Exploring the Role of Mycorrhizal and Rhizobium Inoculation with Organic and Inorganic Fertilizers on the Nutrient Uptake and Growth of Acacia mangium Saplings in Acidic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Soil for Poly Pots
2.2. Physicochemical Properties of Initial Soil
2.3. Preparation of Soil Mixture and Poly Pots
2.4. Pre-Sowing Treatment, Inoculation, and Sowing of Seed
2.5. Details of Experiment
2.6. The Observation during the Growth Period
2.7. InfectivityTest
2.8. Plant and Soil Analysis
2.9. Statistical Analysis
3. Results
3.1. Plant Height and Stem Diameter
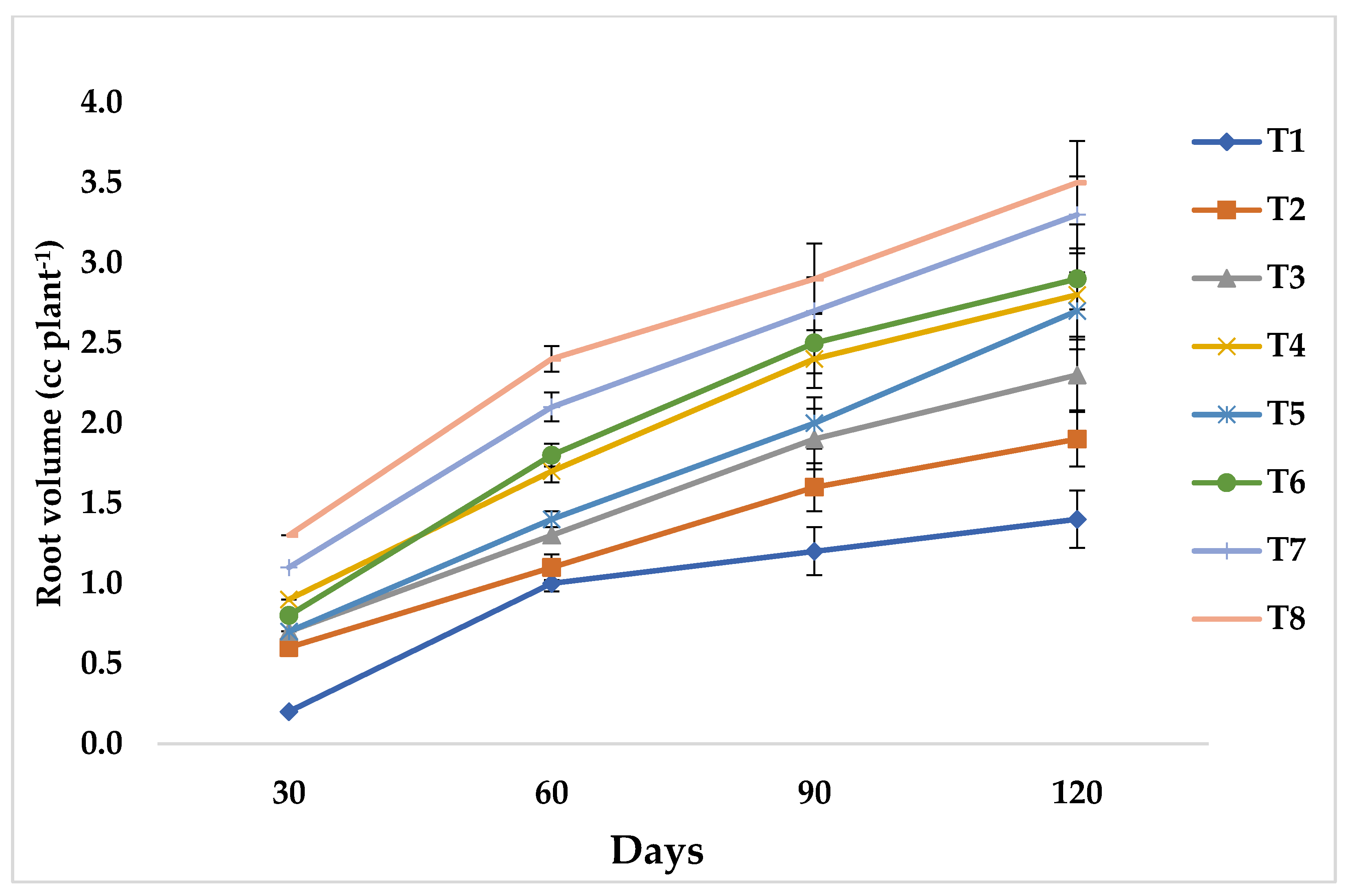
3.2. Effect of Mycorrhiza Inoculation on Root Characteristics
3.3. Total Biomass
3.4. Mycorrhizal Inoculation and Infection
3.5. Mycorrhizal Infection of AcaciamangiumRoots
3.6. Plant Nutrient Concentration and Uptake
3.6.1. Macro-Nutrient
3.6.2. Micronutrient
3.7. Post-Harvest Soilproperties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiti, S.K. Raising of Saplings for Forest Trees, Ecorestoration of the Coalmine Degraded Lands; Springer: Berlin/Heidelberg, Germany, 2013; p. 133. [Google Scholar]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004; p. 402. [Google Scholar]
- Zapata, F.; Zaharah, A.R. Phosphorus availability from phosphate rock and sewage sludge as influenced by addition of soluble phosphate fertilizer. Nutr. Cycl. Agroecosyst. 2002, 63, 43–48. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; Cruz-Ramırez, A.; Herrera-Estella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.H.; Liu, X.; Pan, X.; Herbert, S.J. Phosphorus application affects the soybean root response to water deficit at the initial flowering and full pod stages. Soil Sci. Plant Nutr. 2005, 51, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.C.; Niu, F.R.; Duan, D.P.; Xu, W.Z.; Huang, J. Root morphological characteristics of Lespedeza davurica (L.) intercropped with Bothriochloa ischaemum (L.) Keng under water stress and P application conditions. Pak. J. Bot. 2012, 44, 1857–1864. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Galiana, A.; Gnahoua, G.M.; Chaumont, J.; Lesueur, D.; Prin, Y.; Mallet, B. Improvement of nitrogen fixation in Acacia mangium through inoculation with rhizobium. Agrofor. Syst. 1998, 40, 297–307. [Google Scholar] [CrossRef]
- Mason, P.A.; Wilson, J. Harnessing symbiotic associations: Vesicular arbuscular mycorrhizas. In Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources, Proceedings of the ITE Symposium, 16–19 October 1994, Dallas, TX, USA; Leaky, R.R.B., Newton, A.C., Eds.; Institute of Transportation Engineers: Washington, DC, USA, 1994; pp. 165–175. [Google Scholar]
- Ghosh, S.; Kanp, U.K.; Verma, N.K. Effects of four arbuscular mycorrhizae on Acacia mangium wild. Seedlings in lateritic soil. Indian J. Plant Physiol. 2008, 13, 375–380. [Google Scholar]
- Ullah, M.S.; Islam, M.S.; Islam, M.A.; Haque, T. Effects of organic manures and chemical fertilizers on the yield of brinjal and soil properties. J. Bangladesh Agril. Univ. 2008, 6, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Mumtaz, E. Application of agro-waste products as organic and value added biofertilizer for improving plant growth. J. Pharm. Clin. Res. 2014, 8, 35–41. [Google Scholar]
- Rahman, M.M.; Islam, M.N.; Roni, M.Z.K.; Gani, O.; Jamal-Uddin, A.F.M. Vermicompost and mustard oil cake as an alternative fertilizer for strawberry production. Int. J. Bus. Soc. 2018, 6, 78–84. [Google Scholar]
- Sethi, D.; Mohanty, S.; Pradhan, M.; Dash, S.; Das, R. Effect of LD slag application on yield, yield attributes and protein content of groundnut kernel in an acid soil of Bhubaneswar. Int. J. Farm Sci. 2017, 7, 79–82. [Google Scholar]
- Pattanayak, S.K.; Sarkar, A. Sustainable management of Acid Soils: Technologies and their Transfer. Indian J. Fert. 2016, 12, 16–24. [Google Scholar]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Schouteden, N.; Waele, D.D.; Panis, B.; Christine, M.V. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, W.J.C. Soil Sterilization; George Allen & Unwin Ltd.: London, UK, 1956. [Google Scholar]
- Grace, C.; Stribley, D. A safer procedure for routine staining of vesicular- arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 1160–1162. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet -sieving and decanting. Trans. Brit. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular- arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 10th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1960. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Part 2: Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Intel Science Publishers, Inc.: New York, NY, USA, 1950; p. 70. [Google Scholar]
- Black, C.A. Methods of Soil Analysis: Physical and Mineralogical Properties, including Statistics of Measurement and Sampling: Part 2; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Woodruff, C.M. Testing soils for lime requirement by means of a buffered solution and the glass electrode. Soil Sci. 1984, 66, 53–63. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.W.; Asija, G.L. A rapid procedure for estimation of available nitrogen in soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Chesnin, L.; Yein, C.H. Turbidimetric Determination of Available Sulphur. Soil Sci Soc. Am. Proc. 1951, 15, 149–151. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.L. Development of DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wahid, F.; Sharif, M.; Ali, A.; Fahad, S.; Adnan, M.; Noor, M.; Mian, I.A.; Khan, I.A.; Alam, M.; Saeed, M.; et al. Plant-microbes interactions and functions in changing climate. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2020; pp. 397–419. [Google Scholar]
- Shockley, F.W.; McGraw, R.L.; Garrett, H.E. Growth and nutrient concentration of two native forage legumes inoculated with Rhizobium and Mycorrhiza in Missouri, USA. Agrofor. Syst. 2004, 60, 137–142. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Recipocal rewards stabilize cooperation in the mycorhhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kumar, R.; Anal, A.K.D. Spore population, colonization, species diversity and factors influencing the association of arboscular mycorrhizal fungi with litchi tree in India. J. Environ. Biol. 2016, 37, 91–100. [Google Scholar]
- Siviero, M.A.; Motta, A.M.; Lima, D.S.; Birolli, R.R.; Huh, S.Y.; Santinoni, I.A.; Murate, L.S.; Castro, C.M.A.; Miyauchi, M.Y.H.; Zangaro, W.; et al. Interaction among N-fixing bacteria and AM fungi in Amazonian legume tree (Schizolobium amazonicum) in field conditions. Appl. Soil Ecol. 2008, 39, 144–152. [Google Scholar] [CrossRef]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular Mycorrhiza Stimulates Biological Nitrogen Fixation in Two Medicago spp. through Improved Phosphorus Acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, B.; Adnan, M.; Munsif, F.; Fahad, S.; Saeed, M.; Wahid, F.; Arif, M.; Amanullah, J.; Wang, D.; Saud, S.; et al. Substituting urea by organic wastes for improving maize yield in alkaline soil. J. Plant Nutr. 2019, 42, 2423–2434. [Google Scholar] [CrossRef]
- Wahid, F.; Sharif, M.; Fahad, S.; Adnan, M.; Khan, I.A.; Aksoy, E.; Ali, A.; Sultan, T.; Alam, M.; Saeed, M.; et al. Arbuscular mycorrhizal fungi improve the growth and phosphorus uptake of mung bean plants fertilized with composted rock phosphate fed dung in alkaline soil environment, J. Plant Nutr. 2019, 42, 1760–1769. [Google Scholar] [CrossRef]
- Adnan, M.; Zahir, S.; Fahad, S.; Arif, M.; Mukhtar, A.; Imtiaz, A.K.; Mian, I.A.; Basir, A.; Ullah, H.; Arshad, M.; et al. Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci. Rep. 2017, 7, 16131. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Satter, M.A.; Hanafi, M.M.; Mahmud, T.M.M.; Azizah, H. Role of Arbuscular Mycorrhiza and Phosphorus in Acacia mangium-Peanut Agroforestry System for Rejuvenation of Tin Tailings. J. Sustain. Agric. 2006, 28, 55–68. [Google Scholar] [CrossRef]
- Mathur, N.; Vyas, A. Methods in Physiological Plant Pathology; Shivakami Publication: Madras, India, 1996; p. 215. [Google Scholar]
- Draszawka-Bołzan, B. Effect of pH and soil environment. World News Natl. Sci. 2017, 8, 50–60. [Google Scholar]
- Khabaz-Saberi, H.; Rengel, Z.; Wilson, R.; Setter, T.L. Variation for tolerance to high concentration of ferrous iron (Fe2+) in Australian hexaploid wheat. Euphytica 2010, 172, 275–283. [Google Scholar] [CrossRef]
- Kluber, L.A.; Carrino-Kyker, S.R.; Coyle, K.P.; DeForest, J.L.; Hewins, C.R.; Shaw, A.N.; Smemo, K.A.; Burke, D.J. Mycorrhizal Response to Experimental pH and P Manipulation in Acidic Hardwood Forests. PLoS ONE 2012, 7, e48946. [Google Scholar] [CrossRef] [Green Version]
- Aghili, F.; Gamper, H.A.; Eikenberg, J.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Schulin, R.; Jansa, J.; Frossard, E. Green Manure Addition to Soil Increases Grain Zinc Concentration in Bread Wheat. PLoS ONE 2014, 9, e101487. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral nutrition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Khan, A.; Sharif, M.; Ali, A.; Shah, S.N.M.; Mian, I.A.; Wahid, F.; Jan, B.; Adnan, M.; Nawaz, S.; Ali, N. Potential of AM fungi in phytoremediation of heavy metals and effect on yield of wheat crop. Am. J. Plant. Sci. 2014, 5, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Nduwumuremyi, A.; Habimana, S.; Alexis, T.; Mupenzi, J. Soil acidity analysis and estimation of lime requirement for rectifying soil acidity. Int. Invent. J. Agric. Soil Sci. 2014, 2, 22–26. [Google Scholar]
- Kim, B.; Pagay, V.; Cho, K.; Na, Y.; Yun, B.; Choi, K.; Jung, S.; Choi, H. Effect of oil cake application on soil and leaf nutrition and on fruit yields in non-astringent persimmon (Diospyros × kaki Thunb.) trees. J. Hortic. Sci. Biotechnol. 2015, 90, 203–209. [Google Scholar] [CrossRef]
- Yanai, J.; Araki, S.; Kyuma, K. Effects of plant growth on the dynamics of the soil solution composition in the root zone of maize in four Japanese soils. Soil Sci. Plant Nutr. 1995, 4, 195–206. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elments: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Munns, D.N. Acid soils tolerance in legumes and rhizobia. Adv. Plant Nutr. 1986, 2, 63–91. [Google Scholar]
- Abubakari, F.; Tetteh, F.M.; Abubakari, F.; Tuffour, H.O.; Aduwu, A. Strategies for Improving Nodulation and Nitrogen Fixation of Leguminous Crops to Enhance Production in Small holder Farming Systems. J. Global Agric. Ecol. 2016, 4, 185–190. [Google Scholar]
- Borie, F.; Rubio, R.; Morales, A.; Curaqueo, G.; Cornejo, P. Arbuscular mycorrhizae in agricultural and forest ecosystems in Chile. J. Soil Sci. Plant Nutr. 2010, 10, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Wahid, F.; Sharif, M.; Shah, F.; Ali, A.; Adnan, M.; Rafiullah; Shah, S.; Danish, S.; Ali, M.A.; Ahmed, N.; et al. Mycorrhiza and Phosphate Solubilizing Bacteria: Potential Bioagents for Sustainable Phosphorus Management in Agriculture. Phyton 2022, 91, 257–278. [Google Scholar] [CrossRef]





| Treatments | Sapling Height (cm) | Stem Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Days | ||||||||
| 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | |
| T1 | 5.2 ± 0.09 g | 16.5 ± 0.03 g | 39.9 ± 0.09 f | 66.4 ± 1.21 f | 0.51 ± 0.02 b | 1.77 ± 0.12 e | 2.97 ± 0.24 d | 4.06 ± 0.34 de |
| T2 | 6.6 ± 0.09 f | 21.4 ± 0.15 f | 47.4 ± 0.17 e | 76.9 ± 0.61 e | 0.49 ± 0.02 bc | 1.19 ± 0.09 g | 2.98 ± 0.26 d | 3.98 ± 0.29 e |
| T3 | 7.6 ± 0.06 d | 25.1 ± 0.18 c | 52.5 ± 0.12 c | 84.6 ± 0.60 d | 0.54 ± 0.03 b | 1.65 ± 0.19 ef | 3.19 ± 0.29 c | 3.95 ± 0.36 e |
| T4 | 7.1 ± 0.15 e | 24.0 ± 0.12 d | 52.1 ± 0.34 c | 85.1 ± 0.59 d | 0.51 ± 0.02 b | 1.83 ± 0.18 de | 2.74 ± 0.27 e | 4.57 ± 0.28 b |
| T5 | 6.6 ± 0.06 f | 22.8 ± 0.20 e | 49.8 ± 0.21 d | 82.6 ± 0.64 d | 0.48 ± 0.02 bc | 1.86 ± 0.14 de | 2.98 ± 0.26 d | 4.14 ± 0.37 d |
| T6 | 9.4 ± 0.07 b | 23.7 ± 0.12 d | 56.7 ± 1.01 b | 89.2 ± 0.61 c | 0.44 ± 0.01 c | 2.00 ± 0.21 c | 3.14 ± 0.28 c | 4.27 ± 0.38 |
| T7 | 8.6 ± 0.09 c | 26.8 ± 0.23 b | 58.1 ± 0.55 b | 91.6 ± 0.90 b | 0.94 ± 0.05 a | 2.21 ± 0.20 b | 3.32 ± 0.31 b | 4.66 ± 0.32 b |
| T8 | 10.1 ± 0.21 a | 28.1 ± 0.17 a | 59.9 ± 0.30 a | 98.5 ± 0.91 a | 0.96 ± 0.04 a | 3.00 ± 0.25 a | 3.65 ± 0.34 a | 5.18 ± 0.42 a |
| Treatments | Concentration (%) | Uptake (mg plant−1) | ||||||
| N | P | K | N | P | K | |||
| T1 | 0.48 ± 0.02 h | 0.154 ± 0.03 g | 0.39 ± 0.01 f | 16.4 ± 1.2 e | 5.3 ± 0.04 d | 13.4 ± 1.23 f | ||
| T2 | 0.51 ± 0.03 g | 0.191 ± 0.04 f | 0.42 ± 0.02 e | 21.2 ± 1.3 e | 7.9 ± 0.07 c | 17.3 ± 1.41 e | ||
| T3 | 0.60 ± 0.05 f | 0.216 ± 0.04 d | 0.42 ± 0.02 e | 27.1 ± 1.5 d | 9.8 ± 0.07 c | 19.1 ± 1.55 e | ||
| T4 | 0.63 ± 0.05 e | 0.230 ± 0.05 bc | 0.46 ± 0.05 c | 29.7 ± 1.7 cd | 10.8 ± 0.09 bc | 21.8 ± 1.57 d | ||
| T5 | 0.69 ± 0.05 d | 0.208 ± 0.04 e | 0.47 ± 0.05 c | 33.9 ± 1.9 c | 10.3 ± 0.09 c | 23.1 ± 1.63 cd | ||
| T6 | 0.88 ± 0.09 b | 0.234 ± 0.06 b | 0.44 ± 0.03 d | 49.6 ± 2.4 b | 13.2 ± 0.15 b | 24.7 ± 1.65 c | ||
| T7 | 0.87 ± 0.09 c | 0.227 ± 0.05 c | 0.53 ± 0.06 b | 52.8 ± 2.8 b | 13.8 ± 0.17 b | 32.0 ± 1.65 b | ||
| T8 | 0.99 ± 0.12 a | 0.308 ± 0.08 a | 0.54 ± 0.04 a | 71.0 ± 3.2 a | 22.2 ± 0.29 a | 39.1 ± 1.74 a | ||
| Treatments | Concentration (%) | Uptake (mg plant−1) | ||||||
| Ca | Mg | S | Ca | Mg | S | |||
| T1 | 0.09 ± 0.006 f | 0.073 ± 0.006 f | 0.054 ± 0.004 f | 3.2 ± 0.41 c | 2.5 ± 0.12 d | 1.9 ± 0.03 f | ||
| T2 | 0.10 ± 0.009 e | 0.087 ± 0.009 e | 0.069 ± 0.009 e | 4.3 ± 0.44 c | 3.6 ± 0.11 d | 2.9 ± 0.09 f | ||
| T3 | 0.19 ± 0.013 b | 0.134 ± 0.016 b | 0.099 ± 0.010 c | 8.4 ± 0.67 b | 6.1 ± 0.29 c | 4.5 ± 0.11 e | ||
| T4 | 0.17 ± 0.010 d | 0.121 ± 0.014 c | 0.156 ± 0.017 b | 7.8 ± 0.55 b | 5.7 ± 0.15 c | 7.4 ± 0.14 c | ||
| T5 | 0.17 ± 0.011 c | 0.103 ± 0.009 d | 0.087 ± 0.008 d | 8.5 ± 0.69 b | 5.1 ± 0.12 c | 4.3 ± 0.09 e | ||
| T6 | 0.18 ± 0.012 b | 0.157 ± 0.023 a | 0.104 ± 0.009 c | 10.3 ± 0.73 b | 8.9 ± 0.42 b | 5.9 ± 0.11 d | ||
| T7 | 0.17 ± 0.010 cd | 0.137 ± 0.019 b | 0.178 ± 0.031 a | 10.2 ± 0.72 b | 8.3 ± 0.34 b | 10.8 ± 0.21 b | ||
| T8 | 0.20 ± 0.013 a | 0.166 ± 0.045 a | 0.185 ± 0.037 a | 14.0 ± 0.81 a | 12.0 ± 0.44 a | 13.3 ± 0.27 a | ||
| Treatments | Concentration (µg g−1 of the plant) | Uptake (µg plant−1) | ||||||
| Fe | Mn | Cu | Zn | Fe | Mn | Cu | Zn | |
| T1 | 34.3 ± 1.25 b | 34.2 ± 1.11 c | 3.6 ± 0.05 b | 15.6 ± 0.43 c | 117.1 ± 2.81 f | 117.0 ± 2.01 e | 12.4 ± 0.33 e | 53.4 ± 1.28 e |
| T2 | 42.6 ± 1.34 a | 39.4 ± 1.13 a | 4.6 ± 0.07 a | 16.4 ± 0.61 c | 176.1 ± 2.99 c | 162.7 ± 2.38 c | 18.8 ± 0.39 c | 67.6 ± 2.29 d |
| T3 | 27.3 ± 1.11 f | 25.3 ± 0.93 e | 2.3 ± 0.02 cd | 14.5 ± 0.40 d | 123.4 ± 1.16 f | 114.4 ± 1.93 e | 10.4 ± 0.27 f | 65.6 ± 2.27 d |
| T4 | 28.8 ± 1.14 e | 40.2 ± 1.26 a | 3.8 ± 0.03 b | 16.7 ± 0.66 ab | 135.7 ± 1.19 e | 189.4 ± 2.99 d | 18.1 ± 0.35 c | 78.6 ± 2.45 c |
| T5 | 26.8 ± 0.79 f | 39.6 ± 1.13 a | 2.7 ± 0.02 c | 16.5 ± 0.60 bc | 132.2 ± 1.99 e | 195.4 ± 3.01 d | 13.4 ± 0.33 d | 81.3 ± 2.99 c |
| T6 | 28.1 ± 0.89 e | 27.9 ± 1.10 d | 2.1 ± 0.03 d | 13.4 ± 0.44 e | 158.2 ± 2.15 d | 157.4 ± 2.50 c | 12.00.32 ± e | 75.8 ± 2.76 c |
| T7 | 30.1 ± 0.13 d | 40.4 ± 1.29 a | 3.9 ± 0.04 b | 17.0 ± 0.49 a | 182.7 ± 3.45 b | 245.6 ± 4.19 b | 23.8 ± 0.42 b | 103.3 ± 3.36 b |
| T8 | 32.9 ± 0.14 c | 38.7 ± 1.25 b | 3.9 ± 0.05 b | 16.9 ± 0.50 ab | 236.2 ± 3.99 a | 278.3 ± 4.44 a | 27.9 ± 0.48 a | 121.4 ± 3.94 a |
| Treatments | pH | EC | OC | Available | Exchangeable | ||||
| N | P | K | S | Ca | Mg | ||||
| (1:2.5) | (dS m−1) | (g kg−1) | (mg kg−1) | cmol (p+) kg−1 soil | |||||
| T1 | 4.95 ± 0.011 f | 0.10 ± 0.015 bc | 5.1 ± 0.23 c | 93 ± 1.7 b | 10 ± 1.4 e | 48 ± 1.2 e | 6 ± 1.1 e | 1.14 ± 0.02 e | 0.24 ± 0.04 d |
| T2 | 4.90 ± 0.011 f | 0.11 ± 0.003 b | 5.4 ± 0.25 b | 87 ± 1.1 de | 15 ± 1.5 cd | 150 ± 2.4 a | 6 ± 1.1 e | 1.15 ± 0.02 e | 0.27 ± 0.05 c |
| T3 | 5.11 ± 0.014 c | 0.11 ± 0.007 b | 5.5 ± 0.23 b | 88 ± 0.9 d | 19 ± 1.1 c | 146 ± 2.0 b | 7 ± 1.1 de | 1.25 ± 0.03 b | 0.37 ± 0.09 a |
| T4 | 5.07 ± 0.009 d | 0.10 ± 0.005 bc | 5.7 ± 0.25 a | 85 ± 2.0 ef | 16 ± 1.2 d | 146 ± 2.2 b | 11 ± 1.5 b | 1.23 ± 0.03 c | 0.33 ± 0.08 b |
| T5 | 5.00 ± 0.009 e | 0.11 ± 0.013 b | 5.5 ± 0.22 b | 83 ± 1.6 f | 18 ± 1.1 c | 133 ± 1.6 d | 8 ± 1.0 d | 1.20 ± 0.02 d | 0.29 ± 0.07 c |
| T6 | 5.03 ± 0.013 de | 0.12 ± 0.008 a | 5.4 ± 0.21 b | 98 ± 1.6 b | 21 ± 1.1 b | 140 ± 1.8 c | 9 ± 1.1 cd | 1.22 ± 0.04 cd | 0.32 ± 0.09 b |
| T7 | 5.15 ± 0.010 b | 0.09 ± 0.004 c | 5.3 ± 0.20 bc | 105 ± 1.8 a | 21 ± 0.5 b | 138 ± 1.6 c | 12 ± 1.6 b | 1.26 ± 0.05 b | 0.34 ± 0.12 b |
| T8 | 5.40 ± 0.009 a | 0.08 ± 0.005 c | 5.7 ± 0.23 a | 95 ± 2.2 c | 23 ± 1.5 a | 147 ± 1.5 ab | 13 ± 1.7 a | 1.30 ± 0.05 a | 0.39 ± 0.17 a |
| Initial | 5.29 | 0.11 | 5.2 | 94 | 10 | 106 | 9 | 1.32 | 0.42 |
| Treatments | Available | ||||||||
| Fe | Mn | Cu | Zn | ||||||
| (mg kg−1) | |||||||||
| T1 | 65.21 ± 1.13 a | 2.65 ± 0.09 c | 1.48 ± 0.02 a | 2.42 ± 0.02 a | |||||
| T2 | 63.99 ± 0.38 b | 2.72 ± 0.18 a | 1.44 ± 0.01 a | 2.39 ± 0.01 a | |||||
| T3 | 48.24 ± 0.08 e | 2.47 ± 0.08 f | 1.29 ± 0.01 cd | 2.18 ± 0.02 c | |||||
| T4 | 54.94 ± 0.08 d | 2.67 ± 0.15 b | 1.32 ± 0.01 cd | 2.21 ± 0.01 b | |||||
| T5 | 60.22 ± 0.24 c | 2.56 ± 0.22 d | 1.30 ± 0.02 c | 2.20 ± 0.01 b | |||||
| T6 | 47.19 ± 0.11 e | 2.45 ± 0.10 de | 1.27 ± 0.01 cd | 2.16 ± 0.01 c | |||||
| T7 | 56.73 ± 0.32 d | 2.49 ± 0.08 fe | 1.29 ± 0.01 cd | 2.18 ± 0.01 c | |||||
| T8 | 45.32 ± 0.20 e | 2.41 ± 0.14 g | 1.26 ± 0.01 d | 2.17 ± 0.01 c | |||||
| Initial | 64.42 | 2.76 | 1.45 | 2.39 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, D.; Subudhi, S.; Rajput, V.D.; Kusumavathi, K.; Sahoo, T.R.; Dash, S.; Mangaraj, S.; Nayak, D.K.; Pattanayak, S.K.; Minkina, T.; et al. Exploring the Role of Mycorrhizal and Rhizobium Inoculation with Organic and Inorganic Fertilizers on the Nutrient Uptake and Growth of Acacia mangium Saplings in Acidic Soil. Forests 2021, 12, 1657. https://doi.org/10.3390/f12121657
Sethi D, Subudhi S, Rajput VD, Kusumavathi K, Sahoo TR, Dash S, Mangaraj S, Nayak DK, Pattanayak SK, Minkina T, et al. Exploring the Role of Mycorrhizal and Rhizobium Inoculation with Organic and Inorganic Fertilizers on the Nutrient Uptake and Growth of Acacia mangium Saplings in Acidic Soil. Forests. 2021; 12(12):1657. https://doi.org/10.3390/f12121657
Chicago/Turabian StyleSethi, Debadatta, Sachidananda Subudhi, Vishnu D. Rajput, Konathala Kusumavathi, Tapas Ranjan Sahoo, Subhaprada Dash, Satyabrata Mangaraj, Dhirendra Kumar Nayak, Sushanta Kumar Pattanayak, Tatiana Minkina, and et al. 2021. "Exploring the Role of Mycorrhizal and Rhizobium Inoculation with Organic and Inorganic Fertilizers on the Nutrient Uptake and Growth of Acacia mangium Saplings in Acidic Soil" Forests 12, no. 12: 1657. https://doi.org/10.3390/f12121657
APA StyleSethi, D., Subudhi, S., Rajput, V. D., Kusumavathi, K., Sahoo, T. R., Dash, S., Mangaraj, S., Nayak, D. K., Pattanayak, S. K., Minkina, T., Glinushkin, A. P., & Kalinitchenko, V. P. (2021). Exploring the Role of Mycorrhizal and Rhizobium Inoculation with Organic and Inorganic Fertilizers on the Nutrient Uptake and Growth of Acacia mangium Saplings in Acidic Soil. Forests, 12(12), 1657. https://doi.org/10.3390/f12121657









