Ivabradine–Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review
Abstract
1. Introduction
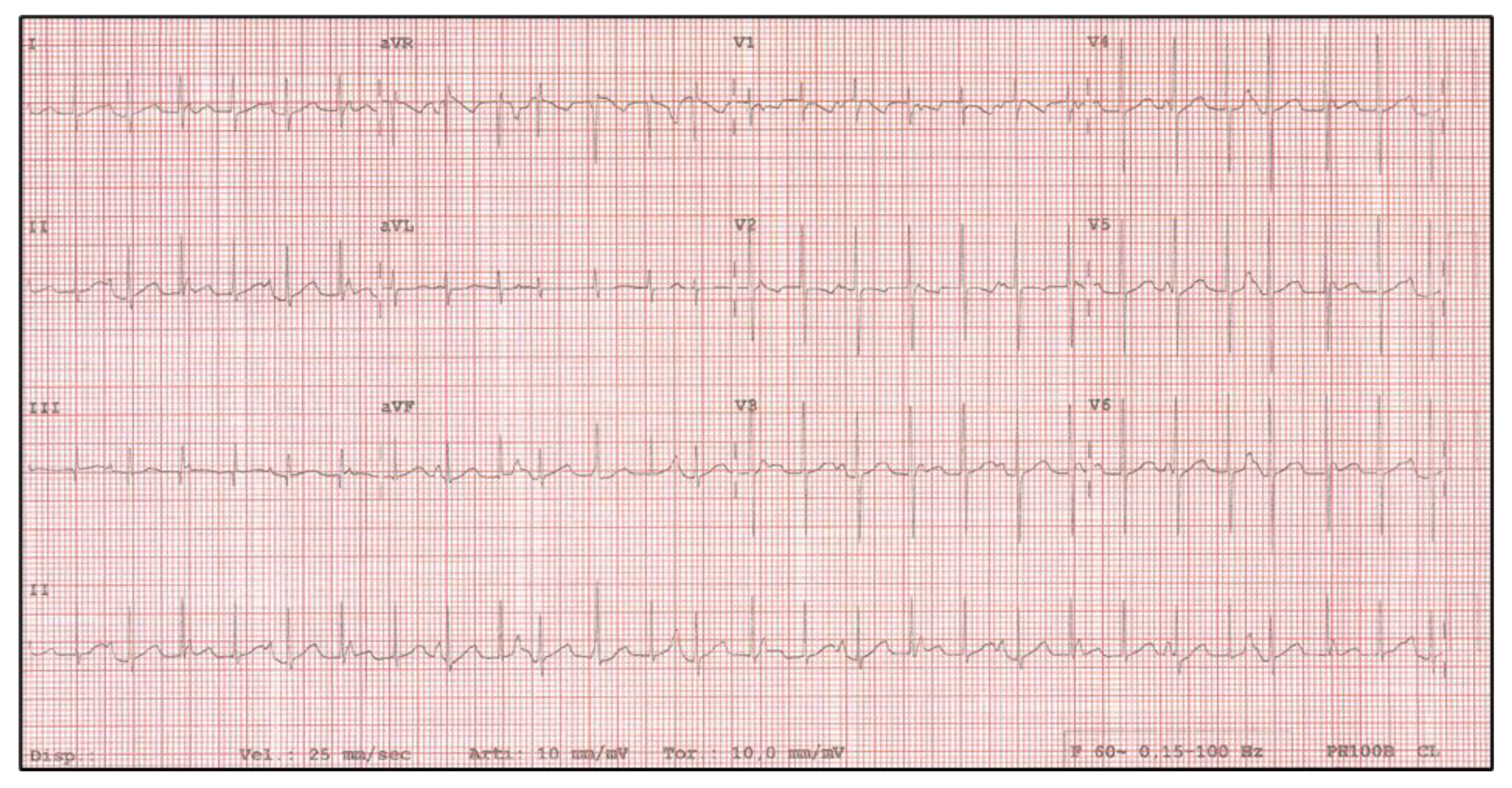
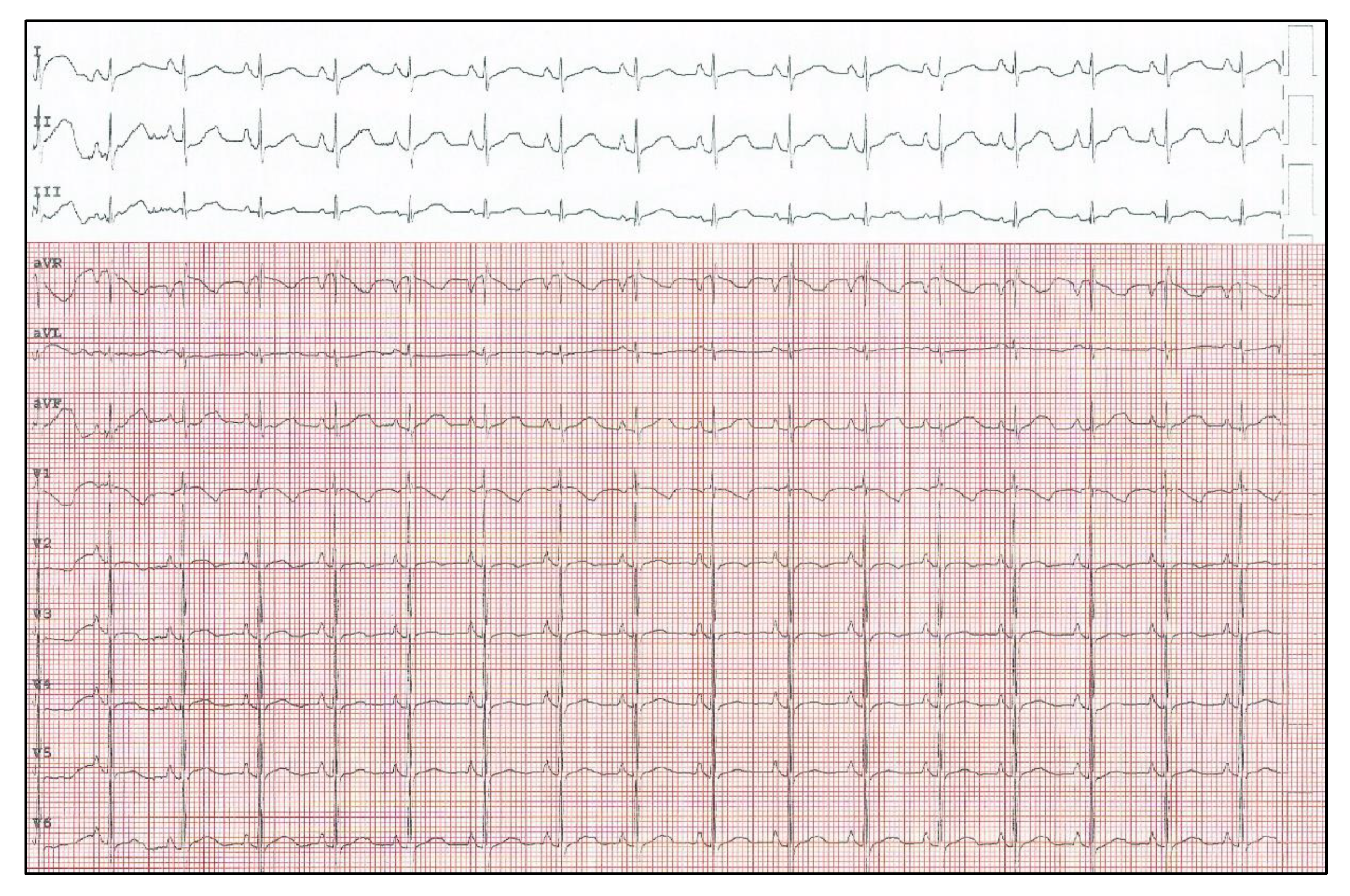
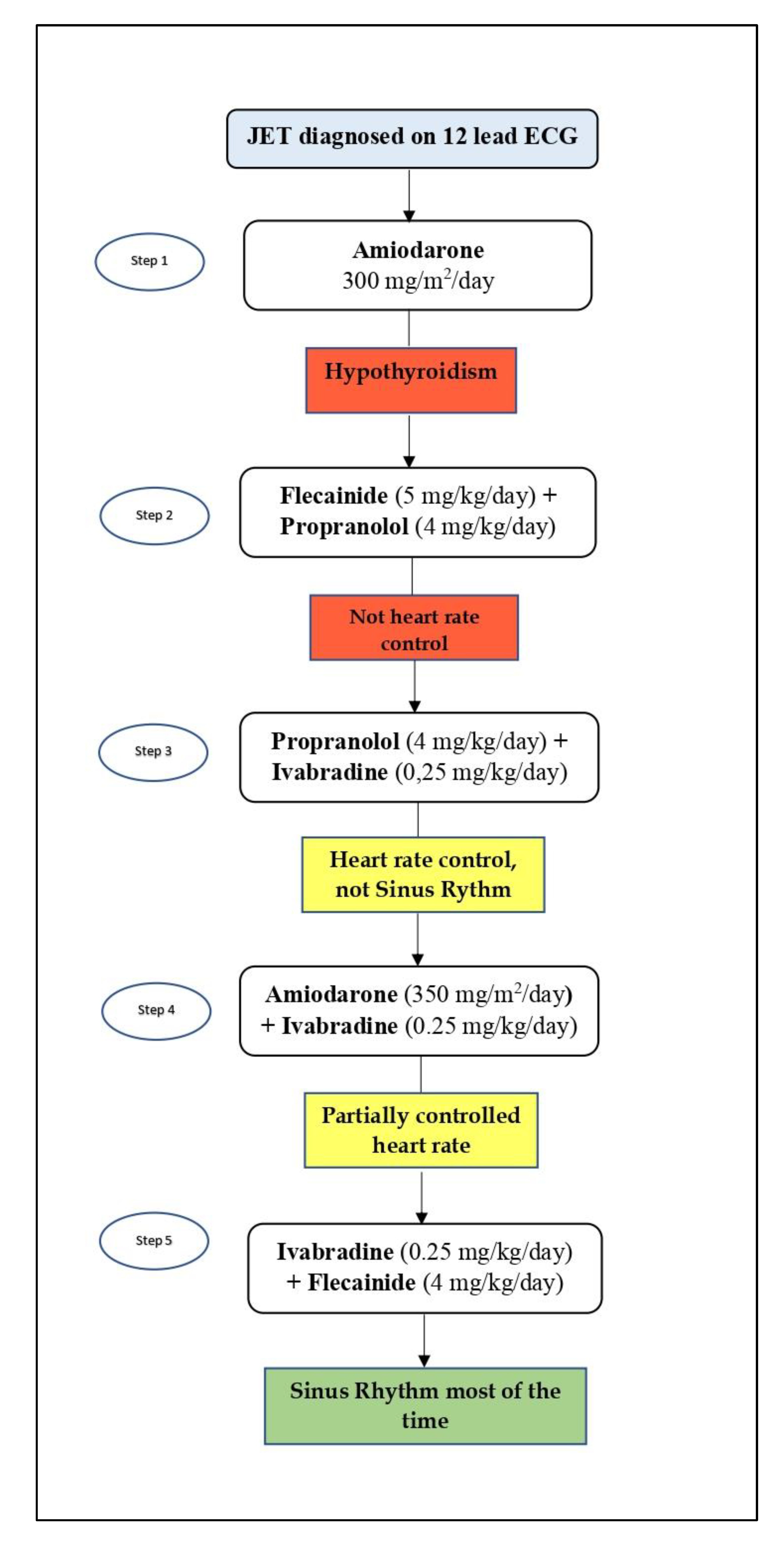
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kylat, R.I.; Samson, R.A. Junctional ectopic tachycardia in infants and children. J. Arrhythm. 2020, 36, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.K.; Van Hare, G.F.; Kertesz, N.J.; Law, I.H.; Bar-Cohen, Y.; Dubin, A.M.; Etheridge, S.P.; Berul, C.I.; Avari, J.N.; Tuzcu, V.; et al. Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J. Am. Coll. Cardiol. 2009, 53, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Blom, N.; Sarquella-Brugada, G.; Blomstrom-Lundqvist, C.; Deanfield, J.; Janousek, J.; Abrams, D.; Bauersfeld, U.; Brugada, R.; Drago, F.; et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace 2013, 15, 1337–1382. [Google Scholar] [CrossRef] [PubMed]
- Siddoway, L.A. Amiodarone: Guidelines for use and monitoring. Am. Fam. Physician 2003, 68, 2189–2196. [Google Scholar]
- Saul, J.P.; Scott, W.A.; Brown, S.; Marantz, P.; Acevedo, V.; Etheridge, S.P.; Perry, J.C.; Triedman, J.K.; Burriss, S.W.; Cargo, P.; et al. Intravenous amiodarone for incessant tachyarrhythmias in children: A randomized, double-blind, antiarrhythmic drug trial. Circulation 2005, 112, 3470–3477. [Google Scholar] [CrossRef]
- Benjamín, M.N.; Infante, J.; Olmedo, J.; Abello, M.; Moltedo, J.M. Taquicardia ectópica congénita de la unión. Tratamiento farmacológico en el primer año de vida” [Congenital junctional ectopic tachycardia. Pharmacologic management during infancy]. Medicina 2011, 71, 521–524. [Google Scholar]
- Villain, E.; Vetter, V.L.; Garcia, J.M.; Herre, J.; Cifarelli, A.; Garson, A. Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation 1990, 81, 1544–1549. [Google Scholar] [CrossRef]
- Sarubbi, B.; Musto, B.; Ducceschi, V.; D’Onofrio, A.; Cavallaro, C.; Vecchione, F.; Musto, C.; Calabrò, R. Congenital junctional ectopic tachycardia in children and adolescents: A 20 year experience based study. Heart 2002, 88, 188–190. [Google Scholar] [CrossRef][Green Version]
- Fenrich, A.L.; Perry, J.C.; Friedman, R.A. Flecainide and amiodarone: Combined therapy for refractory tachyarrhythmias in infancy. J. Am. Coll Cardiol. 1995, 25, 1195–1198. [Google Scholar] [CrossRef]
- Imamura, T.; Tanaka, Y.; Ninomiya, Y.; Yoshinaga, M. Combination of flecainide and propranolol for congenital junctional ectopic tachycardia. Pediatr. Int. 2015, 57, 716–718. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Camm, J.A. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: A new therapeutic perspective in cardiovascular disease. Drugs 2004, 64, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, G.; Dasopoulou, C.; Sakellariou, D.; Tzeis, S.; Koulouris, S.; Kranidis, A.; Kappos, K.; Manolis, A.S. Ivabradine: A selective If current inhibitor in the treatment of stable angina. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 277–282. [Google Scholar] [CrossRef]
- Guglin, M. Heart rate reduction in heart failure: Ivabradine or beta blockers? Heart Fail. Rev. 2013, 18, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, S.; Al-Fayyadh, M.I.; Hamilton, R.M. Potential new indication for ivabradine: Treatment of a patient with congenital junctional ectopic tachycardia. J. Cardiovasc. Electrophysiol. 2013, 24, 822–824. [Google Scholar] [CrossRef]
- Kothari, S.S.; Kidambi, B.R.; Juneja, R. Ivabradine for congenital junctional ectopic tachycardia in siblings. Ann. Pediatr. Cardiol. 2018, 11, 226–228. [Google Scholar] [CrossRef]
- Ergul, Y.; Ozturk, E.; Ozgur, S.; Ozyurt, A.; Cilsal, E.; Guzeltas, A. Ivabradine is an effective antiarrhythmic therapy for congenital junctional ectopic tachycardia-induced cardiomyopathy during infancy: Case studies. Pacing Clin. Electrophysiol. 2018, 41, 1372–1377. [Google Scholar] [CrossRef]
- Horsthuis, T.; Buermans, H.P.; Brons, J.F.; Verkerk, A.O.; Bakker, M.L.; Wakker, V.; Clout, D.E.; Moorman, A.F.; ’t Hoen, P.A.; Christoffels, V.M. Gene expression profiling of the forming atrioventricular node using a novel tbx3-based node-specific transgenic reporter. Circ. Res. 2009, 105, 61–69. [Google Scholar] [CrossRef]
- Dieks, J.K.; Klehs, S.; Müller, M.J.; Paul, T.; Krause, U. Adjunctive ivabradine in combination with amiodarone: A novel therapy for pediatric congenital junctional ectopic tachycardia. Heart Rhythm. 2016, 13, 1297–1302. [Google Scholar] [CrossRef]
- Ríos, M.; Chiesa, P.; Arhcilles, S.; Cuesta, A.; Moltedo, J.M. Uso de la ivrabadina para el tratamiento de la taquicardia ectópica de la unión congénita [Use of ivabradine for the treatment of congenital junctional ectopic tachycardia]. Medicina 2021, 81, 293–296. [Google Scholar] [PubMed]
- Khan, N.; Salvi, P.; Dharod, D.; Chokhandre, M.; Mandrekar, A.; Joshi, S. Use of Ivabradine in the Treatment of Tachyarrhythmias After Surgery for Congenital Heart Diseases. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2395–2400. [Google Scholar] [CrossRef]
- Krishna, M.R.; Kunde, M.F.; Kumar, R.K.; Balaji, S. Ivabradine in Post-operative Junctional Ectopic Tachycardia (JET): Breaking New Ground. Pediatr. Cardiol. 2019, 40, 1284–1288. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, G.; Joshi, S.; Sharma, V. Ivabradine for junctional ectopic tachycardia in post congenital heart surgery. Indian Heart J. 2017, 69, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, G.; Tiwari, N.; Joshi, S.; Sharma, V.; Ramamurthy, R. Ivabradine as an Adjunct for Refractory Junctional Ectopic Tachycardia Following Pediatric Cardiac Surgery: A Preliminary Study. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Niraghatam, H.; Bansal, N.; Singh, S.; Rajashekar, P.; Choudhary, S. Ivabradine—The Final Crusader for Postoperative Junctional Ectopic Tachycardia, a Case Report with Literature Review. World J. Cardiovasc. Surg. 2019, 9, 73–82. [Google Scholar] [CrossRef]
- Sharma, D.; Subramaniam, G.; Sharma, N. Use of ivabradine for treatment of junctional ectopic tachycardia in post congenital heart surgery. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 323–325. [Google Scholar] [CrossRef] [PubMed]
| Author | Number of Patients (N); Gender | Antiarrhythmic Medication Before Ivabradine | Treatment with Ivabradine (Dose) | Outcome, Response to Ivabradine | Antiarrhythmic Medication with Ivabradine | Adverse Reactions | |
|---|---|---|---|---|---|---|---|
| Congenital JET | Al-Ghamdi et al. [14] | N = 1; F | Flecainide, sotalol, procainamide, amiodarone | 2.5 mg once daily | SR, HR control | None | None |
| Dieks et al. [18] | N = 5; 2 M, 3 F | Amiodarone, n = 5 Flecainide, n = 1 Digoxin, n =2 | 0.05–0.1 mg/kg increased up to 0.28 mg/kg/d | HR control, n = 5 SR, n = 3 JR/JET, n = 1 JR/SR, n = 1 | Amiodarone, n = 5 Propranolol, amiodarone, n = 2 Digoxin, amiodarone, flecainide, n = 1 | None | |
| Ergul et al. [16] | N = 3; 2 M, 1 F | Flecainide, amiodarone, digoxin, n = 1 Flecainide, amiodarone, propranolol, n = 2 | 0.1 mg/kg/d | HR control, n = 3 SR, n = 2 JR/SR, n = 1 | Amiodarone, n = 1 Amiodarone, propranolol, flecainide, n = 2 | None | |
| Kothari et al. [15] | N = 2; 1 M, 1 F | Amiodarone, propranolol, flecainide, n = 2 | 0.5 mg/kg/dose | SR, HR control | Amiodarone, propranolol, flecainide, n = 2 | None | |
| Rios et al. [19] | N = 2; 2 M | Amiodarone, flecainide, propranolol, n = 1 Propranolol, amiodarone, n = 1 | 0.05/mg/kg/dose | HR control, n = 2 SR/TN, n = 2 | Amiodarone, n = 2 | None | |
| Postoperative JET | Khan et al. [20] | N = 7 (6 Jet); 5 M, 2 F | Amiodarone, n = 7 | 0.05 mg/kg/dose | SR, n = 4 HR control with persistent slow JET, n = 1 | Amiodarone | None |
| Krishna et al. [21] | N = 8; 4 M, 4 F | Amiodarone, n = 1 Overdrive pacing, n = 5 | 0.05 mg/kg/dose twice daily | SR, HR control, n = 8 | Amiodarone, n = 1 | Bradycardia | |
| Kumar et al. [22] | N = 2; 1 M, 1 F | Amiodarone, esmolol, n = 1 | 0.1 mg/kg/d | SR, n = 2 | It is not clear whether ivabradine was used as a single or adjunctive treatment | N/S | |
| Kumar * et al. [23] | N = 5; 3 M, 2 F | Amiodaron, esmolol, n = 5 | 0.1–0.2 mg/kg/d twice daily | SR, HR control, n = 5 | Amiodarone, n = 2 | None | |
| Sahu et al. [24] | N = 1; F | Magnesium sulfate, digoxin, amiodaron | 0.05 mg/kg twice daily | SR, HR control | None | None | |
| Sharma et al. [25] | N = 4; 2 M, 2 F | Magnesium, n = 4 | 0.1–0.2 mg/kg/dose | SR, HR control, n = 4 | None | Bradycardia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco, G.M.; De Nigris, A.; Pepe, A.; Pagano, A.; Di Nardo, G.; Tipo, V. Ivabradine–Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review. Pediatr. Rep. 2021, 13, 624-631. https://doi.org/10.3390/pediatric13040074
Di Marco GM, De Nigris A, Pepe A, Pagano A, Di Nardo G, Tipo V. Ivabradine–Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review. Pediatric Reports. 2021; 13(4):624-631. https://doi.org/10.3390/pediatric13040074
Chicago/Turabian StyleDi Marco, Giovanni Maria, Angelica De Nigris, Angela Pepe, Annamaria Pagano, Giangiacomo Di Nardo, and Vincenzo Tipo. 2021. "Ivabradine–Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review" Pediatric Reports 13, no. 4: 624-631. https://doi.org/10.3390/pediatric13040074
APA StyleDi Marco, G. M., De Nigris, A., Pepe, A., Pagano, A., Di Nardo, G., & Tipo, V. (2021). Ivabradine–Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review. Pediatric Reports, 13(4), 624-631. https://doi.org/10.3390/pediatric13040074






