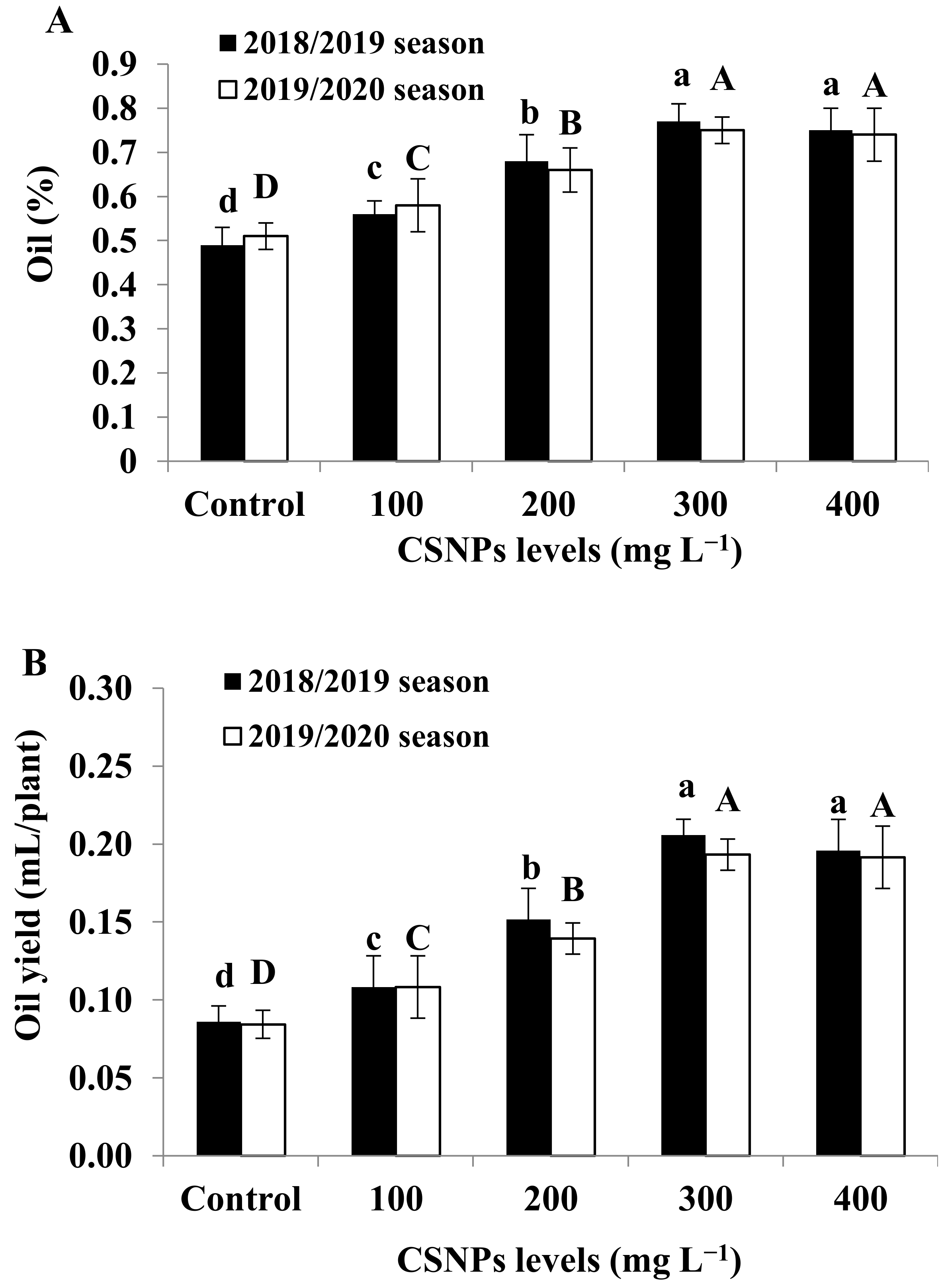
3.4. Essential Oil Content
3.5. Photosynthetic Pigments
3.6. Total Soluble Sugars (TSS)
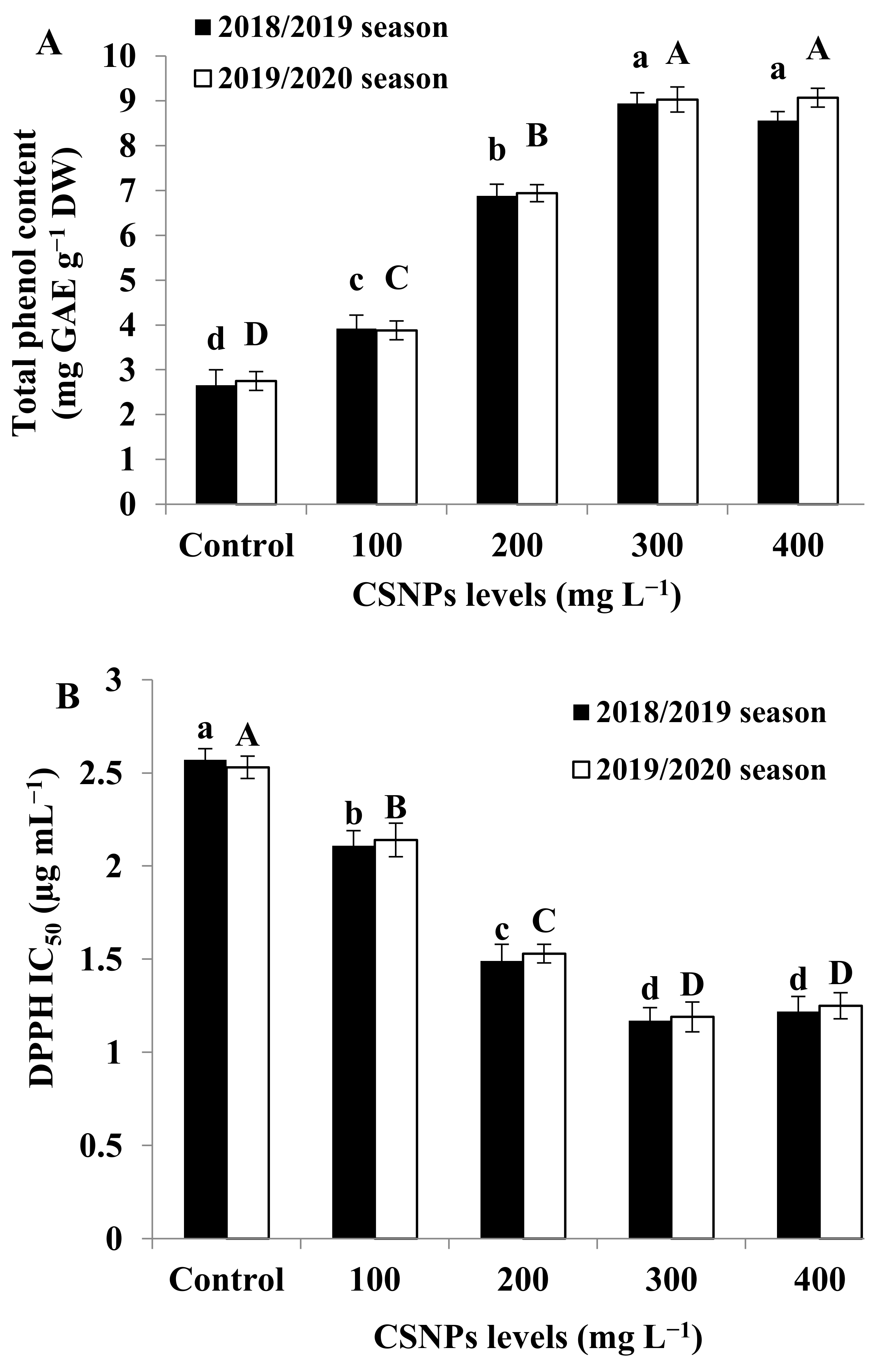
3.7. Total Phenolic Content
3.8. Antioxidant Activity (DPPH)
3.9. N, P and K Contents
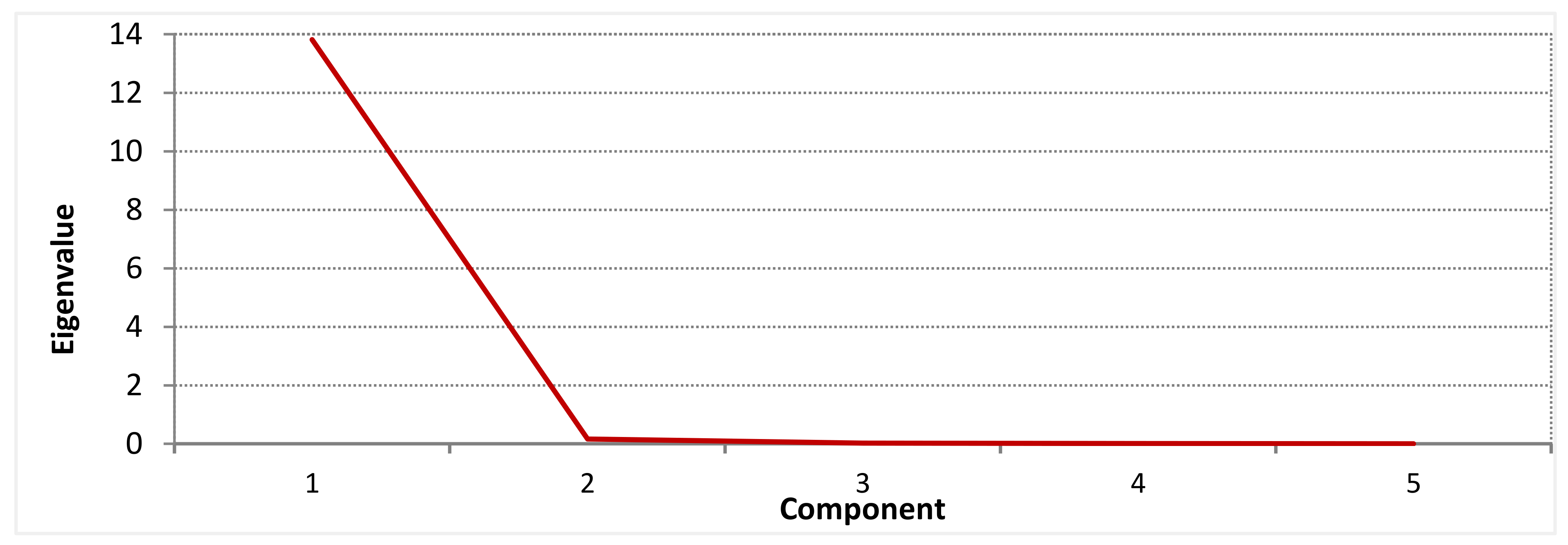
3.10. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook of Practice on a Scientific Basis, 3rd ed.; CRC Press: Stuttgart, Germany, 2004. [Google Scholar]
- Bączek, K.B.; Wiśniewska, M.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z. Arbuscular mycorrhizal fungi in chamomile (Matricaria recutita L.) organic Cultivation. Ind. Crop. Prod. 2019, 140, 111562. [Google Scholar] [CrossRef]
- Mazrou, R. Salt stress alleviation of chamomile plant by Mycorrhizal fungi and salicylic acid. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 5099–5111. [Google Scholar]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.F. Protective effects of 1-methylcyclopropene and salicylic acid on senescence regulation of gladiolus cut spikes. Sci. Hortic. 2014, 179, 146–152. [Google Scholar] [CrossRef]
- Pandey, V.; Patra, D.D. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Hér. under integrated supply of various organic and chemical fertilizers. Ind. Crop. Prod. 2015, 67, 257–263. [Google Scholar] [CrossRef]
- Kahil, A.A.; Hassan, F.A.S.; Ali, E.F. Influence of bio-fertilizers on growth, yield and anthocyanin content of Hibiscus sabdariffa L. plant under Taif region conditions. Annu. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical paramètres. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Fetouh, M.I. Does moringa leaf extract have preservative effect improving the longevity and postharvest quality of gladiolus cut spikes? Sci. Hortic. 2019, 250, 287–293. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Mazrou, R.; Gaber, A.; Hassan, M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F. Bio-production of Nigella sativa L. seeds and oil in Taif area. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 315–328. [Google Scholar]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Pirbalouti, G.A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Bistgani, E.; Zohreh Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Safikhan, S.; Khoshbakht, K.; Chaichi, M.R.; Amini, A.; Motesharezadeh, B. Role of chitosan on the growth, physiological parameters and enzymatic activity of milk thistle (Silybum marianum (L.) Gaertn.) in a pot experiment. J. Appl. Res. Med. Arom. Plants 2018, 10, 49–58. [Google Scholar] [CrossRef]
- Lei, C.; Ma, D.; Pu, G.; Qiu, X.; Du, Z.; Wang, H.; Li, G.; Ye, H.; Liu, B. Foliar application of chitosan activates artemisinin biosynthesis in Artemisia annua L. Ind. Crop. Prod. 2011, 33, 176–182. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.F.; El-Deeb, B. Improvement of postharvest quality of cut rose cv. ‘First Red’ by biologically synthesized silver nanoparticles. Sci. Hortic. 2014, 179, 340–348. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macro. 2021, 177, 517–525. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Weatherley, P.E. Studies in the water relations of the cotton plant. 1. The field measurements of water deficit in leaves. New Phytol. 1950, 49, 8. [Google Scholar] [CrossRef]
- British Pharmacopea. Determination of Volatile Oil in Drugs; Pharmaceutical Press: London, UK, 1963. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Qian, Y.; Tan, D.-X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBF/DREB1 and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal. Res. 2015, 59, 334–342. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- A.O.A.C. Official Method of Analysis, 16th ed.; Association of Official Analytical Chemists International: Washington, DC, USA, 1995; Available online: https://www.worldcat.org/title/official-methods-of-analysis-of-aoac-international/oclc/421897987 (accessed on 13 August 2018).
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of India: New Delhi, India, 1967; pp. 144–197. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part II. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1985. [Google Scholar]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Li, P. Metabolite Profiling of Wheat Seedlings Induced by Chitosan: Revelation of the Enhanced Carbon and Nitrogen Metabolism. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.J.; Hu, J.; Wang, X.; Shao, C. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gornik, K.; Grzesik, M.; Duda, B.R. The effect of chitosan on rooting of grave vine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornam. Plant Res. 2008, 16, 333–343. [Google Scholar]
- Veroneze-Júnior, V.; Martins, M.; Mc Leod, L.; Souza, K.R.D.; Santos-Filho, P.R.; Magalhães, P.C.; Carvalho, D.T.; Santos, M.H.; Souza, T.C. Leaf application of chitosan and physiological evaluation of maize hybrids contrasting for drought tolerance under water restriction. Braz. J. Biol. 2020, 80, 631–640. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X. Physiological Effects of Chitosan Coating on Wheat Growth and Activities of Protective Enzyme with Drought Tolerance. Open J. Soil Sci. 2012, 2, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Kumar, A.; Devi, K.A.; Prajapati, D.; Bhagat, D.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Chitosan nanofertilizer to foster source activity in maize. Int. J. Biol. Macro. 2020, 145, 226–234. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar]
- Yin, H.; Fretté, X.C.; Chrestensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Oreganum vulgare ssp. hirtum). J. Agric. Food Chem. 2011, 60, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kastell, A.; Mewis, I.; Knorr, D.; Smetanska, I. Polysaccharide elicitorsenhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. 2012, 108, 401–409. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, J.; Chen, S.; Yang, F. Antioxidant activity of extracts of black sesame seed (Sesamum indicum L.) by supercritical carbon dioxide extraction. J. Agric. Food Chem. 2004, 52, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Schmidt, G. Post-harvest characteristics of cut carnations as the result of chemical treatments. Acta Agron. Hung. 2004, 52, 125–132. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.F. Effects of salt stress on growth, antioxidant enzyme activity and some other physiological parameters in jojoba (Simmondsia chinensis (Link) Schneider) plant. Aust. J. Crop Sci. 2014, 8, 1615–1624. [Google Scholar]
- Hassan, F.A.S.; Ali, E.F.; Alamer, K.H. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. S. Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic Pathways Regulated by Chitosan Contributing to Drought Resistance in White Clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]

| CSNPs (mg L−1) | 2018/2019 Season | 2019/2020 Season | ||
|---|---|---|---|---|
| Plant Height (cm) | Branch Number/Plant | Plant Height (cm) | Branch Number/Plant | |
| Control | 41.58 ± 0.97 d | 11.33 ± 0.55 c | 39.85 ± 1.04 d | 10.66 ± 0.47 c |
| 100 | 44.72 ± 0.88 c | 11.72 ± 0.61 c | 42.67 ± 0.82 c | 11.17 ± 0.51 c |
| 200 | 49.17 ± 1.03 b | 12.89 ± 0.49 b | 47.29 ± 0.91 b | 12.38 ± 0.43 b |
| 300 | 54.54 ± 0.92 a | 14.28 ± 0.64 a | 52.68 ± 1.06 a | 14.86 ± 0.61 a |
| 400 | 53.22 ± 0.83 a | 14.45 ± 0.57 a | 52.13 ± 0.86 a | 14.45 ± 0.54 a |
| LSD 0.05 | 1.79 | 0.61 | 1.67 | 0.59 |
| CSNPs (mg L−1) | 2018/2019 Season | 2019/2020 Season | ||||
|---|---|---|---|---|---|---|
| Total Chlorophyll (mg g−1 FW) | Carotenoids (mg g−1 FW) | TSS (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Carotenoids (mg g−1 FW) | TSS (mg g−1 FW) | |
| Control | 0.89 ± 0.03 c | 0.31 ± 0.02 d | 11.36 ± 0.52 d | 0.91 ± 0.02 d | 0.33 ± 0.04 d | 12.27 ± 0.38 d |
| 100 | 0.93 ± 0.04 c | 0.34 ± 0.04 c | 13.48 ± 0.48 c | 0.96 ± 0.04 c | 0.36 ± 0.02 c | 13.79 ± 0.45 c |
| 200 | 1.03 ± 0.03 b | 0.39 ± 0.03 b | 16.52 ± 0.54 b | 1.14 ± 0.03 b | 0.42 ± 0.05 b | 16.12 ± 0.40 b |
| 300 | 1.25 ± 0.05 a | 0.43 ± 0.02 a | 20.15 ± 0.46 a | 1.28 ± 0.05 a | 0.45 ± 0.03 a | 20.89 ± 0.52 a |
| 400 | 1.23 ± 0.06 a | 0.42 ± 0.04 a | 19.89 ± 0.49 a | 1.29 ± 0.04 a | 0.44 ± 0.02 a | 20.09 ± 0.43 a |
| LSD 0.05 | 0.036 | 0.017 | 0.87 | 0.037 | 0.019 | 0.96 |
| CSNPs (mg L−1) | 2018/2019 Season | 2019/2020 Season | ||||
|---|---|---|---|---|---|---|
| N (%) | P (%) | K (%) | N (%) | P (%) | K (%) | |
| Control | 1.52 ± 0.07 e | 0.28 ± 0.03 d | 1.62 ± 0.05 d | 1.53 ± 0.03 d | 0.30 ± 0.06 c | 1.59 ± 0.06 d |
| 100 | 1.67 ± 0.06 d | 0.32 ± 0.02 c | 1.76 ± 0.04 c | 1.69 ± 0.05 c | 0.33 ± 0.04 c | 1.74 ± 0.05 c |
| 200 | 1.89 ± 0.05 c | 0.37 ± 0.05 b | 1.92 ± 0.05 b | 1.88 ± 0.04 b | 0.38 ± 0.05 b | 1.93 ± 0.04 b |
| 300 | 2.23 ± 0.07 a | 0.42 ± 0.04 a | 2.45 ± 0.06 a | 2.21 ± 0.06 a | 0.43 ± 0.04 a | 2.48 ± 0.05 a |
| 400 | 2.11 ± 0.08 b | 0.41 ± 0.03 a | 2.40 ± 0.03 a | 2.17 ± 0.05 a | 0.40 ± 0.06 a | 2.45 ± 0.03 a |
| LSD 0.05 | 0.05 | 0.04 | 0.07 | 0.06 | 0.03 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazrou, R.; Ali, E.F.; Hassan, S.; Hassan, F.A.S. A Pivotal Role of Chitosan Nanoparticles in Enhancing the Essential Oil Productivity and Antioxidant Capacity in Matricaria chamomilla L. Horticulturae 2021, 7, 574. https://doi.org/10.3390/horticulturae7120574
Mazrou R, Ali EF, Hassan S, Hassan FAS. A Pivotal Role of Chitosan Nanoparticles in Enhancing the Essential Oil Productivity and Antioxidant Capacity in Matricaria chamomilla L. Horticulturae. 2021; 7(12):574. https://doi.org/10.3390/horticulturae7120574
Chicago/Turabian StyleMazrou, Ragia, Esmat F. Ali, Sabry Hassan, and Fahmy A. S. Hassan. 2021. "A Pivotal Role of Chitosan Nanoparticles in Enhancing the Essential Oil Productivity and Antioxidant Capacity in Matricaria chamomilla L." Horticulturae 7, no. 12: 574. https://doi.org/10.3390/horticulturae7120574
APA StyleMazrou, R., Ali, E. F., Hassan, S., & Hassan, F. A. S. (2021). A Pivotal Role of Chitosan Nanoparticles in Enhancing the Essential Oil Productivity and Antioxidant Capacity in Matricaria chamomilla L. Horticulturae, 7(12), 574. https://doi.org/10.3390/horticulturae7120574








