The Influence of Parental Heat-Stress Priming on Drought-Tolerant Maize Progenies’ Field Performance
Abstract
:1. Introduction
2. Materials and Methods
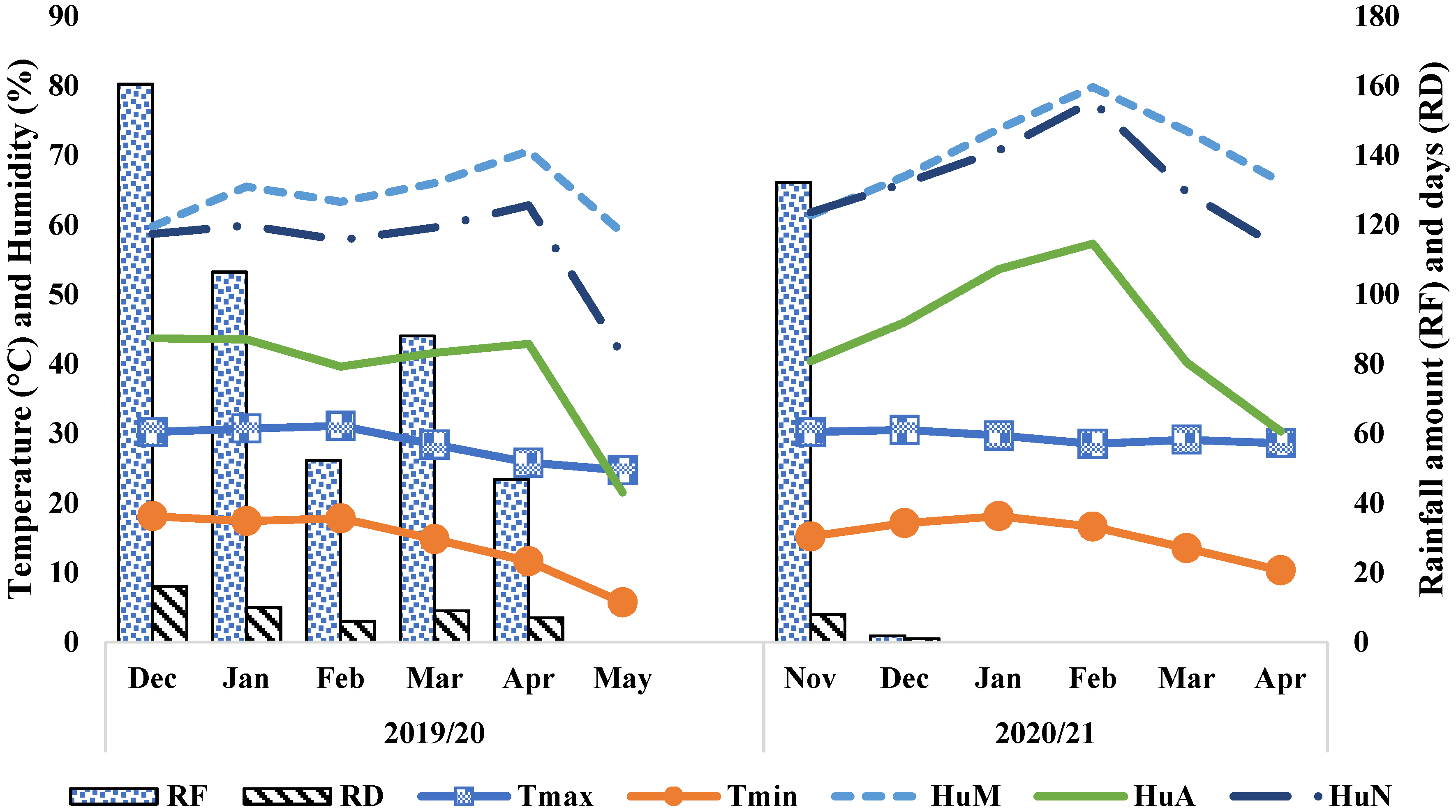
2.1. Description of the Study Site
2.2. Experimental Design, Treatments and Cultural Practices
2.2.1. Parental Evaluation
2.2.2. Progeny Field Evaluation
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Analysis of Variance
3.2. Effect of Parental Heat Stress on the Progenies’ Growth and Yield Attributes
3.3. Varietal Influence on the Progenies’ Growth and Yield Attributes
3.4. Effect of Parental Soil Amendment on the Progenies’ Growth and Yield Attributes
3.5. Interaction of Parental Heat Stress, Maize Variety and Soil Amendment on the Progenies’ Growth and Yield Attributes
4. Discussion
4.1. Effect of Parental Heat Stress on the Progenies’ Growth and Yield Attributes
4.2. Varietal Influence on the Progenies’ Growth and Yield Attributes
4.3. Effect of Parental Soil Amendment on the Progenies’ Growth and Yield Attributes
4.4. Interaction of Parental Heat Stress, Maize Variety and Soil Amendment on the Progenies’ Growth and Yield Attributes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, N.S.; Titov, V.; Ayemere, I.; Byeon, B.; Ilnytskyy, Y.; Kovalchuk, I. Multigenerational exposure to heat stress induces phenotypic resilience, and genetic and epigenetic variations in Arabidopsis thaliana offspring. bioRxiv 2020. [Google Scholar] [CrossRef]
- Colicchio, J.M.; Herman, J. Empirical patterns of environmental variation favor adaptive transgenerational plasticity. Ecol. Evol. 2020, 10, 1648–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Heat stress effect on the grain yield of three drought-tolerant maize varieties under varying growth conditions. Plants 2021, 10, 1532. [Google Scholar] [CrossRef]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Maize response to combined heat and water stresses under varying growth conditions. Agron. J. 2021, 1–18. [Google Scholar] [CrossRef]
- Balla, K.; Karsai, I.; Kiss, T.; Horváth, Á.; Berki, Z.; Cseh, A.; Bónis, P.; Árendás, T.; Veisz, O. Single versus repeated heat stress in wheat: What are the consequences in different developmental phases? PLoS ONE 2021, 16, e0252070. [Google Scholar] [CrossRef] [PubMed]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyoshi, K.; Katano, K.; Yunose, M.; Suzuki, N. Memory of 5-min heat stress in Arabidopsis thaliana. Plant Signal. Behav. 2020, 15, 1778919. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Whittle, C.; Otto, S.; Johnston, M.O.; Krochko, J. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 2009, 87, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.; Sultan, S. Adaptive Transgenerational Plasticity in Plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eichten, S.R.; Schmitz, R.J.; Springer, N.M. Epigenetics: Beyond chromatin modifications and complex genetic regulation. Plant Physiol. 2014, 165, 933–947. [Google Scholar] [CrossRef] [Green Version]
- Kushawaha, A.K.; Khan, A.; Sopory, S.K.; Sanan-Mishra, N. Priming by high temperature stress induces microRNA regulated heat shock modules indicating their involvement in thermopriming response in rice. Life 2021, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Kushawaha, A.K.; Khan, A.; Sopory, S.K.; Sanan-Mishra, N. Light regulated Osa-miR169e is implicated during priming under high temperature stress in rice. Am. J. Plant Sci. 2019, 10, 1662–1674. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Liu, L.; Qian, K.; Chen, J.; Zhang, Y.; Xie, P.; Xu, M.; Hu, Z.; Yan, W.; Wu, Y.; et al. Plant DNA methylation is sensitive to parent seed N content and influences the growth of rice. BMC Plant Biol. 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.P.; Li, Y.; Song, X.X.; Ou, X.F.; Xing, S.C.; Ma, J.; Von Wettstein, D.; Liu, B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.). J. Plant Physiol. 2011, 168, 1685–1693. [Google Scholar] [CrossRef]
- Bilichak, A.; Kovalchuk, I. Transgenerational response to stress in plants and its application for breeding. J. Exp. Bot. 2016, 67, 2081–2092. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 41. [Google Scholar]
- Niu, S.; Du, X.; Wei, D.; Liu, S.; Tang, Q.; Bian, D.; Zhang, Y.; Cui, Y.; Gao, Z. Heat stress after pollination reduces kernel number in maize by insufficient assimilates. Front. Genet. 2021, 12, 728166. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.u.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, K.; Kuchanur, P.H.; Koirala, K.B.; Tripathi, M.P.; Patil, A.; Sudarsanam, V.; Das, R.R.; Chaurasia, R.; Pandey, K.; Vemuri, H.; et al. Genomic regions associated with heat stress tolerance in tropical maize (Zea mays L.). Sci. Rep. 2021, 11, 13730. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Influence of heat stress, variations in soil type, and soil amendment on the growth of three drought–tolerant maize varieties. Agronomy 2021, 11, 1485. [Google Scholar] [CrossRef]
- Mokhtarpour, H.; Teh, C.B.; Saleh, G.; Selamat, A.B.; Asadi, M.E.; Kamkar, B. Non-destructive estimation of maize leaf area, fresh weight, and dry weight using leaf length and leaf width. Commun. Biometry Crop Sci. 2010, 5, 19–26. [Google Scholar]
- Wang, X.; Xin, C.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Heat priming induces trans-generational tolerance to high temperature stress in wheat. Front. Plant Sci. 2016, 7, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, S.E.; Barton, K.; Wilczek, A.M. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 2009, 90, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Bhandari, K.; Siddique, K.H.M.; Nayyar, H. Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016, 2, 1134380. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Yao, Y.; Kovalchuk, I. Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e27971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edreira, J.I.R.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Differences in crop growth, biomass partitioning and reserves use. Field Crop. Res. 2012, 130, 87–98. [Google Scholar] [CrossRef]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E.; Horgan-Kobelski, T.; Riggs, C. Adaptive transgenerational plasticity in an annual plant: Grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 2012, 52, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Virlouvet, L.; Liu, N.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. 2014, 14, 141. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, Ø.; Dæhlen, O.G.; Østreng, G.; Skrøppa, T. Daylength and temperature during seed production interactively affect adaptive performance of Picea abies progenies. New Phytol. 2005, 168, 589–596. [Google Scholar] [CrossRef]
- Sun, C.; Ali, K.; Yan, K.; Fiaz, S.; Dormatey, R.; Bi, Z.; Bai, J. Exploration of epigenetics for improvement of drought and other stress resistance in crops: A review. Plants 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Inyang, P.; Ene, C.O.; Emmanuel, A.; Chukwudi, U.P.; Ikeogu, U.N. Environmental impact and genetic expressions of new drought-tolerant maize genotypes in derived savannah agro-ecology. Not. Sci. Biol. 2021, 13, 10691. [Google Scholar] [CrossRef]
- Uba, C.U.; Agbo, C.U.; Chukwudi, U.P.; Efusie, A.A.; Muojiama, S.O. Field evaluation of yield and yield component traits of breeding lines of maize over two seasons in derived Savannah agro-ecology. Not. Sci. Biol. 2018, 10, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.J.; Donelson, J.M.; Schunter, C.; Ravasi, T.; Gaitán-Espitia, J.D. Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180174. [Google Scholar] [CrossRef] [PubMed]


| Traits | ENV | MVA | SAM | ENV. MVA | ENV. SAM | MVA. SAM | ENV. MVA. SAM |
|---|---|---|---|---|---|---|---|
| Emergence percentage | n.s | n.s | * | n.s | * | * | * |
| Leaf area at 10 WAP | n.s | * | * | * | * | * | * |
| Leaf chlorophyll content at 10 WAP | n.s | n.s | n.s | * | n.s | * | * |
| Number of leaves at 10 WAP | n.s | n.s | n.s | n.s | * | * | * |
| Plant height at 10 WAP | n.s | * | * | * | * | * | * |
| Stem diameter at 10 WAP | * | ** | n.s | * | * | * | * |
| Days until 50% silking | n.s | n.s | * | * | * | * | * |
| Days until 50% tasselling | * | * | * | ** | * | * | * |
| Tassel silk interval | n.s | n.s | n.s | n.s | n.s | n.s | n.s |
| Stover dry weight | n.s | n.s | n.s | n.s | n.s | * | * |
| Number of cobs | n.s | n.s | * | n.s | * | * | * |
| Length of cobs | n.s | n.s | n.s | * | ** | n.s | * |
| Width of cobs | * | * | n.s | * | * | * | * |
| Cob Column | * | n.s | n.s | * | * | * | * |
| Weight of cobs | n.s | n.s | n.s | * | ** | * | * |
| Number of grains | n.s | n.s | * | n.s | ** | * | * |
| Weight of grains | n.s | n.s | n.s | * | ** | * | * |
| Shelling Percentage | n.s | n.s | n.s | * | n.s | * | * |
| 100-Seed Weight | n.s | * | * | * | * | * | * |
| Environment | WAP | HS | NHS | F-LSD (0.05) |
|---|---|---|---|---|
| Emergence percentage (%) | 81.2 | 82.6 | n.s. | |
| Leaf area (cm2) | 4 | 285.0 | 284.0 | n.s. |
| 7 | 662.5 | 654.5 | n.s. | |
| 10 | 699.6 | 714.6 | n.s. | |
| Leaf chlorophyll content (CCI) | 4 | 38.7 | 37.4 | n.s. |
| 7 | 43.1 | 38.5 | 4.3 | |
| 10 | 53.3 | 51.3 | n.s. | |
| Number of leaves | 4 | 9.1 | 9.2 | n.s. |
| 7 | 15.3 | 15.5 | n.s. | |
| 10 | 17.9 | 18.1 | n.s. | |
| Plant height (cm) | 4 | 39.8 | 39.5 | n.s. |
| 7 | 139.9 | 136.4 | n.s. | |
| 10 | 250.5 | 257.6 | n.s. | |
| Stem diameter (mm) | 4 | 15.5 | 16.2 | n.s. |
| 7 | 33.5 | 31.9 | n.s. | |
| 10 | 32.1 | 30.2 | 1.7 |
| Maize Variety | WAP | WE3 | WE5 | ZM1 | F-LSD (0.05) |
|---|---|---|---|---|---|
| Emergence percentage (%) | 83.0 | 81.9 | 80.9 | n.s. | |
| Leaf area (cm2) | 4 | 268.2 | 300.6 | 284.7 | 29.6 |
| 7 | 647.1 | 699.3 | 629.1 | 46.8 | |
| 10 | 696.6 | 744.9 | 679.8 | 38.5 | |
| Leaf chlorophyll content (CCI) | 4 | 37.0 | 38.8 | 38.4 | n.s. |
| 7 | 35.7 | 43.7 | 43.1 | 5.3 | |
| 10 | 50.8 | 51.4 | 54.7 | n.s. | |
| Number of leaves | 4 | 8.9 | 9.1 | 9.4 | n.s. |
| 7 | 15.3 | 15.5 | 15.6 | n.s. | |
| 10 | 18.0 | 17.9 | 17.9 | n.s. | |
| Plant height (cm) | 4 | 38.3 | 41.4 | 39.3 | n.s. |
| 7 | 131.6 | 137.0 | 145.9 | 13.5 | |
| 10 | 262.4 | 252.6 | 247.2 | 11.7 | |
| Stem diameter (mm) | 4 | 14.8 | 16.4 | 16.4 | 1.4 |
| 7 | 31.4 | 35.2 | 31.5 | 2.5 | |
| 10 | 28.9 | 33.2 | 31.4 | 2.1 |
| Soil Amendment | WAP | MF | MPM | PM | F-LSD (0.05) |
|---|---|---|---|---|---|
| Emergence percentage (%) | 82.3 | 88.5 | 75.0 | 9.5 | |
| Leaf area (cm2) | 4 | 268.2 | 300.6 | 284.7 | 29.6 |
| 7 | 647.1 | 699.3 | 629.1 | 46.8 | |
| 10 | 696.6 | 744.9 | 679.8 | 38.5 | |
| Leaf chlorophyll content (CCI) | 4 | 36.4 | 37.2 | 40.5 | n.s. |
| 7 | 42.5 | 41.5 | 38.6 | n.s. | |
| 10 | 51.3 | 52.9 | 52.6 | n.s. | |
| Number of leaves | 4 | 8.7 | 9.2 | 9.6 | 0.5 |
| 7 | 15.3 | 15.7 | 15.3 | n.s. | |
| 10 | 17.9 | 18.3 | 17.7 | n.s. | |
| Plant height (cm) | 4 | 38.5 | 40.3 | 40.2 | n.s. |
| 7 | 137.0 | 141.3 | 136.1 | n.s. | |
| 10 | 248.9 | 263.0 | 250.3 | 11.7 | |
| Stem diameter (mm) | 4 | 14.7 | 16.0 | 16.9 | 1.4 |
| 7 | 32.0 | 33.6 | 32.4 | n.s. | |
| 10 | 30.0 | 31.4 | 32.1 | n.s. |
| Treatment Interactions | EP (%) | Leaf Area (cm2) | Leaf Chlorophyll Content (CCI) | Number of Leaves | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 WAP | 7 WAP | 10 WAP | 4 WAP | 7 WAP | 10 WAP | 4 WAP | 7 WAP | 10 WAP | ||
| HS-WE3-MF-P1 | 91.7 | 256.3 | 590.6 | 664.3 | 34.4 | 42.0 | 51.7 | 9.0 | 15.8 | 19.7 |
| HS-WE3-MPM-P1 | 93.8 | 275.7 | 743.0 | 765.8 | 35.5 | 39.3 | 53.7 | 9.8 | 15.8 | 17.7 |
| HS-WE3-PM-P1 | 54.2 | 229.1 | 579.9 | 609.7 | 42.0 | 35.5 | 52.0 | 7.8 | 13.4 | 16.0 |
| HS-WE5-MF-P1 | 87.5 | 317.3 | 733.8 | 747.2 | 33.2 | 44.2 | 46.4 | 9.0 | 15.6 | 17.3 |
| HS-WE5-MPM-P1 | 79.2 | 352.3 | 729.8 | 819.0 | 45.9 | 48.4 | 62.6 | 10.0 | 16.2 | 19.0 |
| HS-WE5-PM-P1 | 83.3 | 321.0 | 707.7 | 728.6 | 38.0 | 38.8 | 53.9 | 9.2 | 15.2 | 16.7 |
| HS-ZM1-MF-P1 | 83.3 | 271.8 | 724.5 | 755.3 | 41.9 | 57.7 | 62.0 | 8.6 | 14.8 | 17.0 |
| HS-ZM1-MPM-P1 | 97.9 | 264.8 | 566.7 | 609.0 | 38.3 | 43.2 | 45.6 | 9.0 | 15.4 | 19.0 |
| HS-ZM1-PM-P1 | 60.4 | 276.7 | 586.1 | 597.9 | 39.3 | 39.1 | 51.4 | 9.2 | 15.8 | 18.3 |
| NHS-WE3-MF-P1 | 89.6 | 236.2 | 640.1 | 731.1 | 30.6 | 29.5 | 41.1 | 8.2 | 15.4 | 18.3 |
| NHS-WE3-MPM-P1 | 93.8 | 257.1 | 628.8 | 654.0 | 31.5 | 30.4 | 53.8 | 8.2 | 15.6 | 18.0 |
| NHS-WE3-PM-P1 | 75.0 | 354.8 | 700.0 | 755.0 | 48.1 | 37.5 | 52.6 | 10.6 | 15.6 | 18.3 |
| NHS-WE5-MF-P1 | 72.9 | 235.0 | 688.0 | 714.6 | 38.3 | 44.5 | 47.3 | 8.2 | 14.6 | 17.3 |
| NHS-WE5-MPM-P1 | 79.2 | 256.5 | 677.2 | 751.4 | 33.8 | 41.6 | 51.1 | 8.6 | 15.0 | 18.3 |
| NHS-WE5-PM-P1 | 89.6 | 321.5 | 659.2 | 708.5 | 43.4 | 44.6 | 46.8 | 9.8 | 16.2 | 19.0 |
| NHS-ZM1-MF-P1 | 68.7 | 303.9 | 640.0 | 706.4 | 40.0 | 36.9 | 59.5 | 9.0 | 15.6 | 17.7 |
| NHS-ZM1-MPM-P1 | 87.5 | 296.3 | 572.5 | 659.5 | 38.4 | 45.8 | 50.8 | 9.6 | 16.0 | 17.7 |
| NHS-ZM1-PM-P1 | 87.5 | 294.7 | 684.6 | 750.6 | 32.1 | 35.9 | 58.7 | 10.8 | 15.8 | 18.0 |
| F-LSD (0.05) | 23.18 | 72.52 | 114.61 | 94.32 | 12.13 | 12.86 | 13.12 | 1.13 | 1.32 | 1.69 |
| CV (%) | 17.0 | 1.4 | 3.3 | 3.5 | 25.3 | 7.6 | 8.8 | 9.8 | 6.8 | 2.7 |
| Treatment Interactions | Plant Height (cm) | Stem Diameter (mm) | DS50 | DT50 | TSI | SDWt (g Plant−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 WAP | 7 WAP | 10 WAP | 4 WAP | 7 WAP | 10 WAP | |||||
| HS-WE3-MF-P1 | 40.2 | 151.8 | 279.3 | 16.2 | 31.4 | 28.8 | 70.3 | 68.0 | 2.3 | 314 |
| HS-WE3-MPM-P1 | 40.4 | 158.8 | 276.7 | 15.8 | 34.7 | 32.6 | 69.0 | 66.7 | 2.3 | 273 |
| HS-WE3-PM-P1 | 28.0 | 109.8 | 223.7 | 11.2 | 27.5 | 27.1 | 68.7 | 67.3 | 1.3 | 182 |
| HS-WE5-MF-P1 | 43.4 | 134.8 | 240.0 | 16.1 | 38.0 | 31.3 | 68.7 | 65.7 | 3.0 | 254 |
| HS-WE5-MPM-P1 | 48.8 | 139.8 | 254.7 | 17.9 | 37.7 | 36.7 | 69.3 | 67.3 | 2.0 | 282 |
| HS-WE5-PM-P1 | 40.4 | 153.2 | 248.0 | 17.4 | 36.3 | 36.3 | 71.0 | 68.7 | 2.3 | 274 |
| HS-ZM1-MF-P1 | 39.2 | 135.2 | 230.0 | 14.4 | 31.7 | 30.1 | 70.3 | 67.3 | 3.0 | 319 |
| HS-ZM1-MPM-P1 | 36.6 | 147.0 | 271.3 | 15.4 | 32.7 | 33.5 | 71.0 | 68.0 | 3.0 | 333 |
| HS-ZM1-PM-P1 | 41.5 | 128.6 | 231.0 | 15.5 | 31.0 | 32.4 | 72.0 | 69.7 | 2.3 | 300 |
| NHS-WE3-MF-P1 | 37.0 | 122.6 | 260.0 | 12.6 | 27.4 | 25.3 | 69.3 | 67.3 | 2.0 | 313 |
| NHS-WE3-MPM-P1 | 36.6 | 102.2 | 267.7 | 12.6 | 32.1 | 25.3 | 69.3 | 68.0 | 1.3 | 265 |
| NHS-WE3-PM-P1 | 47.4 | 144.4 | 267.3 | 20.3 | 35.1 | 34.2 | 73.3 | 69.7 | 3.7 | 256 |
| NHS-WE5-MF-P1 | 33.0 | 128.6 | 233.7 | 13.1 | 32.5 | 30.7 | 68.7 | 66.0 | 2.7 | 284 |
| NHS-WE5-MPM-P1 | 38.4 | 141.4 | 268.7 | 15.4 | 31.9 | 31.4 | 68.0 | 65.0 | 3.0 | 297 |
| NHS-WE5-PM-P1 | 44.4 | 124.0 | 270.3 | 18.4 | 34.5 | 32.8 | 68.7 | 67.3 | 1.3 | 424 |
| NHS-ZM1-MF-P1 | 38.2 | 149.0 | 250.3 | 15.7 | 31.3 | 33.5 | 68.7 | 65.0 | 3.7 | 325 |
| NHS-ZM1-MPM-P1 | 40.8 | 158.8 | 239.0 | 18.7 | 32.1 | 29.0 | 66.7 | 63.0 | 3.7 | 287 |
| NHS-ZM1-PM-P1 | 39.6 | 156.6 | 261.7 | 18.8 | 30.1 | 29.6 | 69.0 | 66.3 | 2.7 | 299 |
| F-LSD (0.05) | 8.11 | 33.09 | 28.62 | 3.40 | 5.75 | 5.09 | 2.95 | 2.57 | n.s. | 164 |
| CV (%) | 16.2 | 19.0 | 3.2 | 17.0 | 14.0 | 7.0 | 2.6 | 2.3 | 67.7 | 33.7 |
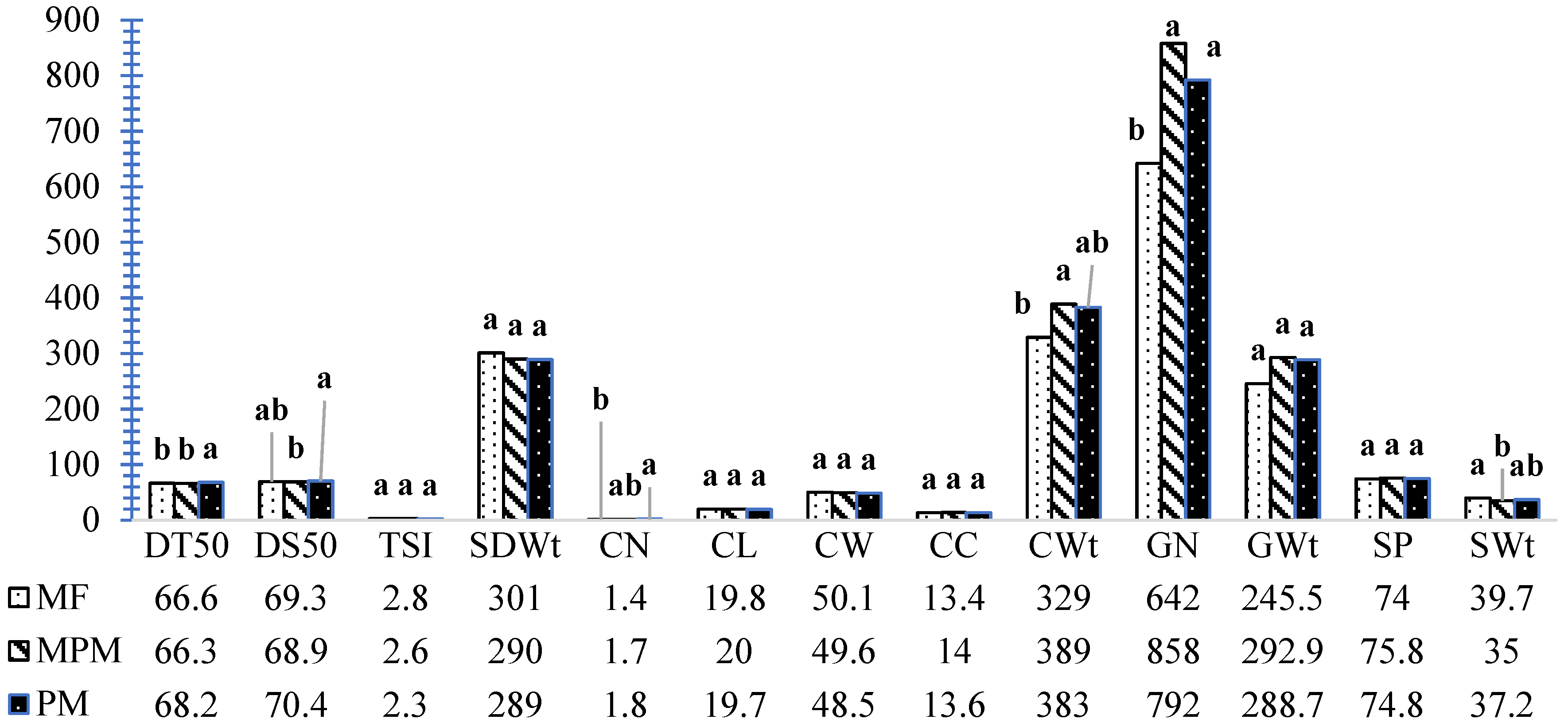
| SN | Treatment Interactions | CN | CL | CW | CC | CWt | GN | GWt | SP | SWt |
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (mm) | (g plant−1) | (g Plant−1) | (%) | (g Plant−1) | |||||
| H1 | HS-WE3-MF-P1 | 1.8 | 18.8 | 48.5 | 13.6 | 365 | 793 | 273.6 | 73.5 | 34.9 |
| H2 | HS-WE3-MPM-P1 | 1.8 | 20.8 | 49.4 | 13.8 | 394 | 847 | 304.2 | 77.3 | 36.7 |
| H3 | HS-WE3-PM-P1 | 1.2 | 17.7 | 49.0 | 12.4 | 248 | 511 | 185.2 | 76.2 | 38.8 |
| H4 | HS-WE5-MF-P1 | 1.2 | 19.6 | 49.5 | 13.2 | 270 | 508 | 190.3 | 70.4 | 37.7 |
| H5 | HS-WE5-MPM-P1 | 2.0 | 20.8 | 53.3 | 14.4 | 579 | 1067 | 415.1 | 71.6 | 39.3 |
| H6 | HS-WE5-PM-P1 | 1.8 | 17.9 | 45.7 | 13.4 | 307 | 773 | 239.7 | 77.1 | 31.7 |
| H7 | HS-ZM1-MF-P1 | 1.4 | 19.9 | 49.5 | 12.4 | 336 | 619 | 255.3 | 76.1 | 44.3 |
| H8 | HS-ZM1-MPM-P1 | 1.6 | 21.0 | 48.1 | 14.6 | 350 | 967 | 270.2 | 77.2 | 28.6 |
| H9 | HS-ZM1-PM-P1 | 2.0 | 18.5 | 44.3 | 12.2 | 269 | 502 | 195.0 | 71.3 | 39.5 |
| N1 | NHS-WE3-MF-P1 | 1.4 | 20.1 | 51.9 | 13.0 | 330 | 593 | 252.3 | 76.7 | 43.6 |
| N2 | NHS-WE3-MPM-P1 | 1.4 | 20.9 | 50.7 | 14.6 | 352 | 740 | 273.2 | 77.6 | 38.9 |
| N3 | NHS-WE3-PM-P1 | 1.8 | 22.4 | 54.0 | 15.2 | 590 | 1013 | 423.2 | 68.9 | 41.9 |
| N4 | NHS-WE5-MF-P1 | 1.2 | 20.0 | 49.9 | 14.0 | 291 | 568 | 207.8 | 71.2 | 37.6 |
| N5 | NHS-WE5-MPM-P1 | 1.8 | 17.9 | 48.1 | 14.2 | 371 | 888 | 275.9 | 74.3 | 31.2 |
| N6 | NHS-WE5-PM-P1 | 1.6 | 20.3 | 49.5 | 14.8 | 371 | 839 | 278.2 | 75.5 | 33.2 |
| N7 | NHS-ZM1-MF-P1 | 1.6 | 20.4 | 51.5 | 14.4 | 385 | 770 | 293.7 | 76.2 | 39.8 |
| N8 | NHS-ZM1-MPM-P1 | 1.6 | 18.3 | 48.0 | 12.6 | 285 | 638 | 219.1 | 77.0 | 35.4 |
| N9 | NHS-ZM1-PM-P1 | 2.4 | 21.4 | 48.7 | 13.4 | 514 | 1113 | 410.6 | 79.8 | 38.0 |
| F-LSD (0.05) | 0.7 | 2.6 | 4.8 | 2.0 | 142 | 363.8 | 119.5 | 7.2 | 7.3 | |
| CV (%) | 33.9 | 10.3 | 7.7 | 11.6 | 30.7 | 37.7 | 34.4 | 7.7 | 15.4 |
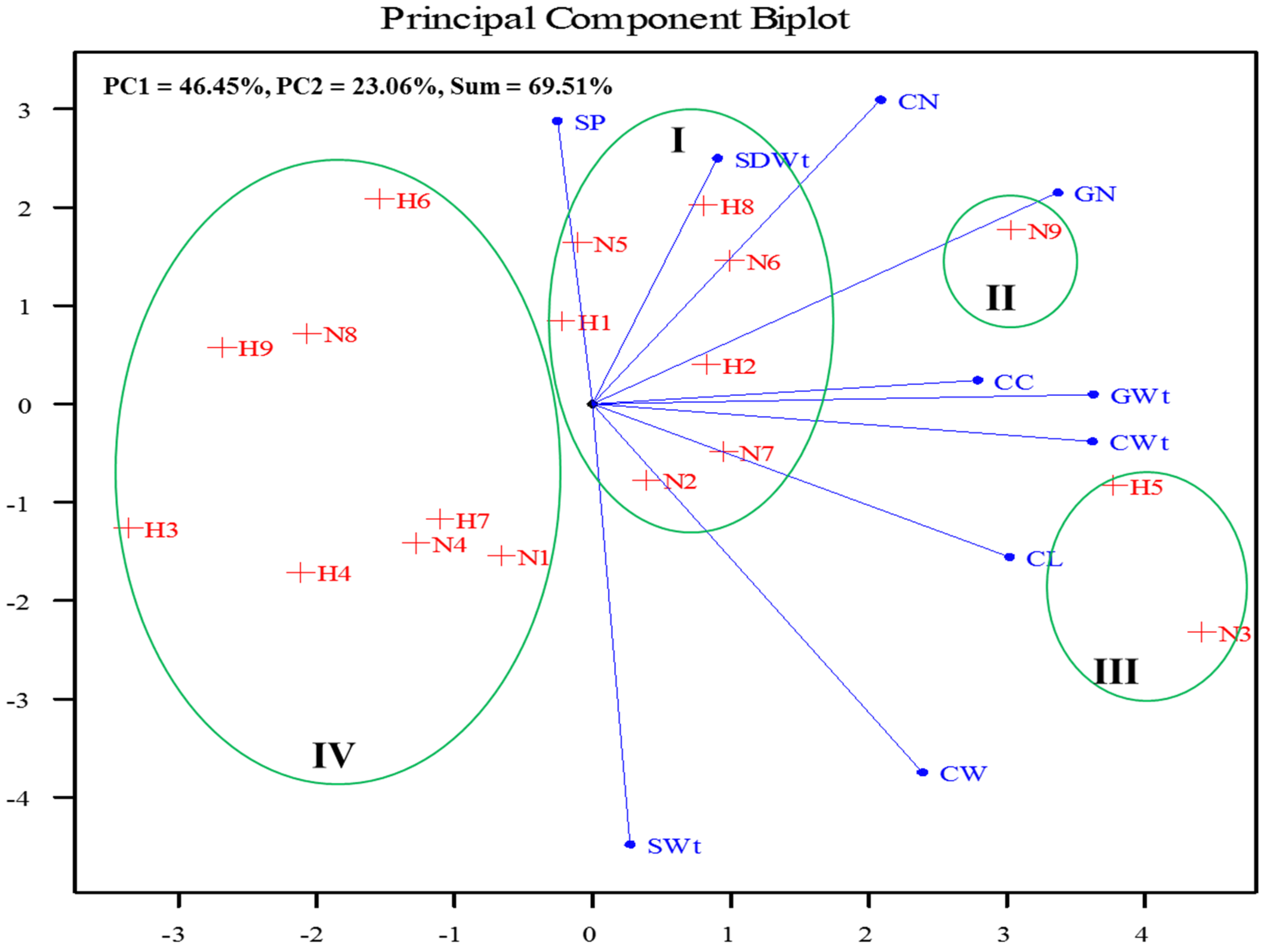
| Attributes | I | II | III | IV | Population Mean |
|---|---|---|---|---|---|
| Stover dry weight (g plant−1) | 318.7 | 299.0 | 269.0 | 276.6 | 293.4 |
| Number of cobs | 1.7 | 2.4 | 1.9 | 1.5 | 1.6 |
| Length of cob (cm) | 20.0 | 21.4 | 21.6 | 19.0 | 19.8 |
| Width of cob (mm) | 49.4 | 48.7 | 53.6 | 48.5 | 49.4 |
| Cob Column | 14.3 | 13.4 | 14.8 | 12.9 | 13.7 |
| Weight of cob (g plant−1) | 369.7 | 514.0 | 584.5 | 292.0 | 367.1 |
| Number of grains | 834.9 | 1113.0 | 1040.0 | 589.0 | 763.8 |
| Weight of grains (g plant−1) | 281.3 | 410.6 | 419.2 | 218.1 | 275.7 |
| Shelling Percentage (%) | 75.9 | 79.8 | 70.3 | 74.5 | 74.9 |
| 100-Seed Weight (g plant−1) | 34.8 | 38.0 | 40.6 | 38.6 | 37.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. The Influence of Parental Heat-Stress Priming on Drought-Tolerant Maize Progenies’ Field Performance. Agriculture 2021, 11, 1229. https://doi.org/10.3390/agriculture11121229
Chukwudi UP, Kutu FR, Mavengahama S. The Influence of Parental Heat-Stress Priming on Drought-Tolerant Maize Progenies’ Field Performance. Agriculture. 2021; 11(12):1229. https://doi.org/10.3390/agriculture11121229
Chicago/Turabian StyleChukwudi, Uchechukwu Paschal, Funso Raphael Kutu, and Sydney Mavengahama. 2021. "The Influence of Parental Heat-Stress Priming on Drought-Tolerant Maize Progenies’ Field Performance" Agriculture 11, no. 12: 1229. https://doi.org/10.3390/agriculture11121229
APA StyleChukwudi, U. P., Kutu, F. R., & Mavengahama, S. (2021). The Influence of Parental Heat-Stress Priming on Drought-Tolerant Maize Progenies’ Field Performance. Agriculture, 11(12), 1229. https://doi.org/10.3390/agriculture11121229








