Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview
Abstract
:1. Introduction
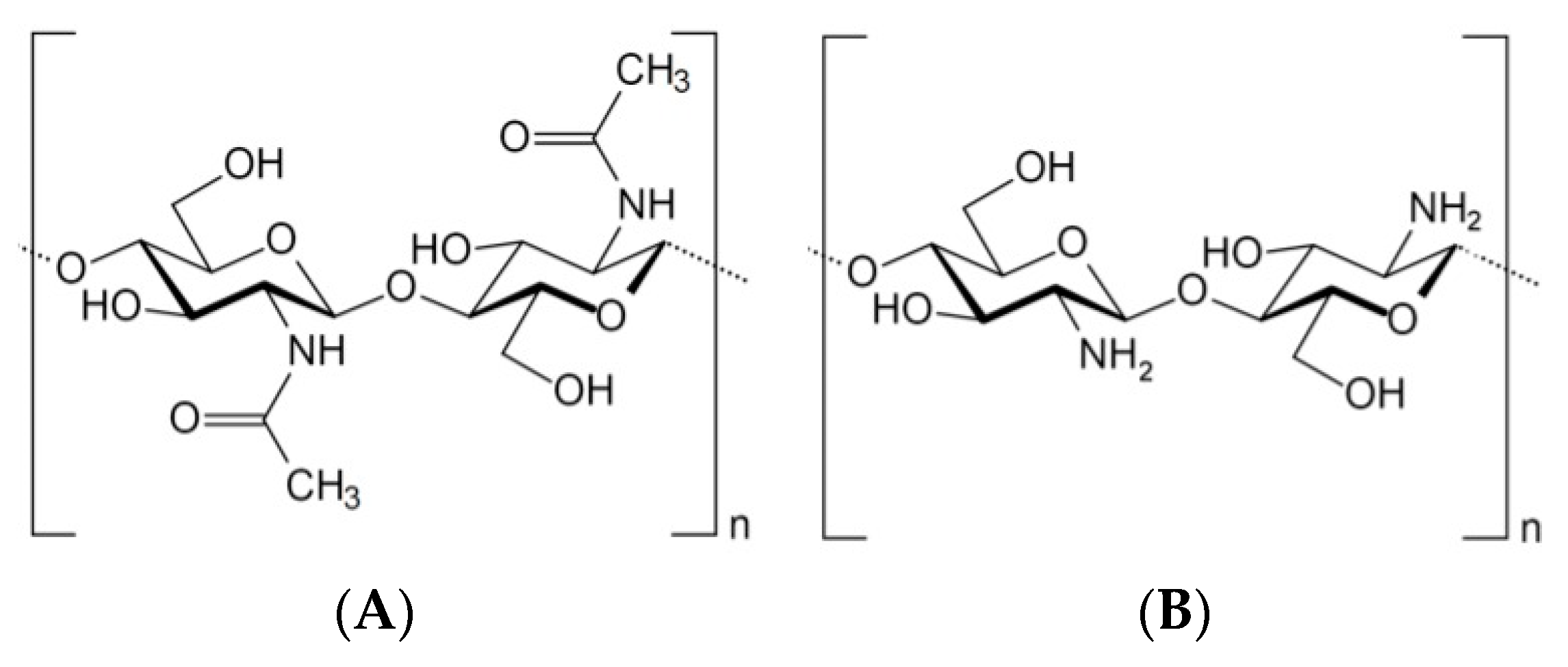
2. Chitin and Chitosan Structural Properties
3. Chitosan Applications in Beverages
3.1. Clarification Agent, Metals and Contaminants Reduction
3.2. Extending the Shelf-Life
4. Chitosan Immobilization
- (i)
- (ii)
- The neutralization method is usually exploited to produce chitosan precipitates, membranes, fibers, and spherical beads of different sizes and porosities. Beads are obtained by dropping a chitosan solution into a solution of NaOH prepared in water-ethanol mixtures once ethanol facilitates the solidification of the beads [124]. Nowadays, this method is also used to produce chitosan films with favorable physical and barrier properties for improving the preservation of chilled meat [128].
- (iii)
- Crosslinking method where an acidic chitosan solution is exposed to straightforward crosslinking by mixing with a crosslinking agent, which results in gelling [124]. Recently, a new emulsification-crosslinking method was developed by Shi et al. [129] for the preparation of chitosan microspheres using an aqueous alkali–urea solution, as an alternative to the commonly used acidic solvents, to dissolve chitosan.
4.1. Chitosan Microorganism’s Immobilization
4.2. Chitosan Enzyme Immobilization
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Mathur, N.K.; Narang, C.K. Chitin and chitosan, versatile polysaccharides from marine animals. J. Chem. Educ. 1990, 67, 938–942. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Gassara, F.; Antzak, C.; Ajila, C.M.; Sarma, S.J.; Brar, S.K.; Verma, M. Chitin and chitosan as natural flocculants for beer clarification. J. Food Eng. 2015, 166, 80–85. [Google Scholar] [CrossRef]
- Ghorbel-Bellaaj, O.; Jridi, M.; Khaled, H.; Jellouli, K.; Nasri, M. Bioconversion of shrimp shell waste for the production of antioxidant and chitosan used as fruit juice clarifier. Int. J. Food Sci. Technol. 2012, 47, 1835–1841. [Google Scholar] [CrossRef]
- Rao, L.; Hayat, K.; Lv, Y.; Karangwa, E.; Xia, S.; Jia, C.; Zhong, F.; Zhang, X. Effect of ultrafiltration and fining adsorbents on the clarification of green tea. J. Food Eng. 2011, 102, 321–326. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; McMillan Press, Ltd.: London, UK, 1992; pp. 85–91. [Google Scholar]
- Soto-Peralta, N.V.; Muller, H.; Knorr, D. Effects of chitosan treatments on the clarity and color of apple juice. J. Food Sci. 1989, 54, 495–496. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef]
- Tastan, O.; Baysal, T. Clarification of pomegranate juice with chitosan: Changes on quality characteristics during storage. Food Chem. 2015, 180, 211–218. [Google Scholar] [CrossRef]
- Tastan, Ö.; Baysal, T. Chitosan as a novel clarifying agent on clear apple juice production: Optimization of process conditions and changes on quality characteristics. Food Chem. 2017, 237, 818–824. [Google Scholar] [CrossRef] [PubMed]
- OIV. International Code of Oenological Practices. International Organisation of Vine and Wine. 2021. Available online: http://www.oiv.int/fr/normes-et-documents-techniques (accessed on 30 October 2021).
- Vincenzi, S.; Polesani, M.; Curioni, A. Removal of specific protein components by chitin enhances protein stability in a white wine. Am. J. Enol. Vitic. 2005, 56, 246–254. [Google Scholar]
- Chagas, R.; Monteiro, S.; Ferreira, R.B. Assessment of potential side effects of common fining agents used for white wine protein stabilization. Am. J. Enol. Vitic. 2012, 63, 574. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Arenas, I.; Ribeiro, M.; Filipe-Ribeiro, L.; Vilamarim, R.; Costa, E.; Siopa, J.; Cosme, F.; Nunes, F.M. Effect of winemaking technology on protein stability, macro-molecular, and phenolic composition of Albariño white wines: Comparative efficiency of chitosan, k-carrageenan and bentonite as heat stabilisers. Foods 2021, 10, 608. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Ferreira, P.; Silva, M.A.; Rodrigues, A.; Amado, O.; Coimbra, J.; et al. Chitosan–genipin film, a sustainable methodology for wine preservation. Green Chem. 2016, 18, 5331–5341. [Google Scholar] [CrossRef]
- Spagna, G.; Pifferi, P.G.; Rangoni, C.; Mattivi, F.; Nicolini, G.; Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int. 1996, 29, 241–248. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Pifferi, P.G. Fining treatments of white wines by means of polymeric adjuvants for their stabilization against browning. J. Agric. Food Chem. 2000, 48, 4619–4627. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Riponi, C. Efficacy of chitosan in inhibiting the oxidation of (+)-catechin in white wine model solutions. J. Agric. Food Chem. 2014, 62, 9868–9875. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, D.; Costa, E.M.; Silva, S.; Pintado, M.M.; Couto, J.A. The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J. Chitin Chitosan Sci. 2013, 1, 240–245. [Google Scholar] [CrossRef]
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of a fungal-origin chitosan preparation on Brettanomyces bruxellensis in the context of wine aging. Wine Stud. 2014, 3, 4574. [Google Scholar] [CrossRef] [Green Version]
- Elmaci, S.B.; Gülgör, G.; Tokatli, M.; Erten, H.; İşci, A.; Özçelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 2015, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. OENO One 2016, 50, 49–56. [Google Scholar] [CrossRef]
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef]
- Milheiro, J.; Ribeiro, L.F.; Cosme, F.; Nunes, F.M. A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B 2017, 1041–1042, 183–190. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Reducing the negative sensory impact of volatile phenols in red wine with different chitosans: Effect of structure on efficiency. Food Chem. 2018, 242, 591–600. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Data on changes in red wine phenolic compounds, headspace aroma compounds and sensory profile after treatment of red wines with chitosans with different structures. Data Brief. 2018, 17, 1201–1217. [Google Scholar] [CrossRef]
- Castro-Marín, A.; Colangelo, D.; Lambri, M.; Riponi, C.; Chinnici, F. Relevance and perspectives of the use of chitosan in winemaking: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Boeris, V.; Micheletto, Y.; Lionzo, M.; da Silveira, N.P.; Pico, G. Interaction behavior between chitosan and pepsin. Carbohydr. Polym. 2011, 84, 459–464. [Google Scholar] [CrossRef]
- Marudova, M.; MacDougall, A.J.; Ring, S.-G. Pectin–chitosan interactions and gel ormation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Savant, V.D.; Torres, J.A. Fourier transform infrared analysis of chitosan based coagulating agents for treatment of surimi waste water. J. Food Technol. 2003, 1, 23–28. Available online: https://medwelljournals.com/abstract/?doi=jftech.2003.23.28 (accessed on 24 November 2021).
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133. [Google Scholar] [CrossRef] [Green Version]
- Khanafari, A.; Marandi, R.; Sanatei, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran. J. Environ. Health Sci. Eng. 2008, 5, 1–24. [Google Scholar]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 1–14. [Google Scholar] [CrossRef]
- Kafetzopoulos, D.; Martinou, A.; Bouriotis, V. Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Nat. Acad. Sci. USA 1993, 90, 2564–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Il’ina, A.V.; Tkacheva, Y.V.; Varlamov, V.P. Depolymerization of High-Molecular-Weight Chitosan by the Enzyme Preparation Celloviridine G20x. Appl. Biochem. Microbiol. 2002, 38, 112–115. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A review. J. Aquatic Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and Chitosan Preparation from Shrimp Shells Using Optimized Enzymatic Deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Chaabouni, M.; Rinaudo, M.; Souard, F.; Vanhaverbeke, C.; Jellouli, K.; Nasri, M. Use of a fractional factorial design to study the effects of experimental factors on the chitin deacetylation. Int. J. Biol. Macromol. 2014, 70, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Edebo, L.; Sjostrom, B.; Taherzadeh, M.J. Extraction and precipitation of chitosan from cell wall of zygomycetes fungi by dilute sulfuric acid. Biomacromolecules 2007, 8, 3786–3790. [Google Scholar] [CrossRef]
- Streit, F.; Koch, F.; Laranjeira, M.C.M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibeka, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Kandra, P.; Challa, M.M.; Jyothi, H.K. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. 2014, 45, 72–81. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.-H.; De Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Abdou, E.S.; Elkholy, S.S.; Elsabee, M.Z.; Mohamed, E. Improved Antimicrobial Activity of Polypropylene Films by Plasma Surface treatment and Modification with Chitosan. J. Appl. Polym. Sci. 2008, 108, 2290–2296. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Close, H.N.; Sin, S.; Yusof, N.S.A.; Hamid, R.A. Rahman Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 2006, 73, 313–319. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Clarification of fruit juice with chitosan. Process Biochem. 2004, 39, 2229–2232. [Google Scholar] [CrossRef]
- Rungsardthong, V.; Wongvuttanakul, N.; Kongpien, N.; Chotiwaranon, P. Application of fungal chitosan for clarification of apple juice. Process Biochem. 2006, 41, 589–593. [Google Scholar] [CrossRef]
- Rizzo, L.; Di Gennaro, A.; Gallo, M.; Belgiorno, V. Coagulation/chlorination of surface water: A comparison between chitosan and metal salts. Sep. Purif. Technol. 2008, 62, 79–85. [Google Scholar] [CrossRef]
- Domingues, R.C.C.; Faria, S.B., Jr.; Silva, R.B.; Cardoso, V.L.; Reis, M.H.M. Clarification of passion fruit juice with chitosan: Effects of coagulation process variables and comparison with centrifugation and enzymatic treatments. Process Biochem. 2012, 47, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Ariffin, A.; Shatat, R.S.; Norulaini, A.N.; Omar, A.M. Synthetic polyelectrolytes of varying charge densities but similar molar mass based on acrylamide and their applications on palm oil mill effluent treatment. Desalination 2005, 173, 201–208. [Google Scholar] [CrossRef]
- Kalve, H.G.; Duran, A. The Use of Chitosan as Clarification Agent. In Proceedings of the International Conference on Engineering and Natural Science, Sarajevo, Serbia, 12–14 July 2016; pp. 2811–2814. [Google Scholar]
- Chen, Y.; Li, C. Studies on the application of chitosan to clarification of grape-fruit juice. Food Sci. Taiwan 1996, 23, 617–628. [Google Scholar]
- Martin-Diana, A.B.; Rico, D.; Barat, J.M.; Barry-Ryan, C. Orange juices enriched with chitosan: Optimisation for extending the shelf-life. Innov. Food Sci. Emerg. Technol. 2009, 10, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Penders, M.H.G.M.; Scollard, D.J.P.; Needham, D.; Pelan, E.G.; Davies, A.P. Some molecular and colloidal aspects of tea cream formation. Food Hydrocoll. 1998, 12, 443–450. [Google Scholar] [CrossRef]
- Leiper, K.A.; Stewart, G.G.; McKeown, I.P.; Nock, T.; Thompson, M.J. Optimising beer stabilisation by the selective removal of tannoids and sensitive proteins. J. Inst. Brew. Distill. 2005, 111, 118–127. [Google Scholar] [CrossRef]
- Rehmanji, M.; Gopal, C.; Mola, A. Beer stabilization technology-clearly a matter of choice. Tech. Q. MBAA 2005, 42, 332–338. [Google Scholar]
- McMurrough, I.; O’Rourke, T. New insight into the mechanism of achieving colloidal stability. Tech. Q. MBAA 1997, 34, 271–277. [Google Scholar]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem. Eng. Sci. 2006, 118, 99–105. [Google Scholar] [CrossRef]
- Masri, M.S.; Reuter, F.W.; Friedman, M. Binding of metal cations by natural substances. J. Appl. Polym. Sci. 1974, 18, 675–681. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. Eur. Food Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Khan, F.I.; Rahman, S.; Queen, A.; Ahamad, S.; Ali, S.; Kim, J.; Hassan, M.I. Implications of molecular diversity of chitin and its derivatives. Appl. Microbiol. Biotechnol. 2017, 101, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, D.; Gao, X.; Zhang, L.; Xu, Y.; Li, Y. Removal of arsenic from “Laminaria japonica Aresch” juice using As(III)-imprinted chitosan resin. Eur. Food Res. Technol. 2011, 232, 911–917. [Google Scholar] [CrossRef]
- Magomedov, Z.B.; Dagestan, M. Extraction of heavy metal cations from wine materials using chitosan. Storage Process. Farm Prod. 2014, 6, 31–34. [Google Scholar]
- Kurtbay, H.M.; Bekçi, Z.; Merdivan, M.; Yurdakoc, K. Reduction of ochratoxin a levels in red wine by bentonite, modified bentonites, and chitosan. J. Agric. Food Chem. 2008, 56, 2541–2545. [Google Scholar] [CrossRef]
- Quintela, S.; Villaran, M.C.; Lopez De Armentia, I.; Elejalde, E. Ochratoxin a removal from red wine by several oenological fining agents: Bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit. Contam. Part A 2012, 29, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalapathy, R.; Packirisamy, A.S.B.; Indira Ramachandran, A.C.; Udhyasooriyan, L.P.; Peter, M.J.; Senthilnathan, K.; Basheer, V.A.; Muthusamy, S. Assessing the effect of chitosan on pesticide removal in grape juice during clarification by gas chromatography with tandem mass spectrometry. Process Biochem. 2020, 94, 305–312. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Chitosan applications for the food industry. In Chitosan: Derivatives, Composites and Applications; Wiley: Hoboken, NJ, USA, 2017; Chapter 8; pp. 183–232. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans. Food J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Castro, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thetiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.; Li, C.; Lee, C.; Chen, H. Influence of micronized chitosan on antioxidative activities in grape juice. Food Nutr. Sci. 2013, 4, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Wang, P.; Li, C.; Li, Z.; Li, P. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorgan. Med. Chem. 2005, 13, 1573. [Google Scholar] [CrossRef]
- Chien, P.-J.; Sheu, F.; Huang, W.-T.; Su, M.-S. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chem. 2007, 102, 1192–1198. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Perez-Berna, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Nah, J.-W.; Park, Y. pH-dependent mode of antibacterial actions of low molecular weight water-soluble chitosan (LMWSC) against various pathogens. Macromol. Res. 2011, 19, 853–860. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodriguez, M.S.; Debbaudt, A.; Zuniga, A.; Casalongue, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.M.J.; Ocio Lagaron, J.M. The use of chitosan in antimicrobial films for food protection. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2010, 5, 1–11. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa Martinez-Rodriguez, A.J. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganism and its mechanism of action. J. Food Prot. 2009, 72, 1735–1738. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.A. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench 2012, 5, 16–23. [Google Scholar]
- Amaral, L.; Silva, D.; Couto, M.; Nunes, C.; Rocha, S.M.; Coimbra, M.A.; Coimbra, A.; Moreira, A. Safety of chitosan processed wine in shrimp allergic patients. Ann. Allergy Asthma Immunol. 2016, 116, 462–463. [Google Scholar] [CrossRef]
- Dalton-Bunnow, M.F. Review of sulfite sensitivity. Am. J. Hosp. Pharm. 1985, 42, 2220–2226. [Google Scholar] [CrossRef]
- Armentia, A. Adverse reactions to wine: Think outside the bottle. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 266–269. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V. Wine features related to safety and consumer health: An integrated perspective. Crit. Rev. Food Sci. Nutr. 2012, 52, 31–54. [Google Scholar] [CrossRef] [Green Version]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Gallego, R.; Puxeu, M.; Nart, E.; Martın, L.; Andorra, I. Evaluation of tempranillo and albarino SO2-free wines produced by different chemical alternatives and winemaking procedures. Food Res. Int. 2017, 102, 647–657. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.-B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2015, 118, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sapers, G.M. Chitosan enhances control of enzymatic browning in apple and pear juice by filtration. J. Food Sci. 1992, 57, 1192–1193. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Strand, S.P.; Lelu, S.; Reitan, N.K.; de Lange Davies, C.; Artursson, P.; Vårum, K.M. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials 2010, 31, 975–987. [Google Scholar] [CrossRef]
- Gao, P.; Xia, G.; Bao, Z.; Feng, C.; Cheng, X.; Kong, M.; Liu, Y.; Chen, X. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016, 91, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Shi, R.; Borgens, R.B.J. Chitosan nanoparticle-based neuronal membrane sealing and neuroprotection following acrolein-induced cell injury. Biol. Eng. 2010, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.-L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2016, 43, 3527–3537. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Canepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Arguelles-Monal, W.; Sarabia-Sainz, A.; Lucero-Acuña, A.; del Castillo-Castro, T.; San Román, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature stimuli-responsive nanoparticles from chitosan-graft-poly (N-vinylcaprolactam) as a drugdeliverysystem. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; LamazaresArcia, E.; Fleitas-Salazar, N.; Gancino Guevara, M.; Mansilla, R.; Gomez-Gaete, C.; Altamirano, C.; Fernández, K.; Ruiz, A.; Toledo Alonso, J.R. Polymeric nanoencapsulation of alpha interferon increases drug bioavailability and induces a sustained antiviral response in vivo. Mater. Sci. Eng. C 2020, 116, 111260. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Jamil, S.N. Synthesis and Optimization of Chitosan Nanoparticles Loaded with l-Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [Green Version]
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization, and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microbial Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Siso, M.I.G.; Lang, E.; Carrenõ-Gómez, B.; Becerra, M.; Espinar, F.O.; Méndez, J.B. Enzyme encapsulation on chitosan microbeads. Process Biochem. 1997, 32, 211–216. [Google Scholar] [CrossRef]
- Essa, D.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Comparative Nanofabrication of PLGA-Chitosan-PEG Systems Employing Microfluidics and Emulsification Solvent Evaporation Techniques. Polymers 2020, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Villicaña-Molina, E.; Pacheco-Contreras, E.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Pectin and chitosan microsphere preparation via a water/oil emulsion and solvent evaporation method for drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 467–475. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.; Goff, H.D.; Zhong, F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Shi, A.; Guan, Y.; Zhang, Y. A new emulsification-crosslinking technique for preparation of physically crosslinked chitosan microspheres. J. Bioact. Compat. Polym. 2020, 35, 289–300. [Google Scholar] [CrossRef]
- Nedović, V.A.; Manojlovic, V.; Bugarski, B.; Willaert, R. State of the art in immobilized/encapsulated cell technology in fermentation processes. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Nedović, V.A., Zuidam, N.J., Eds.; Springer: London, UK, 2010; pp. 119–146. [Google Scholar]
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast Immobilization Systems for Alcoholic Wine Fermentations: Actual Trends and Future Perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.; Etievant, P.X. Quantitative determination of solerone and sotolona in flor sherries by two dimensional-capillary GC. J. High Resol. Chromat. Chromat. Commun. 1991, 14, 133–135. [Google Scholar] [CrossRef]
- Anastas, P.T.; Rodriguez, A.; Winter, T.M.; Coish, P.; Zimmerman, J.B. A review of immobilization techniques to improve the stability and bioactivity of lysozyme. Green Chem. Lett. Rev. 2021, 14, 302–338. [Google Scholar] [CrossRef]
- Klinkenberg, G.; Lystad, K.Q.; Levine, D.W.; Dyrset, N. Cell release from alginate immobilized Lactococcus lactis ssp. lactis in chitosan and alginate coated beads. J. Dairy Sci. 2001, 84, 1118–1127. [Google Scholar] [CrossRef]
- Liouni, M.; Drichoutis, P.; Nerantzis, E.T. Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads, using Saccharomyces cerevisiae entrapped cells. World J. Microbiol. Biotechnol. 2008, 24, 281–288. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2013, 97, 4991–5000. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Wang, D.; Xu, Y. Immobilized Rhodotorula mucilaginosa: A novel urethanase-producing strain for degrading ethyl carbamate. Appl. Biochem. Biotechnol. 2013, 171, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Cecchi, T.; Lombardelli, C.; Maresca, D.; Mauriello, G.; Esti, M. Novel microencapsulated yeast for the primary fermentation of green beer: Kinetic behavior, volatiles, and sensory profile. Food Chem. 2021, 340, 127900. [Google Scholar] [CrossRef]
- Günata, Y.Z.; Bayonove, C.L.; Tapiero, C.; Cordonnier, R.E. Hydrolysis of grape monoterpenyl β-d-glucosides by various β-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for wine fermentation: Current and perspective applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Greco, E.; Manenti, I.; Pifferi, P.G. A mixture of purified glycosidases from Aspergillus niger for oenological application immobilised by inclusion in chitosan gels. Enzym. Microb. Technol. 2002, 30, 80–89. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Ahumada, K.; Martínez-Gil, A.; Moreno-Simunovic, Y.; Illanes, A.; Wilson, L. Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates. Molecules 2016, 21, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef]
- Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Xie, W.; Zeng, D.; Cai, D.; Wang, H.; Zhou, C.; Wang, J.; Li, L. Alkaline phosphatase enzyme-induced biomineralization of chitosan scaffolds with enhanced osteogenesis for bone tissue engineering. Chem. Eng. J. 2019, 371, 618–630. [Google Scholar] [CrossRef]
- Cappannella, E.; Benucci, I.; Lombardelli, C.; Liburdi, K.; Bavaro, T.; Esti, M. Immobilized lysozyme for the continuous lysis of lactic bacteria in wine: Bench-scale fluidized-bed reactor study. Food Chem. 2016, 210, 49–55. [Google Scholar] [CrossRef]
- Han, Q.; Shi, J.; Zhu, J.; Lv, H.; Du, S. Enzymes extracted from apple peels have activity in reducing higher alcohols in Chinese liquors. J. Agric. Food Chem. 2014, 62, 9529–9538. [Google Scholar] [CrossRef]
- Guo, C.; Han, L.; Guo, M.; Li, M.; Yu, L.; Yang, Y. Synthesis of triethylene tetramine-modified water-insoluble corn flour caged in magnetic chitosan resin and its adsorption application for removal of patulin from apple juice. J. Food Sci. 2020, 85, 1371–1379. [Google Scholar] [CrossRef]
- Nouri, M.; Khodaiyan, F. Magnetic Biocatalysts of Pectinase: Synthesis by Macromolecular Cross-Linker for Application in Apple Juice Clarification. Food Technol. Biotechnol. 2020, 58, 391–401. [Google Scholar] [CrossRef]
- Zhang, S.; Bilal, M.; Zdarta, J.; Cui, J.; Kumar, A.; Franco, M.; Ferreira, L.F.R.; Iqbal, H.M. Biopolymers, and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res. Int. 2021, 140, 109979. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M. Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mater. Res. Technol. 2019, 8, 3644–3652. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Cacciotti, I.; Esti, M. Multi-enzymatic Systems Immobilized on Chitosan Beads for Pomegranate Juice Treatment in Fluidized Bed Reactor: Effect on Haze-Active Molecules and Chromatic Properties. Food Bioprocess Technol. 2019, 12, 1559–1572. [Google Scholar] [CrossRef]
- Lima, P.C.; Gazoni, I.; de Carvalho, A.M.G.; Bresolin, D.; Cavalheiro, D.; de Oliveira, D.; Rigo, E. β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. Food Chem. 2021, 349, 129050. [Google Scholar] [CrossRef]
- Facin, B.R.; Melchiors, M.S.; Valério, A.; Oliveira, J.V.; Oliveira, D. Driving Immobilized Lipases as Biocatalysts: 10 Years State of the Art and Future Prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
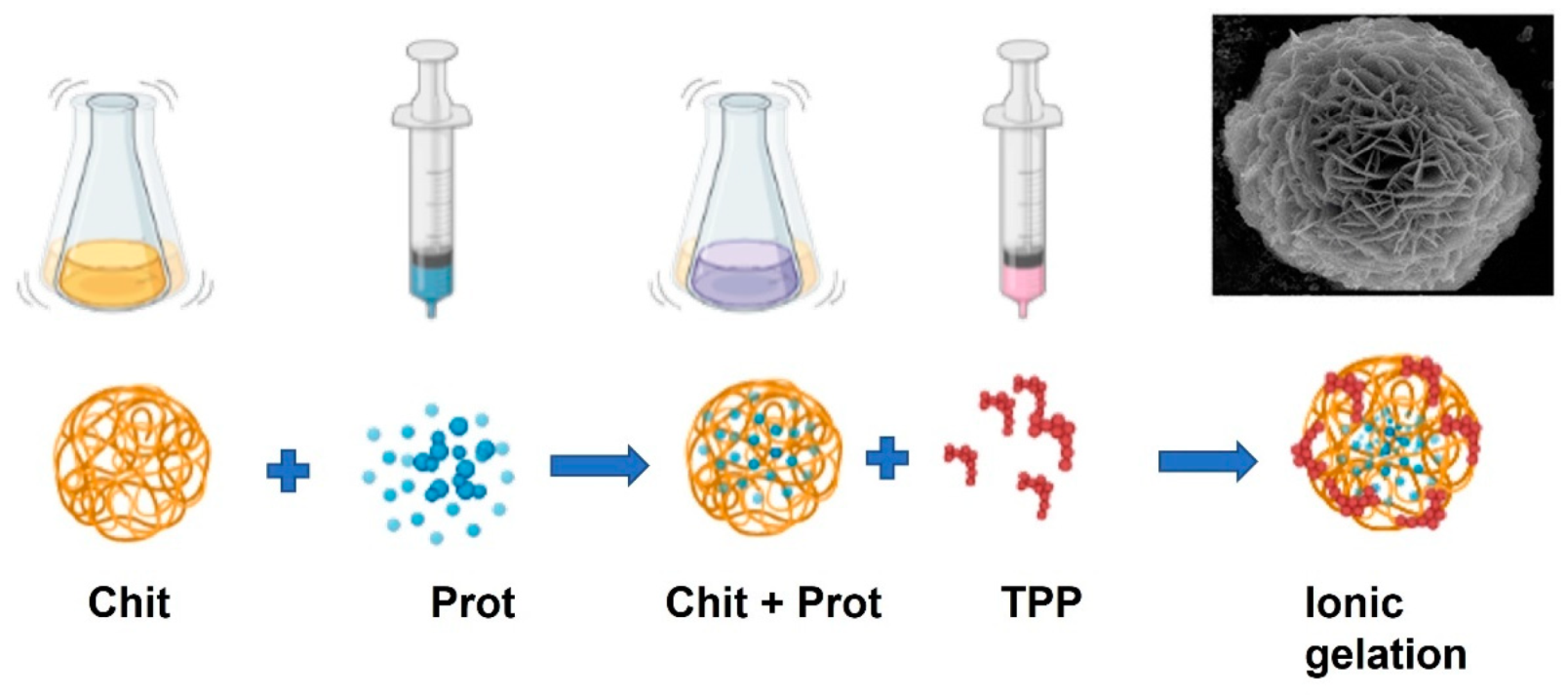
| Chitosan Origin/Type | Beverage | Activity/Advantages | Reference |
|---|---|---|---|
| Chitin and chitosan | Beer | Application of chitin (5 mg/L) and chitosan (5 mg/L) for clarification and haze removal in beer. Chitin and chitosan showed the ability to flocculate beer colloidal particles, being chitosan’s most effective. | [4] |
| Chitosan (deacetylated: 95%, molecular weight 100 kDa) | Green tea infusions | Chitosan coagulates the anionic compounds (pectin, proteins, etc.) from the tea infusion, and chelate metal ions selectively, separating the suspended particles from beverages. Being therefore effective to clarify green tea infusions | [6] |
| Commercial water soluble chitosan (Carboxymethyl chitosan, CAS No. 83512-85-0) deacetylation degree of 90–95%, medium molecular weight | Pomegranate juice | Clarifying agent | [10] |
| Chitosan from squid gladius (Loligo vulgaris) Deacetylation degree—71% | Apple juice | Application as a clarifying agent without affecting nutritional value. Chitosan (400 mg/mL) exhibited high antimicrobial activity against. Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Fusarium solani, Botrytis cinerea, Alternaria solani. | [11] |
| Fungal chitosan (from Agaricus bisporus or Aspergillus niger) | Grape must and wine | Application as a fining agent to facilitate settling and clarification, as well as a treatment to prevent protein haze, allowed, at a maximum dose of 100 g/hL (OIV-OENO 336B/2009; OIV-OENO 337A-2009). | [12] |
| Fungal chitosan (from Agaricus bisporus or Aspergillus niger) | Wine | Application to avoid wine iron and copper instability and to reduce heavy metals (iron, lead, cadmium, and copper) at a maximum dose of 100 g/hL (OIV-OENO 338A/2009), to reduce possible wine contaminants, especially ochraoxin A, at a maximum dose of 500 g/hL (OIV-OENO 338A/2009) and to control the development of undesirable microorganisms, namely Brettanomyces sp. at a maximum dose of 10 g/hL (OIV-OENO 338A/2009). | [12] |
| Chitosan from shrimp shell | Fruit juice | Application of chitosan at low concentration showed to be effective in the clarification of different fruit juices (apple, grape, lemon, and orange juices). | [59] |
| Acid-soluble fungal chitosan (A. glauca var. paradoxa) Degree of deacetylation 86%. | Apple juice | The fungal chitosan is highly effective in reducing juice turbidity. | [60] |
| Chitosan from shrimp shells (Sigma-Aldrich, Iceland) | Passion fruit juice | Clarifying agent | [64] |
| Chitosan from crab shells (Sigma-Aldrich Ireland Ltd., Dublin, Ireland) (0 to 2 g/L) | Orange juices | Extending the shelf-life–it was shown that increasing chitosan concentration extended the quality of the orange juice, reducing enzymatic and non-enzymatic browning and controlling the spoilage during the storage time. | [66] |
| Chitosans with low molecular weight (LMWC, MW = 12 kDa), medium molecular weight (MMWC, MW = 95 kDa) and high molecular weight (HMWC, MW = 318 kDa) | Apple juice | LMWC exhibited stronger scavenging activity toward DPPH radicals, superoxide anion radicals and hydrogen peroxide., therefore could increase anti-oxidant activity in apple juice | [86] |
| Chitosan glutamate (Drammen, Norway). 42% glutamate; deacetylation degree range of 75–85% | Apple juice | Antifungal properties for foods prone to fungal spoilage | [110] |
| Chitosan hydrochloride (SEACURE CL 110) from FMC Biopolymer A/S-Drammen, Norway | Fermented milk | Chitosan coated alginate-beads reduced the final concentrations of free cells, the initial release of free cells, and the rate of lactate production in milk fermented batch-wise to a final pH of 4.7 in five consecutive batch fermentations. | [134] |
| Chitosan Sigma Aldrich (Milano, Italy) | Riesling sparkling wine | Application of encapsulated yeast in chitosan-alginate microcapsules, produced a sparkling wine having sensory properties like those produced by free yeasts (both adapted and non-adapted to ethanol) in terms of aroma, taste, and body. | [136] |
| Low-molecular-weight chitosan (Sigma-Aldrich-St. Louis, MO, USA) | Acidic wine | Significantly reduced the volatile acidity of an acidic wine | [137] |
| Not discriminated | Chinese Rice Wine | Cell immobilization in chitosan-alginate beads was applied to improve R. mucilaginosa cell resistance, and 51.6% of Ethyl Carbamate (EC) in commercial rice wine was removed by the immobilized cells. This was the first to remove EC by urethanase from R. mucilaginosa. | [138] |
| Chitosan (low molecular weight, 75–85% deacetylated) | Pale Ale beer | Application of a commercial brewing yeast (S. cerevisiae Nottingham Ale), entrapped into chitosan–calcium alginate double-layer microcapsules, to produce a Pale Ale beer with an improved flavor profile. | [139] |
| Chitosan from A. niger | Italian wines (Sauvignon blanc and Sangiovese) | Applying the immobilized HEWL (lysozyme from hen egg white) appeared more useful than the free form, in the continuous lysis of lactic bacteria in real white (Sauvignon blanc) and red (Sangiovese) wine. | [152] |
| Chitosan, with a deacetylation level of about 90%, from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China) | Apple juice. | The adsorption performance of the TETA-WICF/MCR obtained toward patulin, in apple juice, demonstrated that was depended on adsorbent dosage, contact time, temperature, and initial patulin concentration. It was also found that the TETA-WICF/MCR had good reusability without any adverse changes in apple juice. | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosme, F.; Vilela, A. Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview. Appl. Sci. 2021, 11, 11427. https://doi.org/10.3390/app112311427
Cosme F, Vilela A. Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview. Applied Sciences. 2021; 11(23):11427. https://doi.org/10.3390/app112311427
Chicago/Turabian StyleCosme, Fernanda, and Alice Vilela. 2021. "Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview" Applied Sciences 11, no. 23: 11427. https://doi.org/10.3390/app112311427








