Purshia plicata Triggers and Regulates Proteins Related to Apoptosis in HeLa Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purshia plicata Extract
2.2. Cell Culture
2.3. Cell Viability
MTT Assay
2.4. Cytotoxic Effect
LDH Assay
2.5. Characterization of Cell Morphologic Changes and Comet Assay (Single Cell Gel Electrophoresis)
2.6. Evaluation of Protein Expression
3. Results
3.1. Cell Viability
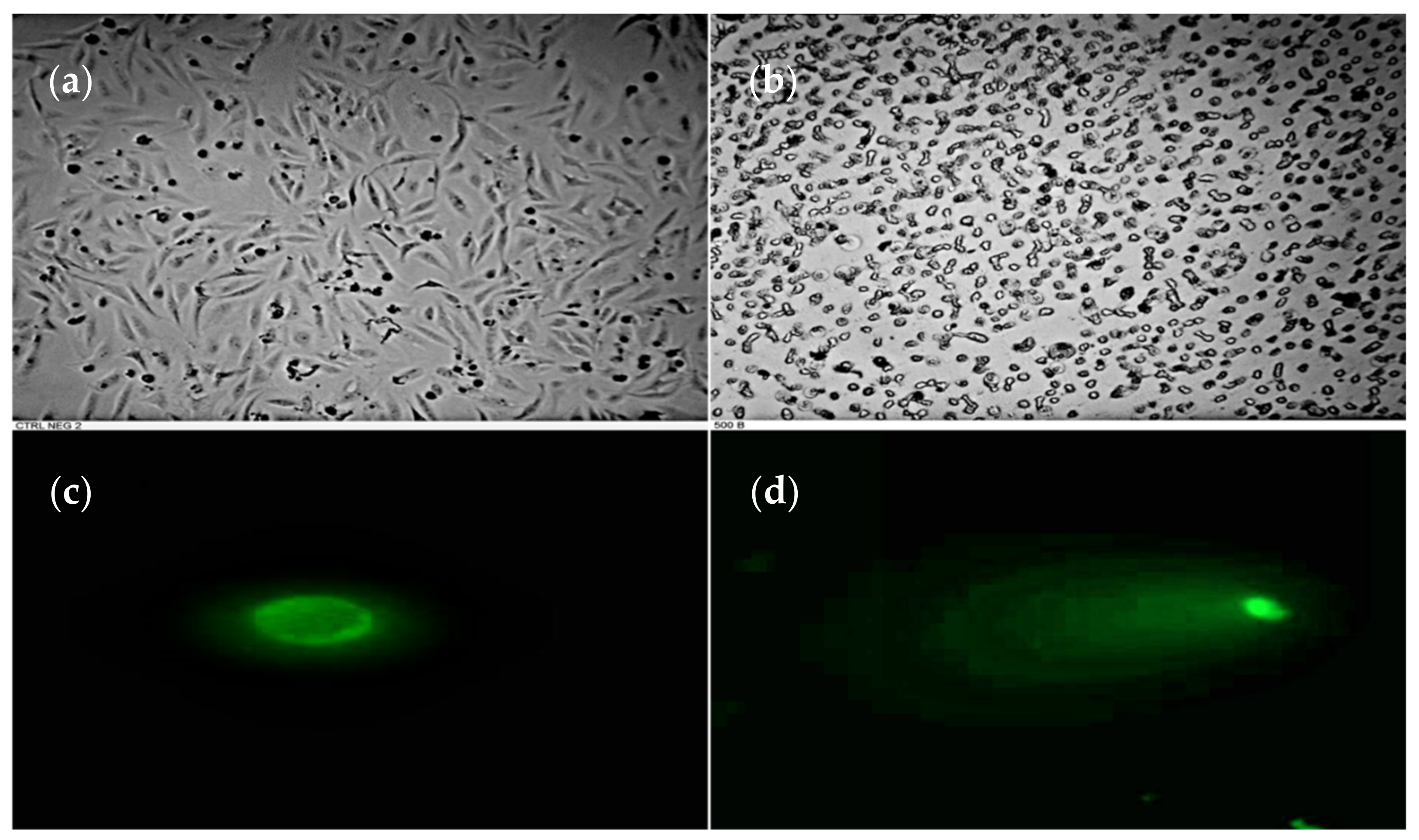
3.2. DNA Fragmentation in HeLa Cell Induced by P. plicata Extract
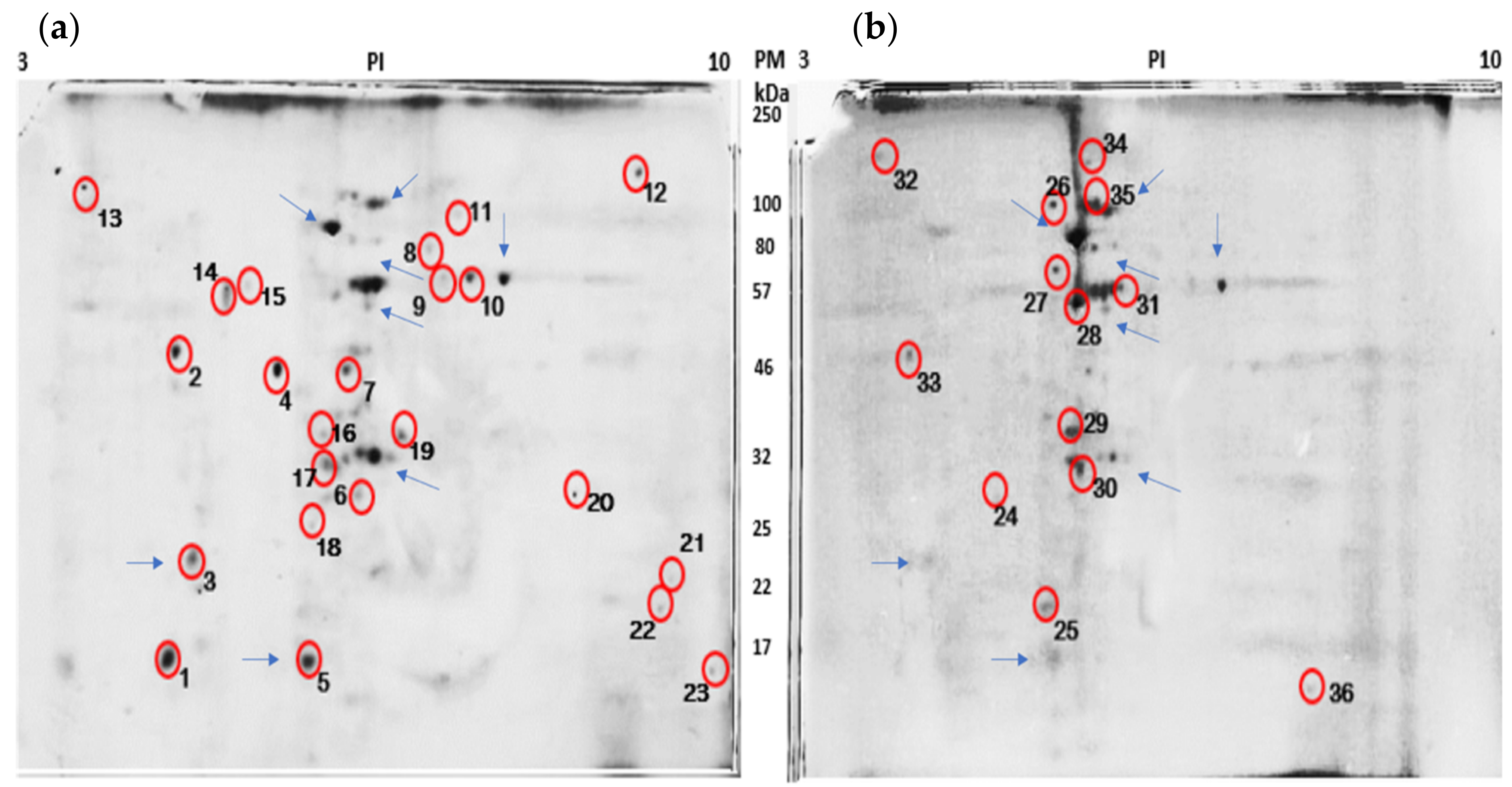
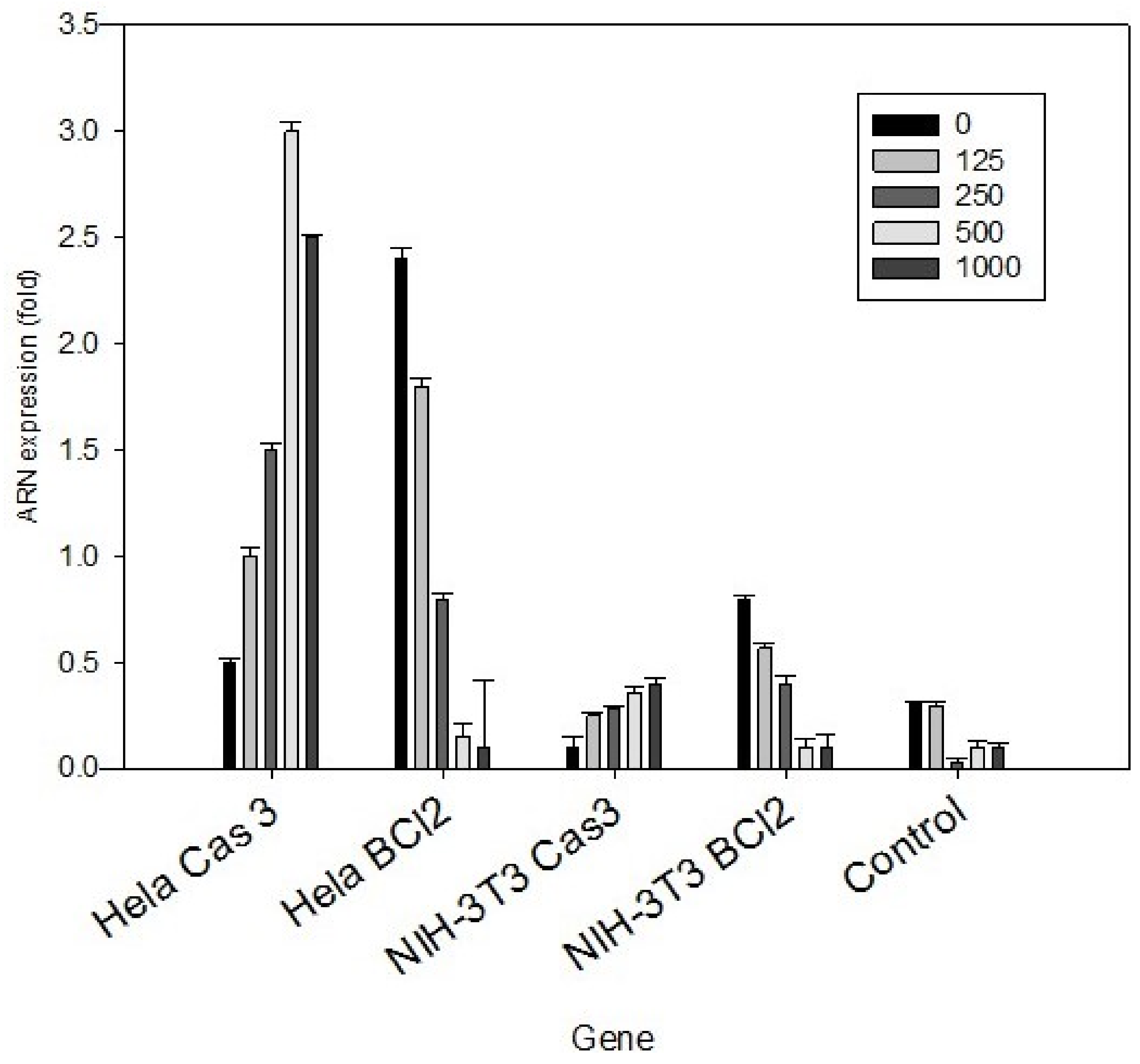
3.3. HeLa Cell Peptides Related to the Apoptotic Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Hashem, M.M.M.; Abo-elmatty, D.M.; Mesbah, N.M.; Abd El-Mawgoud, A.M. Induction of sub-G0 arrest and apoptosis by seed extract of Moringa peregrina (Forssk.) Fiori in cervical and prostate cancer cell lines. J. Integr. Med. 2019, 17, 410–422. [Google Scholar] [CrossRef]
- Zamani, S.; Sohrabi, A.; Rahnamaye-Farzami, M.; Hosseini, S.M. Glutathione S-transferase omega gene polymorphism as a biomarker for human papilloma virus and cervical cancer in Iranian women. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Susianti, S.; Yanwirasti, Y.; Darwin, E.; Jamsari, J. The cytotoxic effects of purple nutsedge (Cyperus rotundus L.) tuber essential oil on the HeLa cervical cancer cell line. Pak. J. Biotechnol. 2018, 15, 4. [Google Scholar]
- Silva, V.A.O.; Alves, A.L.V.; Rosa, M.N.; Silva, L.R.V.; Melendez, M.E.; Cury, F.P.; Gomes, I.N.F.; Tansini, A.; Longato, G.B.; Martinho, O.; et al. Hexane partition from Annona crassiflora Mart. promotes cytotoxity and apoptosis on human cervical cancer cell lines. Investig. New Drugs 2018, 1, 14. [Google Scholar] [CrossRef]
- Munguia-Moreno, J.A.; Diaz-Chavez, J.; Garcia-Villa, E.; Albino-Sanchez, M.E.; Mendoza-Villanueva, D.; Ocadiz-Delgado, R.; Bonilla-Delgado, J.; Marin-Flores, A.; Cortes-Malagon, E.M.; Alvarez-Rios, E.; et al. Early synergistic interactions between the HPV16E7 oncoprotein and 17beta-oestradiol for repressing the expression of Granzyme B in a cervical cancer model. Int. J. Oncol. 2018, 53, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, I.; Kiani, M.H.; Ahmad, M.; Masood, M.I.; Sadaquat, H. Major contributions towards finding a cure for cancer through chemotherapy: A historical review. Tumori 2016, 102, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Chan, L.C. ROS-Mediated Mitochondrial Pathway is Required for Manilkara Zapota (L.) P. Royen Leaf Methanol Extract Inducing Apoptosis in the Modulation of Caspase Activation and EGFR/NF-kappaB Activities of HeLa Human Cervical Cancer Cells. Evid. Based Complementary Altern. Med. 2018, 2018, 6578648. [Google Scholar] [CrossRef]
- Sanchez-Carranza, J.N.; Diaz, J.F.; Redondo-Horcajo, M.; Barasoain, I.; Alvarez, L.; Lastres, P.; Romero-Estrada, A.; Aller, P.; Gonzalez-Maya, L. Gallic acid sensitizes paclitaxel-resistant human ovarian carcinoma cells through an increase in reactive oxygen species and subsequent downregulation of ERK activation. Oncol. Rep. 2018, 39, 3007–3014. [Google Scholar] [CrossRef]
- Vásquez-Cruz, M.; Sosa, V. Assembly and origin of the flora of the Chihuahuan Desert: The case of sclerophyllous Rosaceae. J. Biogeogr. 2019. [Google Scholar] [CrossRef]
- Singh, G.; Passsari, A.K.; Singh, P.; Leo, V.V.; Subbarayan, S.; Kumar, B.; Singh, B.P.; Lalhlenmawia, H.; Kumar, N.S. Pharmacological potential of Bidens pilosa L. and determination of bioactive compounds using UHPLC-QqQLIT-MS/MS and GC/MS. BMC Complementary Altern. Med. 2017, 17, 492. [Google Scholar] [CrossRef]
- García-Alvarado, J.S.; Verde-Star, M.J.; Heredia, N.L. Traditional Uses and Scientific Knowledge of Medicinal Plants from Mexico and Central America. J. Herbs Spices Med. Plants 2001, 8, 37–89. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda, L.; Morlett-Chávez, J.; Meléndez-Renteria, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.; Aguilar, C.N. Solid-State Fermentation with Aspergillus niger GH1 to Enhance Polyphenolic Content and Antioxidative Activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Rached, W.; Zeghada, F.Z.; Bennaceur, M.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Carvalho, A.M.; Marouf, A.; Ferreira, I.C.F.R. Phytochemical analysis and assessment of antioxidant, antimicrobial, anti-inflammatory and cytotoxic properties of Tetraclinis articulata (Vahl) Masters leaves. Ind. Crop. Prod. 2018, 112, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharm. 2007, 159, 79–113. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 215S–217S. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol. Med. 2018, 24, 1–17. [Google Scholar] [CrossRef]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Han, H.W.; Qiu, H.Y.; Hu, C.; Sun, W.X.; Yang, R.W.; Qi, J.L.; Wang, X.M.; Lu, G.H.; Yang, Y.H. Design, synthesis and anti-cancer activity evaluation of podophyllotoxin-norcantharidin hybrid drugs. Bioorg. Med. Chem. Lett. 2016, 26, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
- Chemicals, C. LDH Cytotoxicity Assay Kit. Available online: https://www.caymanchem.com/product/601170/ldh-cytotoxicity-assay-kit (accessed on 2 January 2020).
- Klaude, M.; Eriksson, S.; Nygren, J.; Ahnstrom, G. The comet assay: Mechanisms and technical considerations. Mutat. Res. 1996, 363, 89–96. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Liu, K.; Liu, B.; Xu, W.; Gao, J.; Ding, L.; Tao, L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019, 52, e12543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelle, J.L.; Green, R. One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE). Methods Enzym. 2014, 541, 151–159. [Google Scholar] [CrossRef]
- Thiede, B.; Koehler, C.J.; Strozynski, M.; Treumann, A.; Stein, R.; Zimny-Arndt, U.; Schmid, M.; Jungblut, P.R. High resolution quantitative proteomics of HeLa cells protein species using stable isotope labeling with amino acids in cell culture(SILAC), two-dimensional gel electrophoresis(2DE) and nano-liquid chromatograpohy coupled to an LTQ-OrbitrapMass spectrometer. Mol. Cell Proteom. 2013, 12, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.X.; Song, X.Y.; Ma, C.; Feng, L.X.; Guan, S.H.; Wu, W.Y.; Yang, M.; Jiang, B.H.; Liu, X.; Cui, Y.J.; et al. Effects of triterpenes from Ganoderma lucidum on protein expression profile of HeLa cells. Phytomedicine 2010, 17, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Boeing, J.S.; Barizão, É.O.; Rotta, E.M.; Volpato, H.; Nakamura, C.V.; Maldaner, L.; Visentainer, J.V. Phenolic Compounds from Butia odorata (Barb. Rodr.) Noblick Fruit and Its Antioxidant and Antitumor Activities. Food Anal. Methods 2020, 13, 61–68. [Google Scholar] [CrossRef]
- Liu, W.N.; Shi, J.; Fu, Y.; Zhao, X.H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe(2+)/Cu(2+) Addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewinska, A.; Adamczyk, J.; Pajak, J.; Stoklosa, S.; Kubis, B.; Pastuszek, P.; Slota, E.; Wnuk, M. Curcumin-mediated decrease in the expression of nucleolar organizer regions in cervical cancer (HeLa) cells. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2014, 771, 43–52. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2017, 15, 9845–9851. [Google Scholar] [CrossRef] [Green Version]
- Astirin, O.P.; Artanti, A.N.; Fitria, M.S.; Perwitasari, E.A.; Prayitno, A. Annonaa muricata Linn Leaf Induce Apoptosis in Cancer Cause Virus. J. Cancer Ther. 2013, 4, 1244–1250. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Yang, Y.; Li, Q.; Liu, F. Crude Flavonoids from Caryacathayensis Sargent inhibited HeLa Cells Proliferation through Induction of Apoptosis and Cell Cycle Arrest. Lat. Am. J. Pharm. 2009, 28, 5. [Google Scholar]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; Clevers, H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 997–1014. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F.; Kahn, M. Targeting Wnt signaling: Can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010, 16, 3153–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Kuroda, K.; Ozaki, R.; Ikemoto, Y.; Murakami, K.; Muter, J.; Matsumoto, A.; Itakura, A.; Brosens, J.J.; Takeda, S. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2-RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, U.; Venkatesan, T.; Radhakrishnan, V.; Samuel, S.; Rasappan, P.; Rathinavelu, A. Cell Cycle Arrest and Cytotoxic Effects of SAHA and RG7388 Mediated through p21(WAF1/CIP1) and p27(KIP1) in Cancer Cells. Medicina 2019, 55, 30. [Google Scholar] [CrossRef] [Green Version]

| ID | Compounds | Family |
|---|---|---|
| 1 | Kaempferol | Flavanols |
| 2 | Kaempferol | Flavanols |
| 3 | Quercetina | Flavanols |
| 4 | Catechin | Catechins |
| 5 | Kaempferol | Flavanols |
| 6 | Epicatechin | Catechins |
| 7 | Quercetin | Flavanols |
| 8 | Ellagic acid | |
| 9 | Gallic acid | Hydroxybenzoic acids |
| 10 | Apigenin | Flavones |
| ID | Protein | Sequence |
|---|---|---|
| 1 | Cytochrome C | VLGPDRVLGPDRLPEFSDRNPGVQQRAQEEIDRIASGSAGQSVALESMERLPEFSDRSSLPYIEAVWRFLDDKGGLTDDLPAYAFGFGRMSLAYLAGALVLAAAVLWKGVETR |
| 2 | Apoptosis activation factor 1 | MDAKARNCLLQHREALEKDIKTSYIMDHMISDGFLTISEEEKVRNEPTQQQRAAMLIKMILKKDNDSYVSFYNALLHEGYKDLAALLHDGIPVVSSSSGKDSVSGITSYVRTVLCEGGVPQRPVVFVTRKKLVNAIQQKLSKLKGEPGWVTIHGMAGCGKSVLAAEAVRDHSLLEGCFPGGVHWVSVGKQDKSGLLMKLQNLCTRLDQDESFSQRLPLNIEAKDRLRILMLRKHPRSLLILDDVWDSWVLKAFDSQCQILLTTRDKSVTDSVMGPKYVV PVESSLGKEK GLEILSLFVNMKKADLPEQA HSIIKECKGS LE |
| 3 | Caspases 8 | DATAKIRLVRHSLDSVDPTPRPRSHPQAAPQPQAHTPTASVPSRRRPSTPQAPPPPSMVSVDSPRRPSTPQAPPPPSMVSVDSPRMNASGKASAVASNVHAGPAAAGAMSFGWLGPRLSFGSPR |
| 4 | CREB3 regulatory factor | MLSATPLYGNVHSWMNSERVRMCGASEDRKILVNDGDASKARLELREENPLNHNVVDASTAHRIDGLAALSMDRTGLIREGLRVPGNIVYSSLCGLGSEKGREAATSTLGGLGFSSERNPEMQFKPNTPETVEASAVSGKPPNGFSAIYKTPPGIQKSAVATAEALGLDRPASDKQSPLNINGASYLRLPWVNPYMEGATPAIYPFLDSPNKYSLNMYKALLPQQSYSLAQPLYSPVCTNGERFLYLPPPHYVGPHIPSSLASPMRLSTPSASPAIPPLVHCADKFYGSSVCEPDDESGYDVLANPPGPEDQDDDDDAYSDVFEFEFSETPLLPCYNIQVSVAQGPRNWLLLSDVLKKLKMSSRIFRCNFPNVEIVTIAEAEFYRQVSASLLFSCSKDLEAFNPESKELLDLVEFTNEIQTLLGSSVEWLHPSDLASDNYW |
| 5 | BCL-6 corepressor | MLSATPLYGNVHSWMNSERVRMCGASEDRKILVNDGDASKARLELREENPLNHNVVDASTAHRIDGLAALSMDRTGLIREGLRVPGNIVYSSLCGLGSEKAGGKKQAQPSCAPAPLLPCYNIQVSVAQGPRNWLLLSDVLKKLKMSSRIFRCNFPNVEIVTIAEAEFYRQVSASLLFSCSKDLEAFNPESKELLDLVEFTNEIQTLLGSSVEWL HPSDLASDNY W |
| 6 | Protein BTG2 | MSHGKGTDMLPEIAAAVGFLSSLLRTRGCVSEQRLKVFSGALQEALTEHYKHHWFPEKPSKGSGYRCIRINHKMDPIISRVASQIGLSQPQLHQLLPSELTLWVDPYEVSYRIGEDGSICVLYEEAPLAASCGLLTCKNQVLLGRSSPSK NYVMAVSS |
| 7 | Retinoblastoma | MPSGGDQSPPPPPPPPAAAASDEEEEDDGEAEDAAPPAESPTPQIQQRFDELCSRLNMDEAARAEAWDSYRSMSESYTLEGNDLHWLACALYVACRKSVPTVSKGTVEGNYVSLTRILKCSEQSLIEFFNKMKKWEDMANRTSRDSSPVMRSSSTLPVPQPSSAPPTPTRLTGANSDMEEEERGDLIQFYNNIYIKQIKTFAMKYSQANMDAPPLSPYPFVRTGSPRRIQLSQNHPVYISPHKNETMLSPREKIFYYFSNSPSKRLREINSMIRTGETPTKKRGILLEDGS ESPAKRICPE NHSALLRRLQ DVANDRGSH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Ortiz, P.; Ascacio-Valdés, J.; Vera-Reyes, I.; Esparza-González, C.; Rodríguez-Herrera, R.; Salinas-Santander, M.; del Ángel-Martínez, M.; Morlett-Chávez, A. Purshia plicata Triggers and Regulates Proteins Related to Apoptosis in HeLa Cancer Cells. Plants 2021, 10, 2559. https://doi.org/10.3390/plants10122559
Álvarez-Ortiz P, Ascacio-Valdés J, Vera-Reyes I, Esparza-González C, Rodríguez-Herrera R, Salinas-Santander M, del Ángel-Martínez M, Morlett-Chávez A. Purshia plicata Triggers and Regulates Proteins Related to Apoptosis in HeLa Cancer Cells. Plants. 2021; 10(12):2559. https://doi.org/10.3390/plants10122559
Chicago/Turabian StyleÁlvarez-Ortiz, Patricia, Juan Ascacio-Valdés, Ileana Vera-Reyes, Cecilia Esparza-González, Raúl Rodríguez-Herrera, Mauricio Salinas-Santander, Mayela del Ángel-Martínez, and Antonio Morlett-Chávez. 2021. "Purshia plicata Triggers and Regulates Proteins Related to Apoptosis in HeLa Cancer Cells" Plants 10, no. 12: 2559. https://doi.org/10.3390/plants10122559
APA StyleÁlvarez-Ortiz, P., Ascacio-Valdés, J., Vera-Reyes, I., Esparza-González, C., Rodríguez-Herrera, R., Salinas-Santander, M., del Ángel-Martínez, M., & Morlett-Chávez, A. (2021). Purshia plicata Triggers and Regulates Proteins Related to Apoptosis in HeLa Cancer Cells. Plants, 10(12), 2559. https://doi.org/10.3390/plants10122559






