Growth and Optical Properties of the Whole System of Li(Mn1-x,Nix)PO4 (0 ≤ x ≤ 0.5) Single Crystals
Abstract
:1. Introduction
2. Experimental Section
2.1. Crystal Growth
2.2. Crystal Characterization
3. Results and Discussions
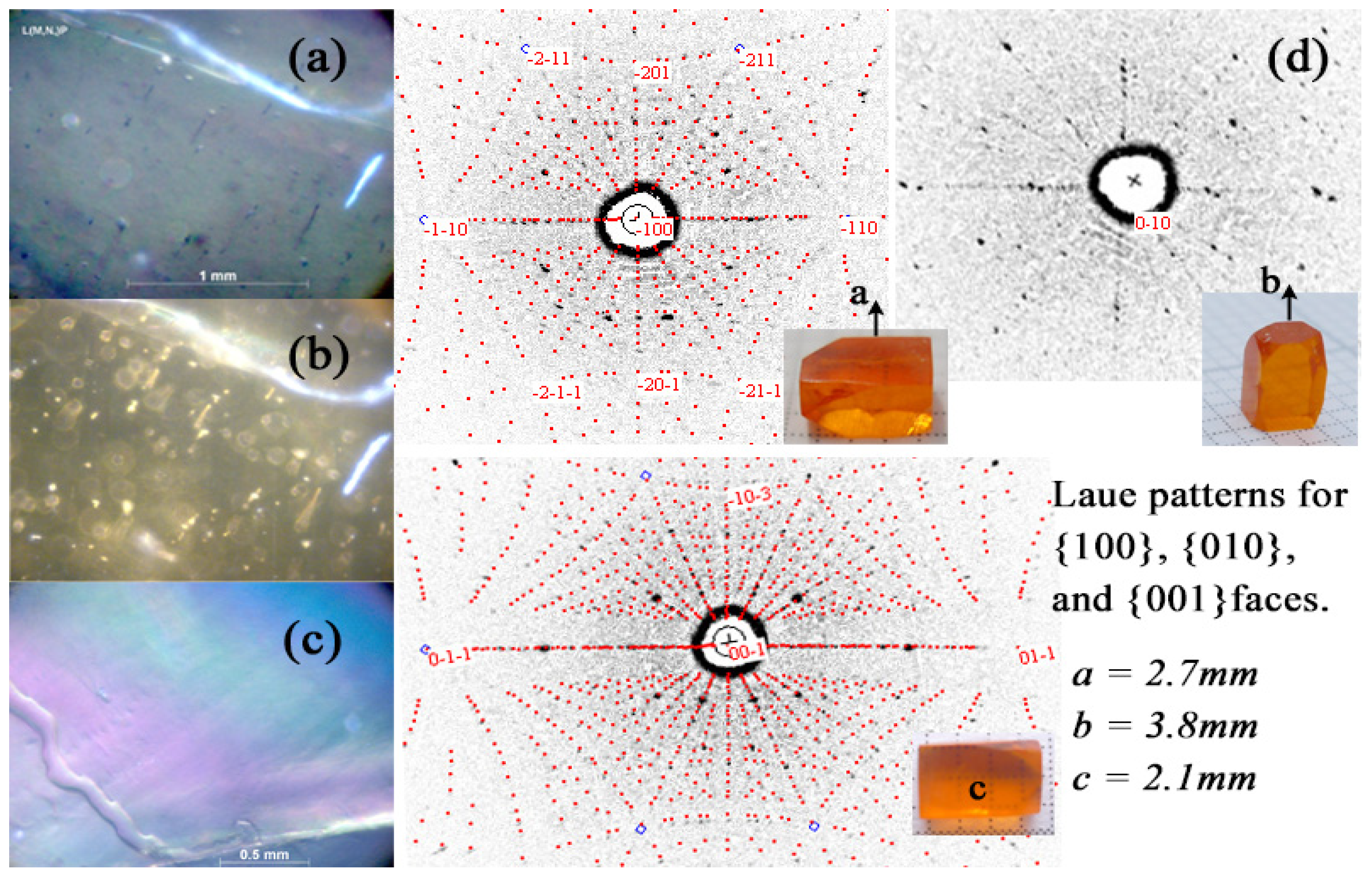
3.1. Crystal Growth
3.2. Crystal Characterization
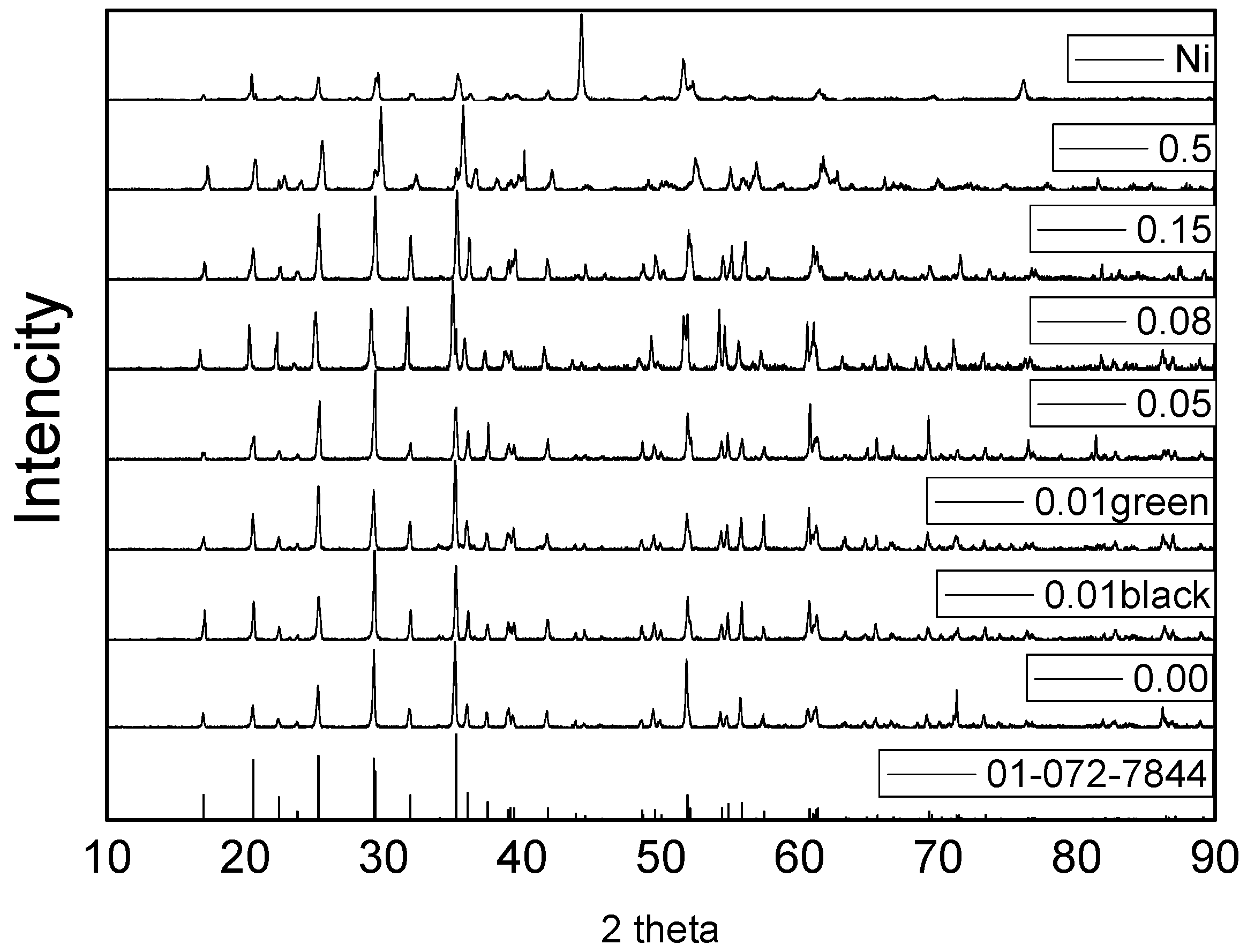
3.3. XRD and Structures
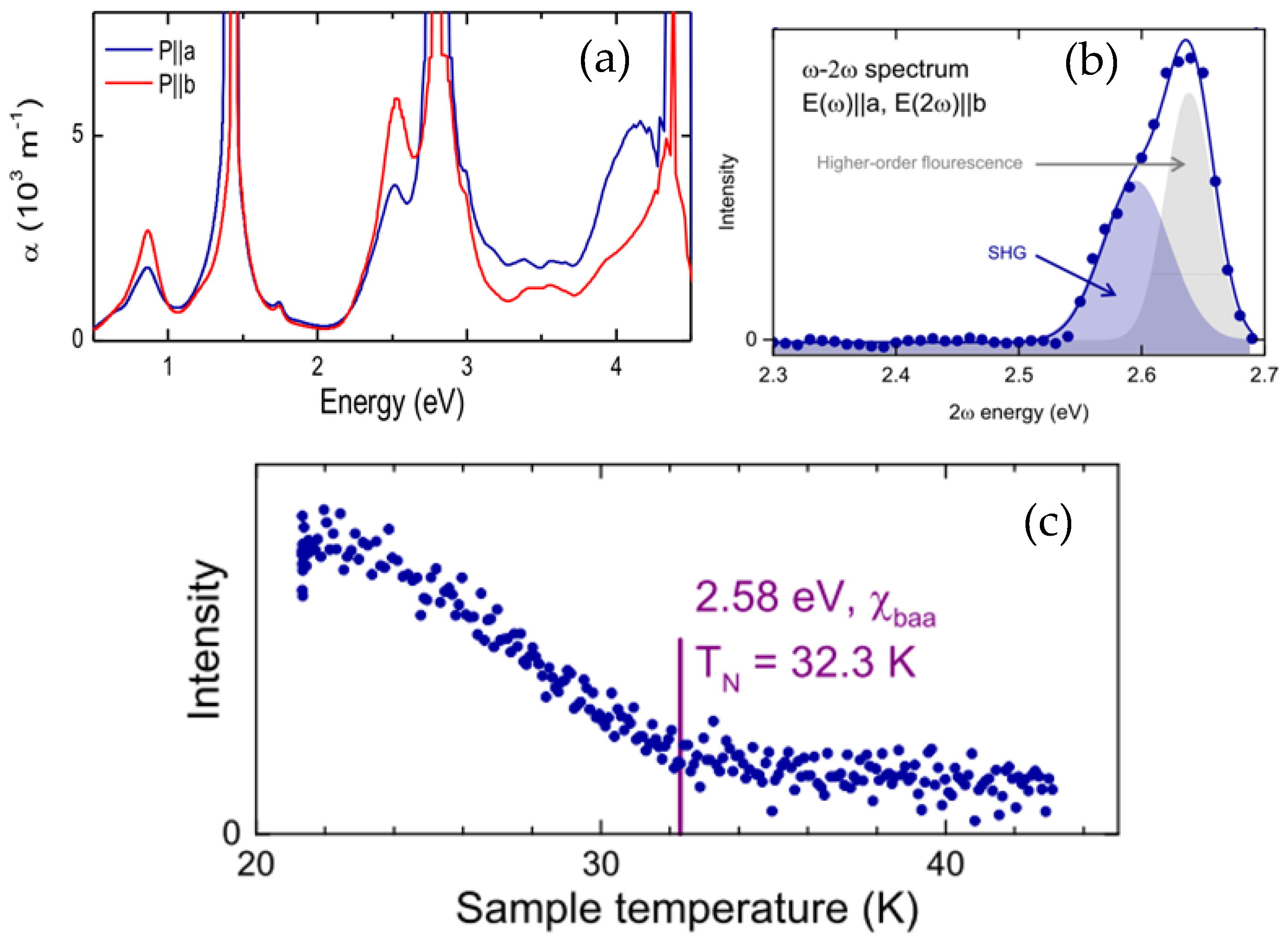
3.4. Linear Optical Properties
3.5. Nonlinear Optical Properties
3.6. The Second Harmonic Generation (SHG) Laser Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Kamat, P.V. Lithium-ion batteries and beyond: Celebrating the 2019 nobel prize in chemistry. ACS Energy Lett. 2019, 4, 2757–2759. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Yang, J.; Wang, P. Cathode Active Material for Lithium-Ion Secondary Battery and Preparation Method Thereof, Cathode Pole Piece for Lithium-Ion Secondary Battery, and Lithium-Ion Secondary Battery. U.S. Patent 10,454,094, 6 June 2019. [Google Scholar]
- Iaconangelo, D. Nobel-winning scientist on 100% renewables, EVs and Murkowski. E&E News Reporter Energywire: Thursday, 17 October 2019. [Google Scholar]
- Omidi, A.H.; Babaei, A.; Ataie, A. Low temperature synthesis of nanostructured LiFePO4/C cathode material for lithium ion batteries. Mater. Res. Bull. 2020, 125, 110807. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.J.; Deng, J.L.; Luo, G.Y.; Wu, F.Z.; Mai, Y.; Li, J.Q. Effect of doped Mn on improving the electrochemical performance of LiFePO4. J. Mater. Sci. Mater. Electron. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Amin, R.; Balaya, P.; Maier, J. Anisotropy of electronic and ionic transport in LiFePO4 single crystals. Electrochem. Solid-State Lett. 2007, 10, A13–A16. [Google Scholar] [CrossRef]
- Menale, C.; D’Annibale, F.; Mazzarotta, B.; Bubbico, R. Thermal management of Lithium-ion batteries: An experimental investigation. Energy 2019, 182, 57–71. [Google Scholar] [CrossRef]
- Kollmeyer, P.J.; Jahns, T.M. Aging and performance comparison of absorbed glass matte, enhanced flooded, PbC, NiZn, and LiFePO4 12V start stop vehicle batteries. J. Power Sources 2019, 441, 227139. [Google Scholar] [CrossRef]
- Sun, Q.F.; Li, X.L.; Zhang, H.Z.; Song, D.W.; Shi, X.X.; Song, J.S.; Li, C.L.; Zhang, L.Q. Resynthesizing LiFePO4/C materials from the recycled cathode via a green full-solid route. J. Alloys Compd. 2020, 818, 153292. [Google Scholar] [CrossRef]
- Li, X.Y.; Dai, K.W.; Wang, Z.P.; Han, W.J. Lithium-ion batteries fault diagnostic for electric vehicles using sample entropy analysis method. J. Energy Storage 2020, 27, 101121. [Google Scholar] [CrossRef]
- Wang, K.; Maljuk, A.; Blum, C.G.; Kolb, T.; Jähne, C.; Neef, C.; Klingeler, R. Growth, characterization, and magnetic properties of a Li(Mn, Ni)PO4 single crystal. J. Cryst. Growth 2014, 386, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Neef, C.; Reiser, A.; Thauer, E.; Klingeler, R. Anisotropic ionic conductivity of LiMn1−xFexPO4 (0≤x≤1) single crystals. Solid State Ion. 2020, 346, 115197. [Google Scholar] [CrossRef]
- Hergett, W.; Neef, C.; Wadepohl, H.; Meyer, H.P.; Abdel-Hafiez, M.M.; Ritter, C.; Thauer, E.; Klingeler, R. High-pressure optical floating-zone growth of Li2FeSiO4 single crystals. J. Cryst. Growth 2019, 515, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, A.S.; Meier, D.; Fiebig, M. Ferroic nature of magnetic toroidal order. Nat. Commun. 2014, 5, 4796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnewuch, S.; Rodriguez, E.E. The fourth ferroic order: Current status on ferrotoroidic materials. J. Solid State Chem. 2019, 271, 175–190. [Google Scholar] [CrossRef]
- Lehmann, J.; Donnelly, C.; Derlet, P.M.; Heyderman, L.J.; Fiebig, M. Poling of an artificial magneto-toroidal crystal. Nat. Nanotech. 2019, 14, 141–144. [Google Scholar] [CrossRef]
- Cheong, S.W.; Fiebig, M.; Wu, W.; Chapon, L.; Kiryukhin, V. Seeing is believing: Visualization of antiferromagnetic domains. npj Quant. Mater. 2020, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Fiebig, M. Order! Order!! Nat. Phys. 2020, 16, 9–10. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Jow, T.R.; Thompson, T.; Sakamoto, J.; Jo, H.; Choe, H. LiCoPO4 mechanical properties evaluated by nanoindentation. Ceram. Int. 2014, 40, 13673–13677. [Google Scholar] [CrossRef]
- Sun, M.; Rousse, G.; Abakumov, A.M.; Saubanère, M.; Doublet, M.L.; Rodríguez-Carvajal, J.; Van Tendeloo, G.; Tarascon, J.M. Li2Cu2O(SO4)2: A possible electrode for sustainable Li-based batteries showing a 4.7 V redox activity vs Li+/Li0. Chem. Mater. 2015, 27, 3077–3087. [Google Scholar]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L. Understanding and development of olivine LiCoPO4 cathode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14483. [Google Scholar] [CrossRef]
- Herrera, J.O.; Camachomontes, H.; Fuentes, L.E.; Álvarezcontreras, L. LiMnPO: Review on synthesis and electrochemical properties. J. Solid State Electrochem. 2015, 12, 663–669. [Google Scholar]
- Wizent, N.; Behr, G.; Löser, W.; Büchner, B.; Klingeler, R. Challenges in the crystal growth of Li2CuO2 and LiMnPO4. J. Cryst. Growth 2011, 318, 995–999. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; UdriŞTioiu, E.G.; Aboul-Enein, H.Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, S.J.; Kim, C.S. Magnetic properties of phospho-olivine Li(Fe1-xMnx)PO4 investigated with Mössbauer spectroscopy. IEEE Trans. Magn. 2012, 48, 1553–1555. [Google Scholar] [CrossRef]



| Measurement Spots | Element | Wt % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|---|
| 1 | Pκ | 34.63 | 48.56 | 0.2532 | 1.0667 | 0.6839 | 1.0024 |
| Mnκ | 60.77 | 48.04 | 0.5760 | 0.9598 | 0.9824 | 1.0052 | |
| Niκ | 4.60 | 3.40 | 0.0424 | 0.9943 | 0.9272 | 1.0000 | |
| Total | 100.00 | 100.00 | |||||
| 1 | Mnκ | 93.72 | 94.10 | 0.9399 | 0.9976 | 0.9990 | 1.0062 |
| Niκ | 6.28 | 5.90 | 0.0584 | 1.0348 | 0.8978 | 1.0000 | |
| Total | 100.00 | 100.00 | |||||
| 2 | Mnκ | 92.64 | 93.08 | 0.9296 | 0.9972 | 0.9989 | 1.0074 |
| Niκ | 7.36 | 6.92 | 0.0684 | 1.0344 | 0.8990 | 1.0000 | |
| Total | 100.00 | 100.00 | |||||
| 3 | Mnκ | 92.53 | 92.97 | 0.9285 | 0.9972 | 0.9988 | 1.0075 |
| Niκ | 7.47 | 7.03 | 0.0695 | 1.0343 | 0.8991 | 1.0000 | |
| Total | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Li, Z.; Wang, K.; Zhang, J.; Liang, L.; Li, L.; Ren, Y.; Liu, Y.; Liu, M.; Xue, D. Growth and Optical Properties of the Whole System of Li(Mn1-x,Nix)PO4 (0 ≤ x ≤ 0.5) Single Crystals. Materials 2021, 14, 7233. https://doi.org/10.3390/ma14237233
Xu B, Li Z, Wang K, Zhang J, Liang L, Li L, Ren Y, Liu Y, Liu M, Xue D. Growth and Optical Properties of the Whole System of Li(Mn1-x,Nix)PO4 (0 ≤ x ≤ 0.5) Single Crystals. Materials. 2021; 14(23):7233. https://doi.org/10.3390/ma14237233
Chicago/Turabian StyleXu, Benyan, Zhenyou Li, Kunpeng Wang, Jianxiu Zhang, Lanju Liang, Longfei Li, Yanbiao Ren, Yong Liu, Meng Liu, and Dongfeng Xue. 2021. "Growth and Optical Properties of the Whole System of Li(Mn1-x,Nix)PO4 (0 ≤ x ≤ 0.5) Single Crystals" Materials 14, no. 23: 7233. https://doi.org/10.3390/ma14237233
APA StyleXu, B., Li, Z., Wang, K., Zhang, J., Liang, L., Li, L., Ren, Y., Liu, Y., Liu, M., & Xue, D. (2021). Growth and Optical Properties of the Whole System of Li(Mn1-x,Nix)PO4 (0 ≤ x ≤ 0.5) Single Crystals. Materials, 14(23), 7233. https://doi.org/10.3390/ma14237233







