Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies
Abstract
:1. Introduction
2. Results
2.1. Experimental Design
- Acute administration at doses of 0.3 and 1 mg/kg respectively, and the PFCs or hippocampi were dissected 30 min after the administration.
- Chronic administration for 14 days at doses of 0.3 mg/kg (MK-801) and 1 mg/kg (scopolamine), and the PFCs or hippocampi were dissected 24 h after the last administration.
- The levels of selected amino acids: L-citrulline, L-glutamate, L-glutamine, L-ornithine and L-arginine and its derivatives: asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and n-monomethyl-L-arginine (NMMA), measured by mass spectrometry.
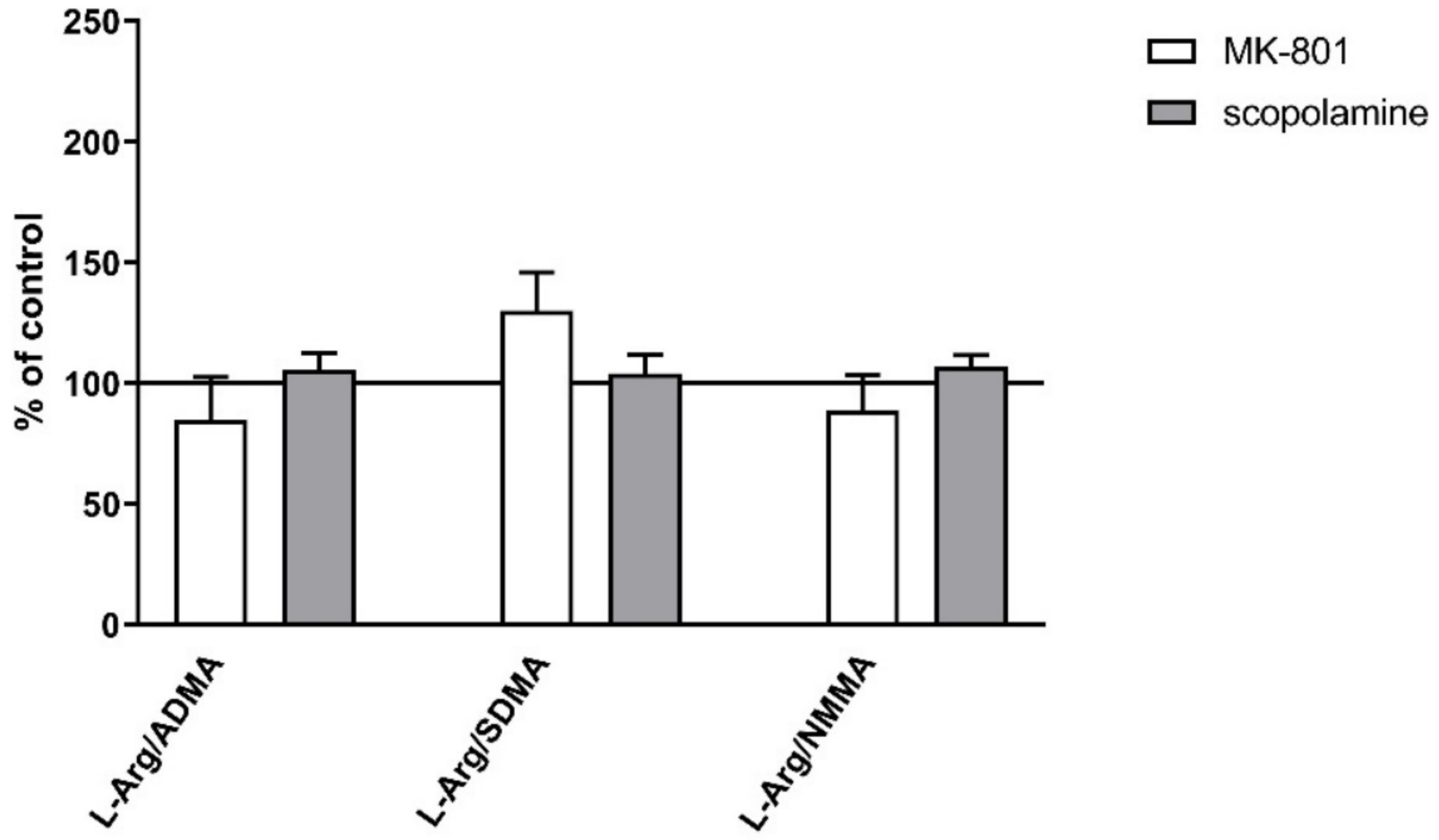
- L-Arg/ADMA, L-Arg/SDMA and L-Arg/NMMA ratios.
- The expression of eNOS, nNOS, DDAH1, PMRT1 and PMRT5, analyzed by Western blotting.
- GluN2B subunit-containing NMDA receptor and cGMP levels, analyzed by Western blotting and ELISA, respectively.
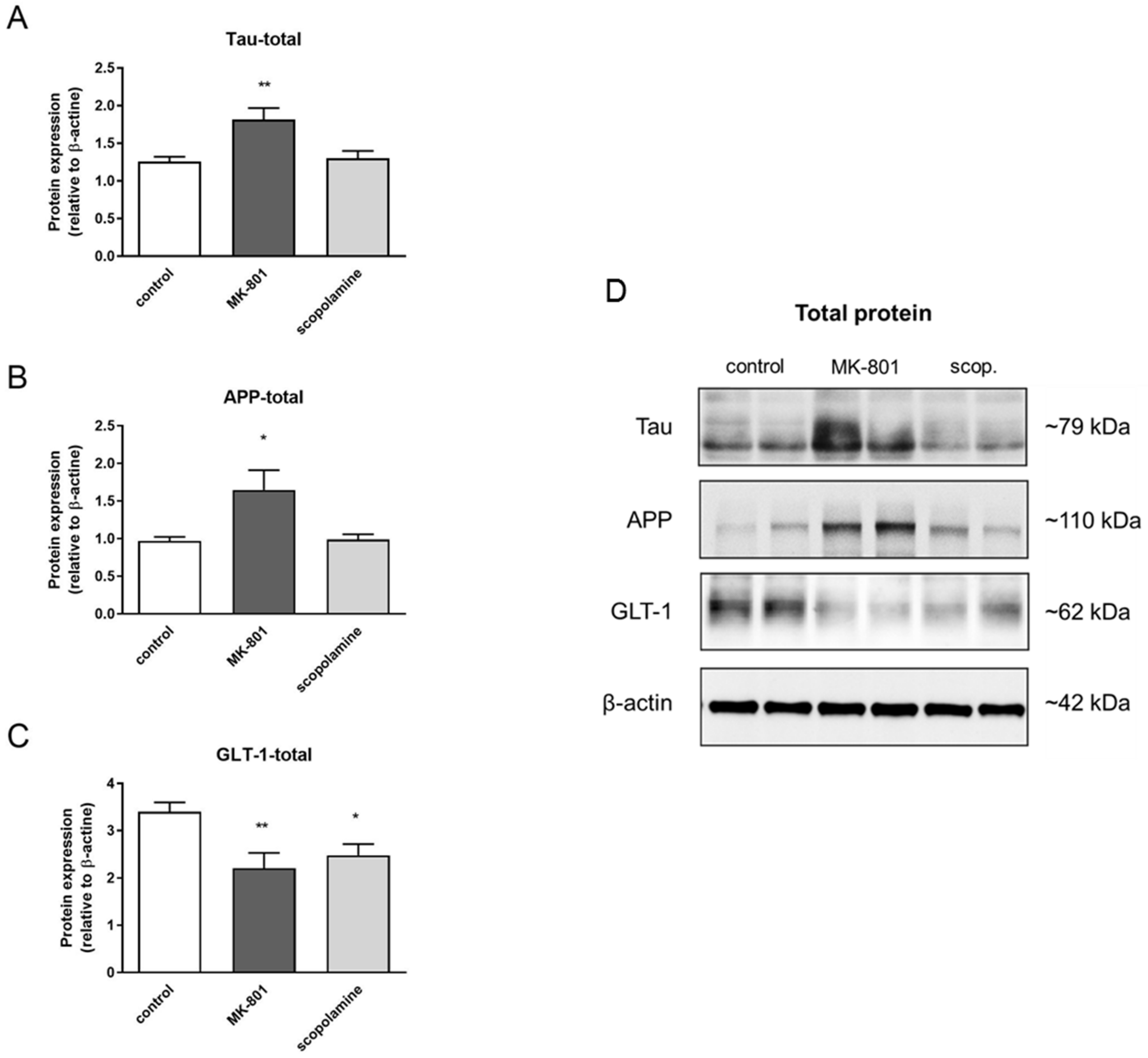
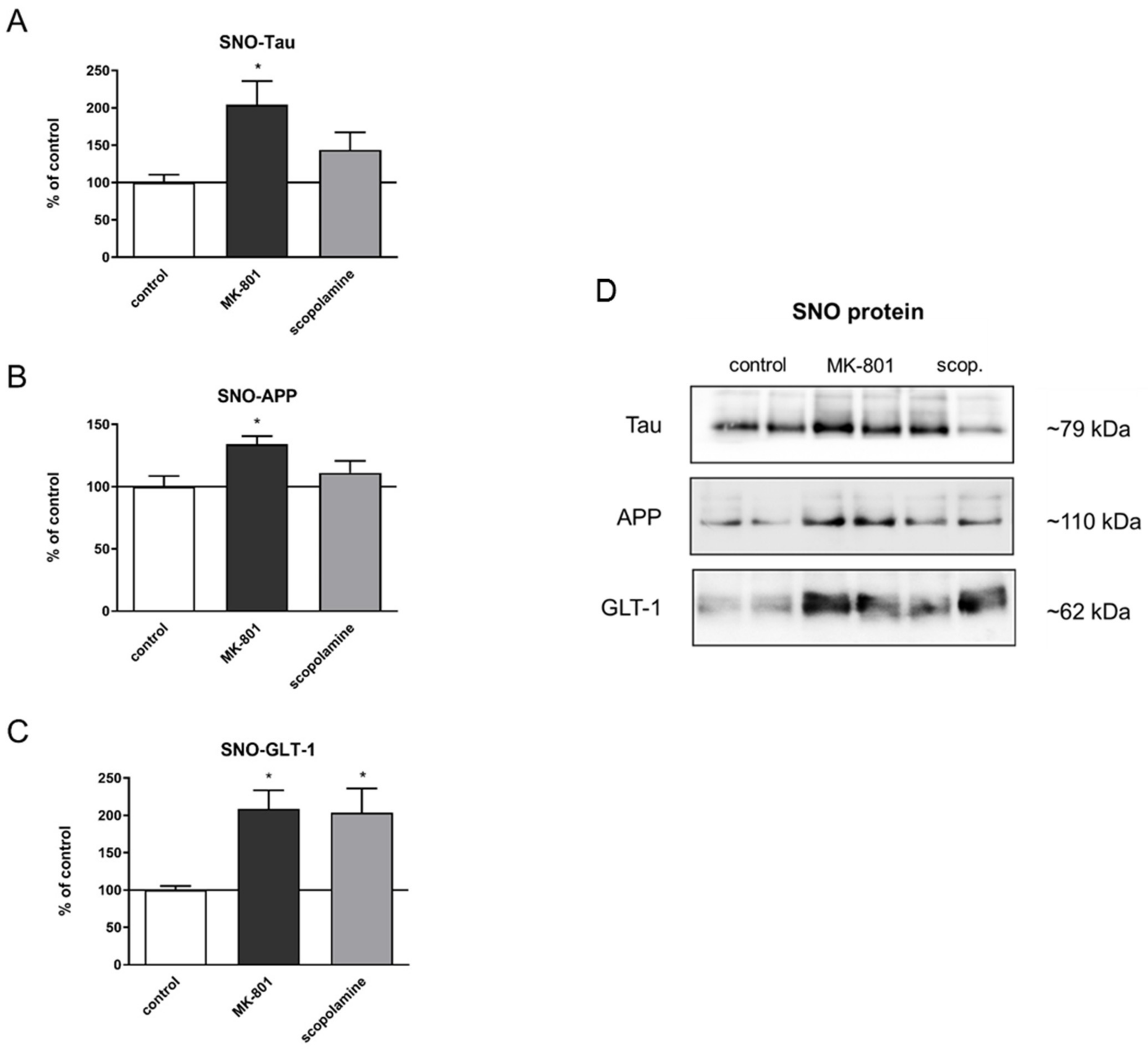
- S-nitrosylation of selected proteins: Tau, apolipoprotein (APP) and GLT-1 transporter (glutamate transporter 1).
2.2. Acute MK-801 or Scopolamine Administration
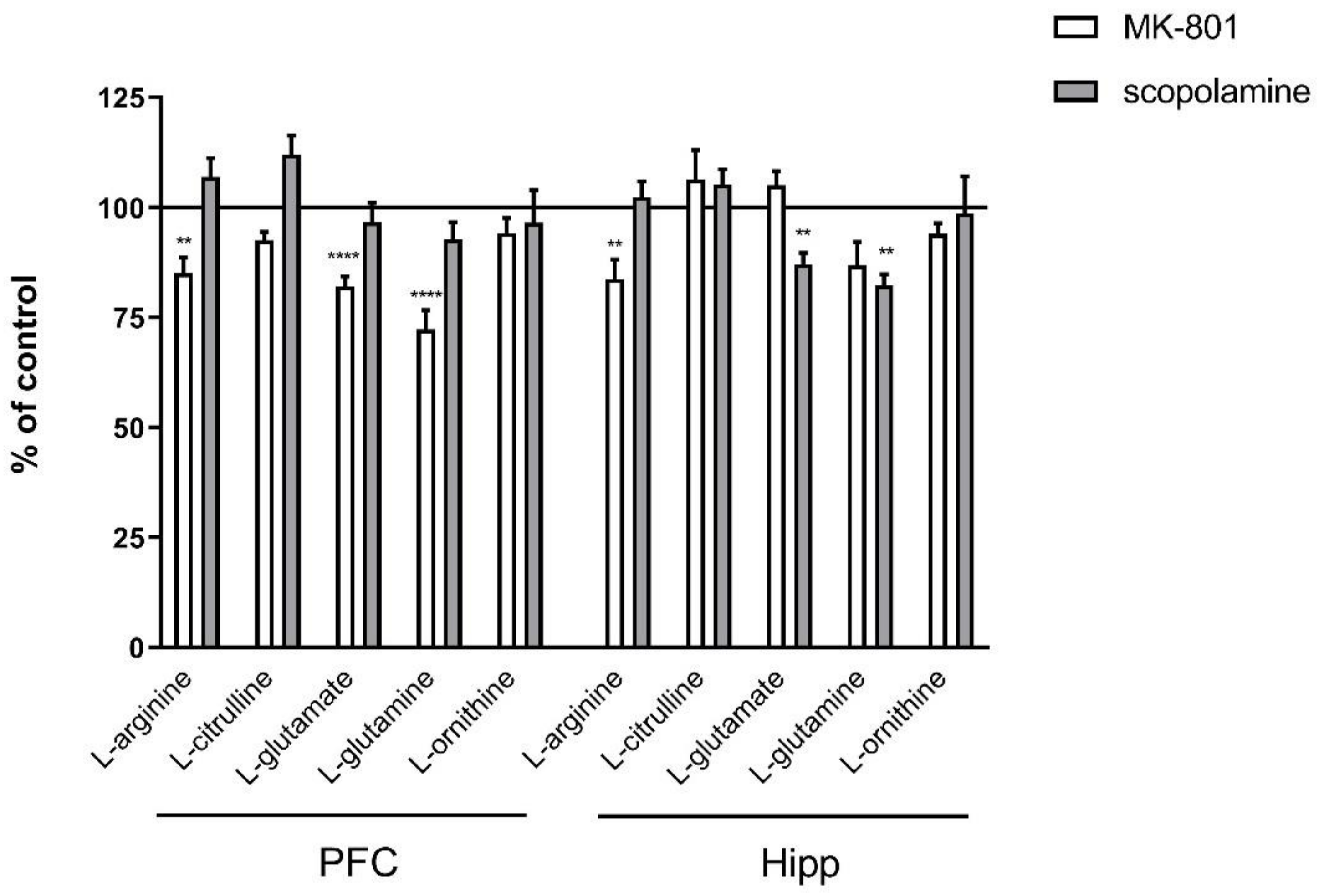
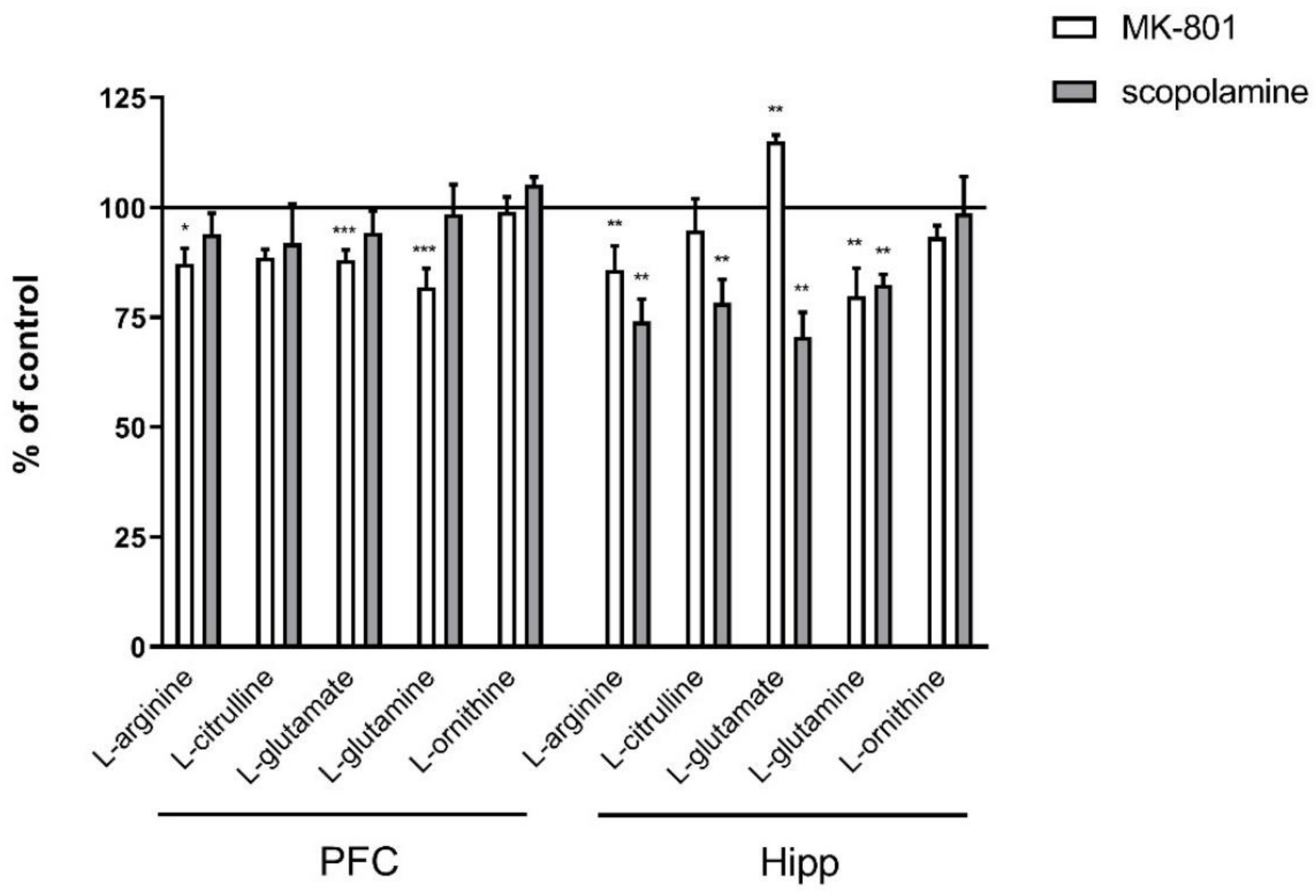
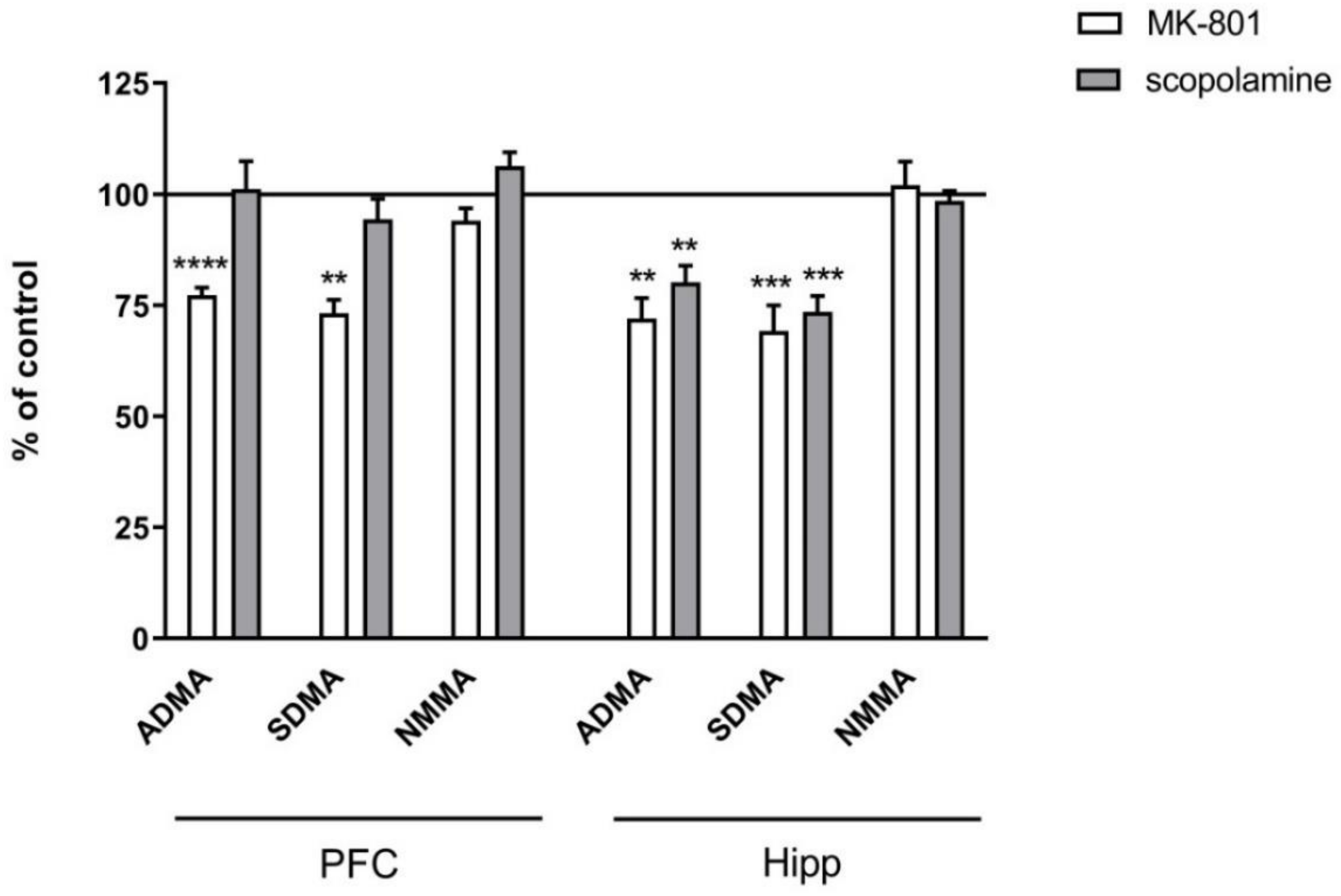
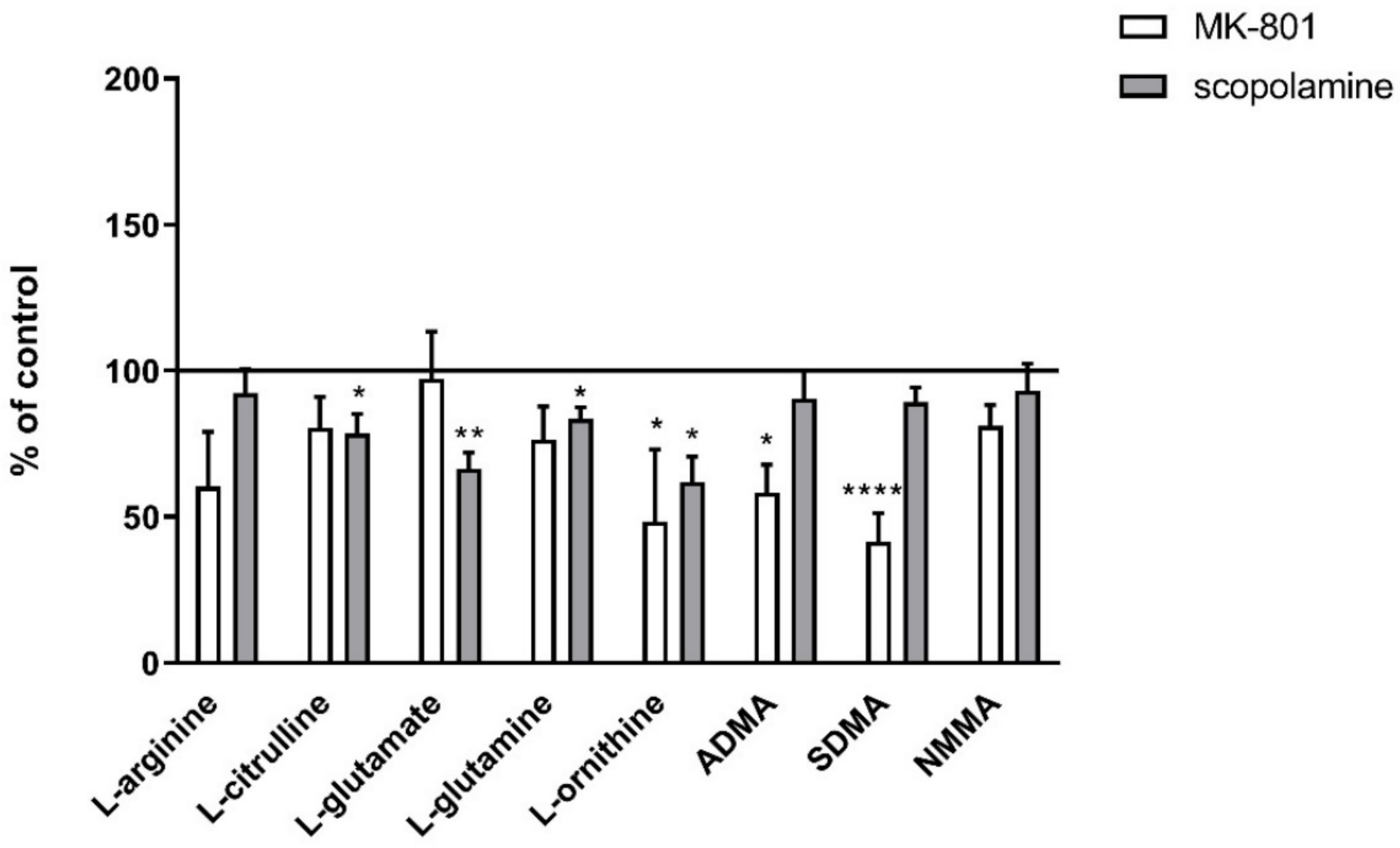
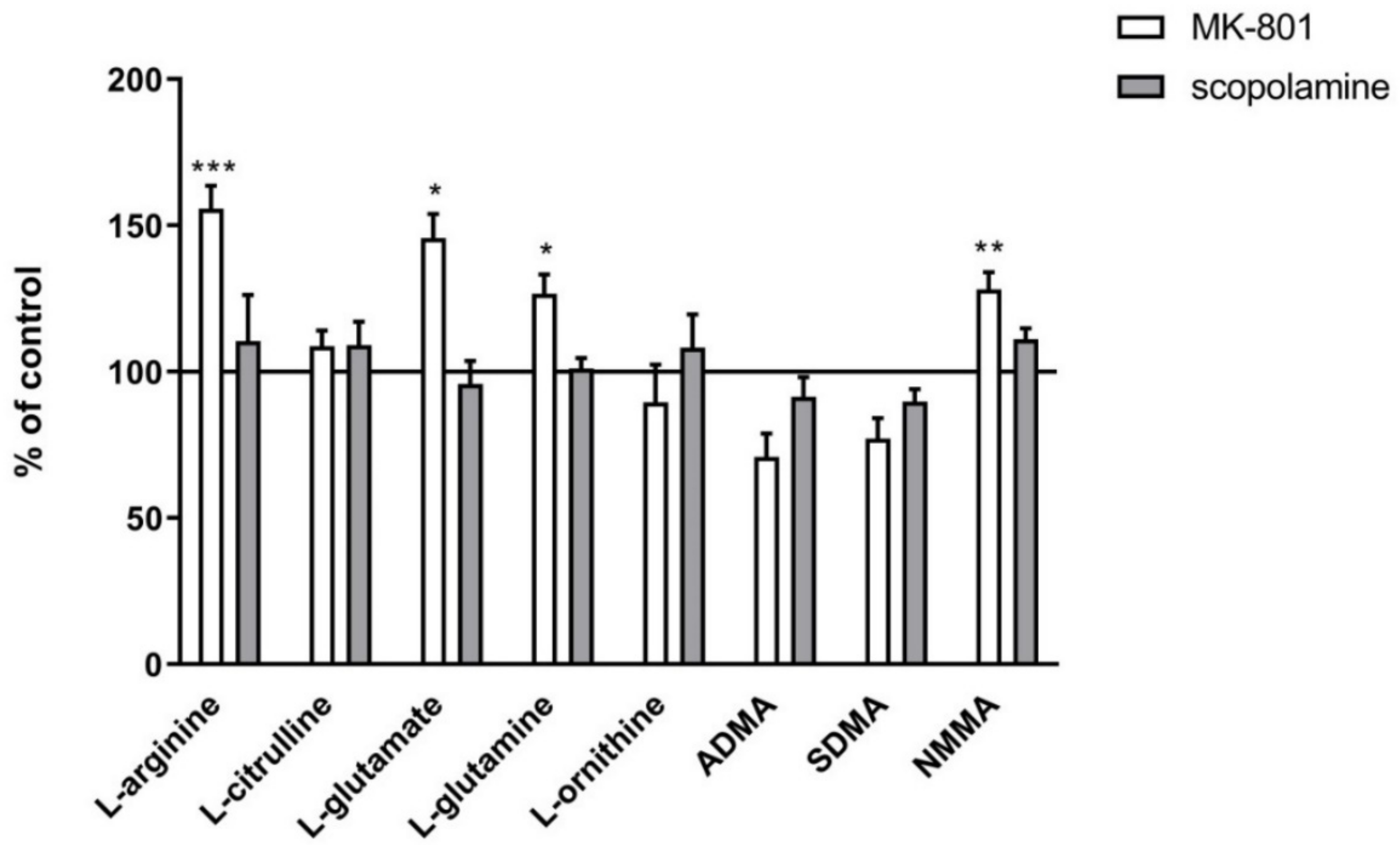
2.2.1. Amino Acids in PFC and Hippocampus
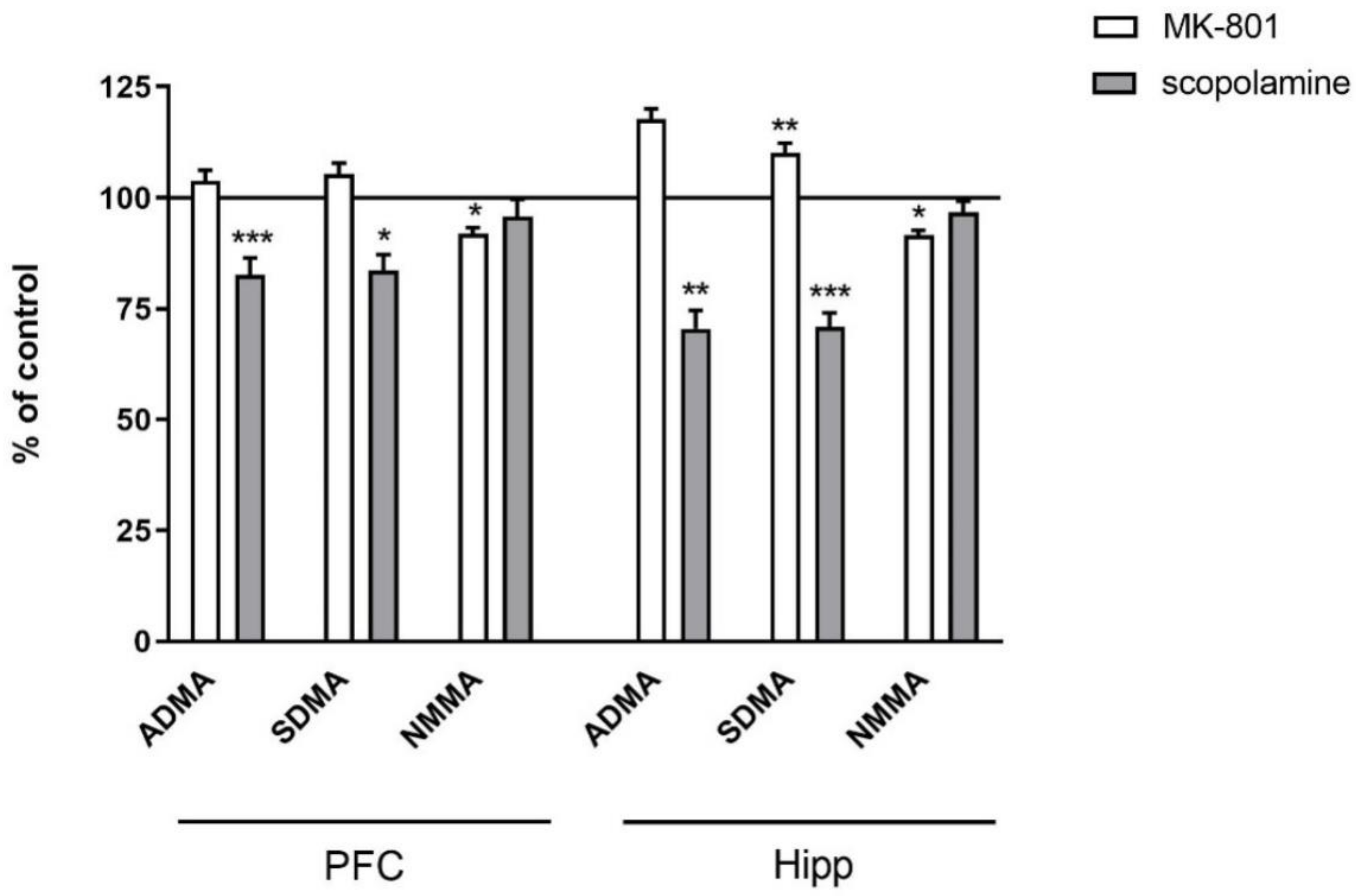
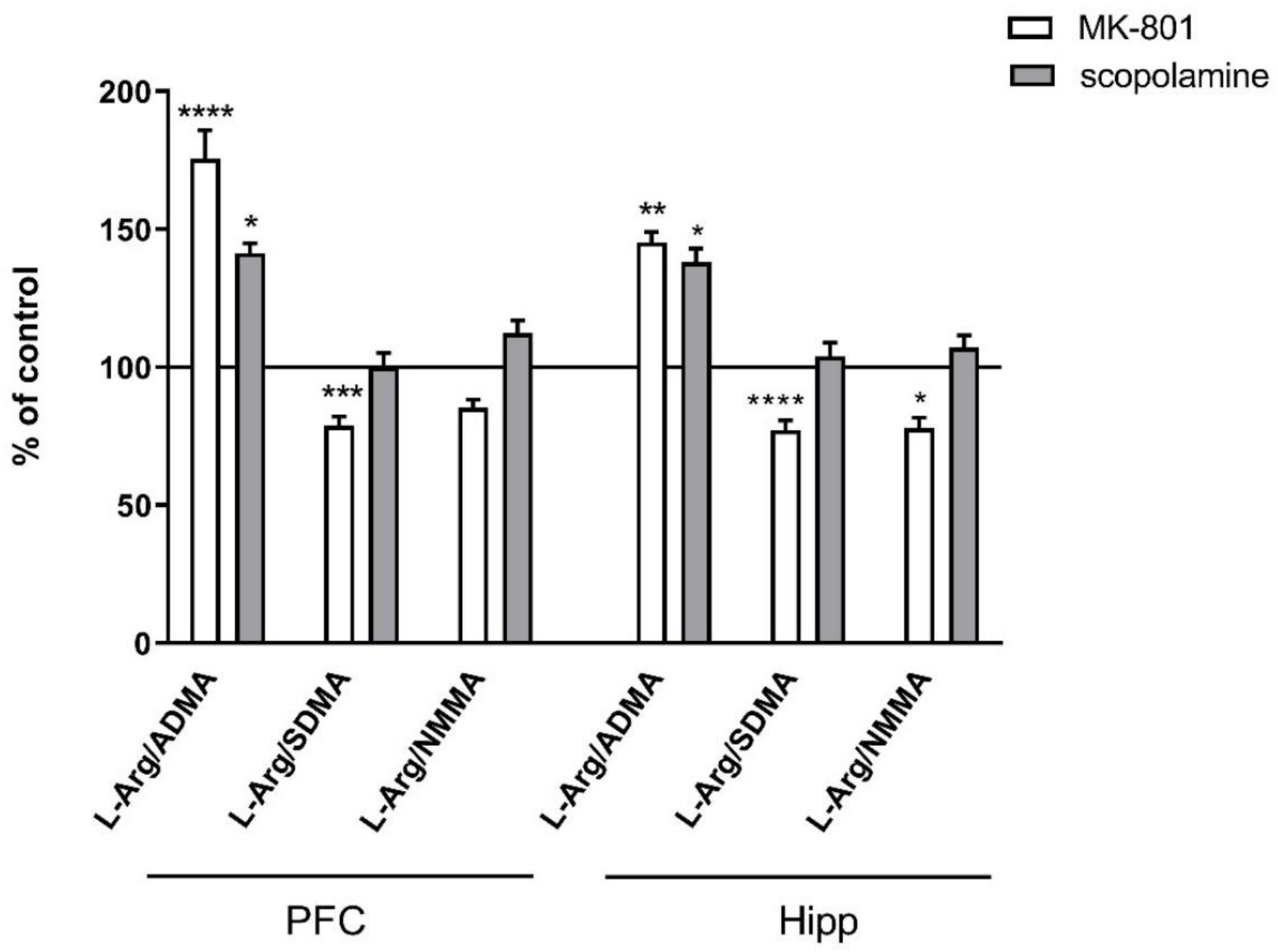
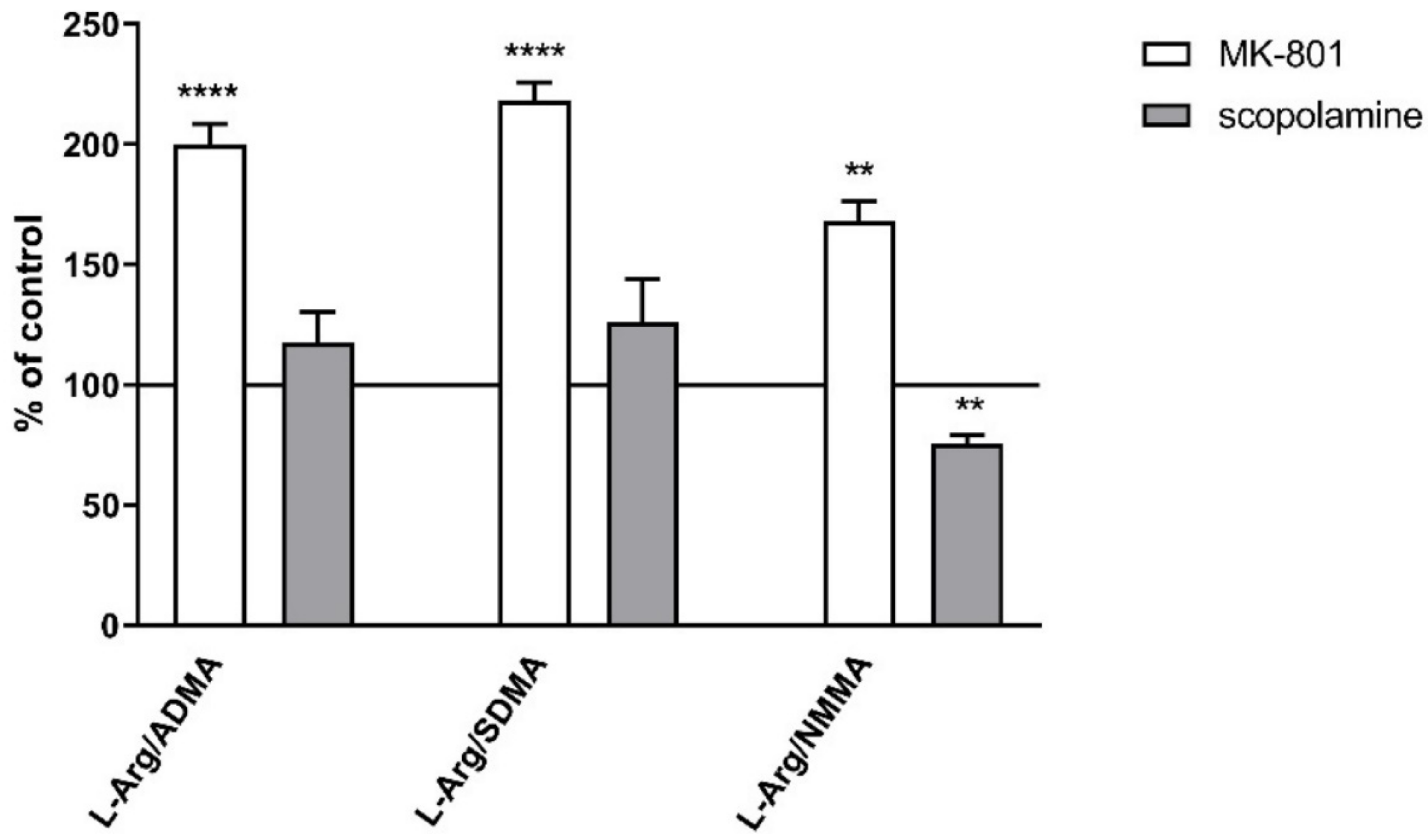
2.2.2. L-Arginine Derivatives in PFC and Hippocampus
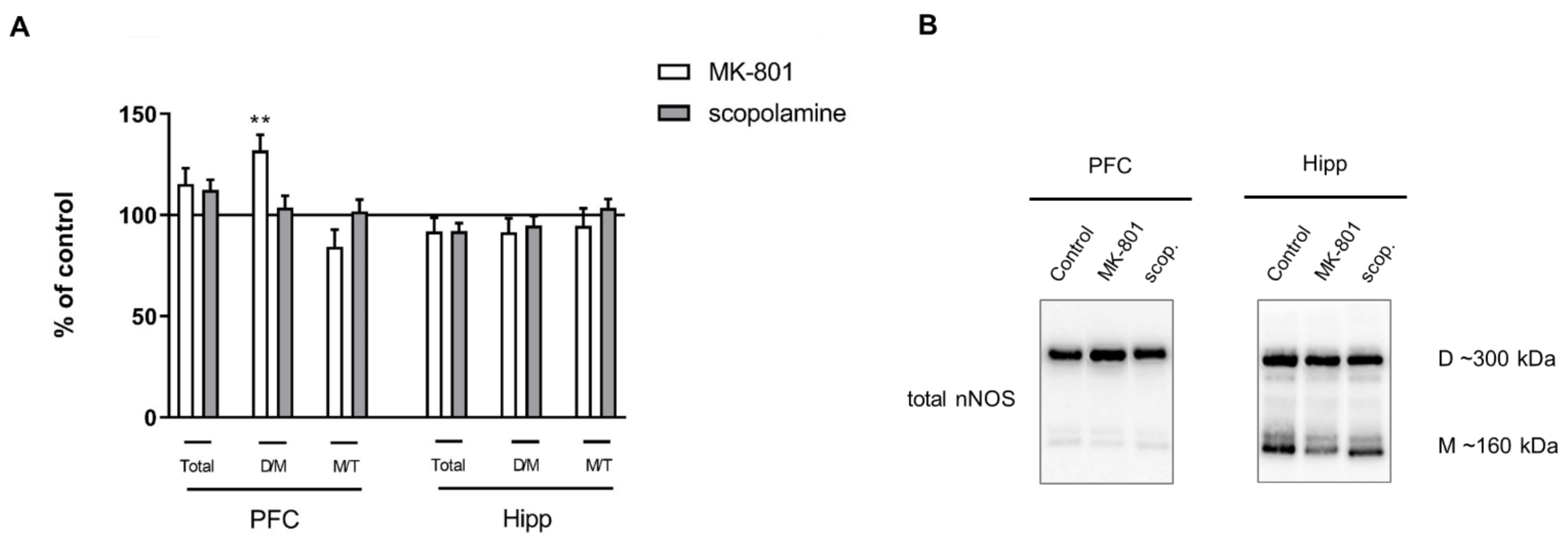
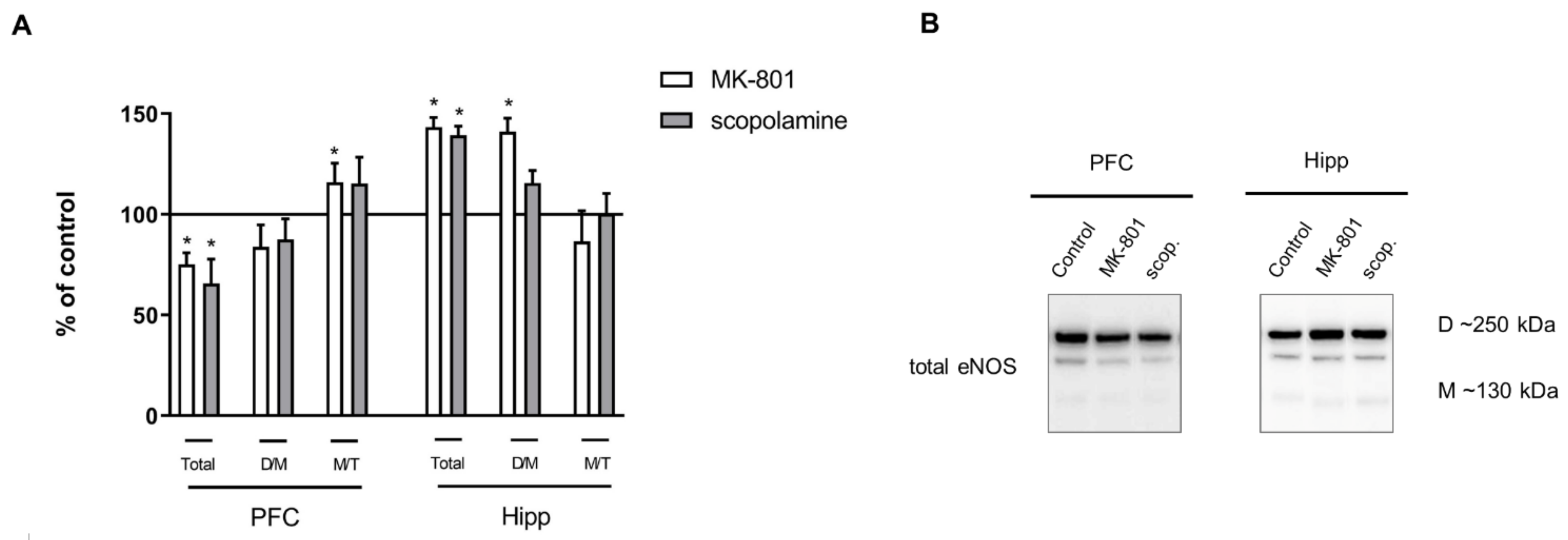
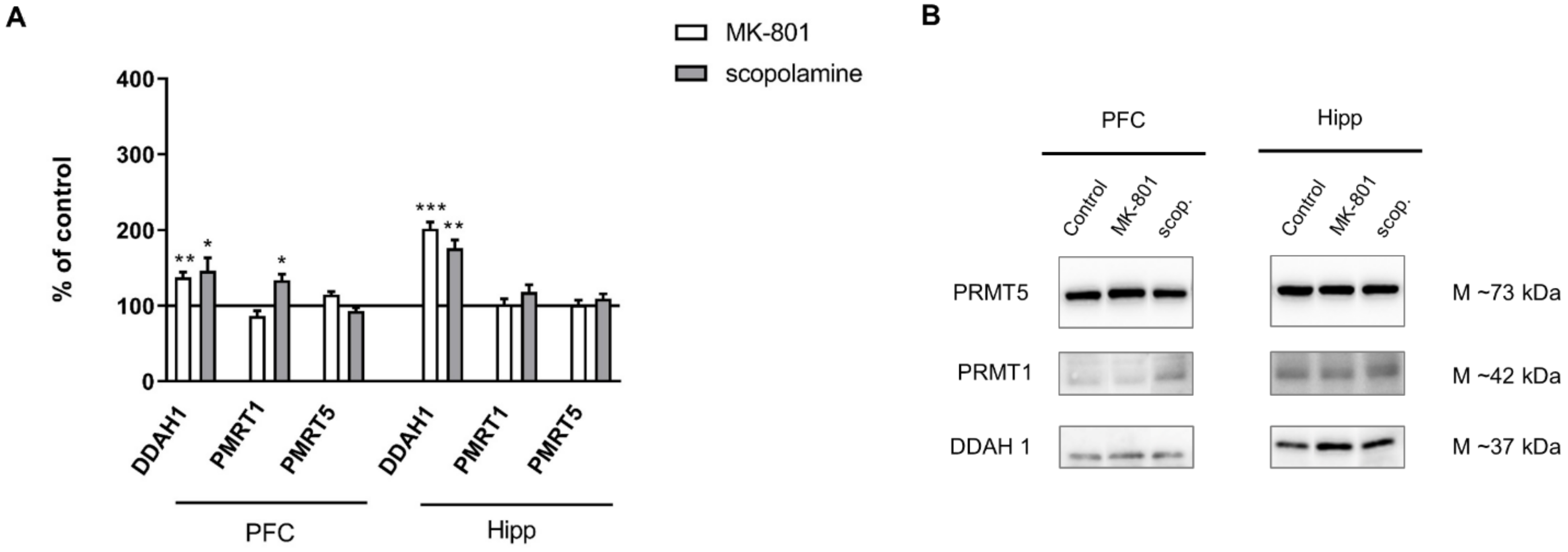
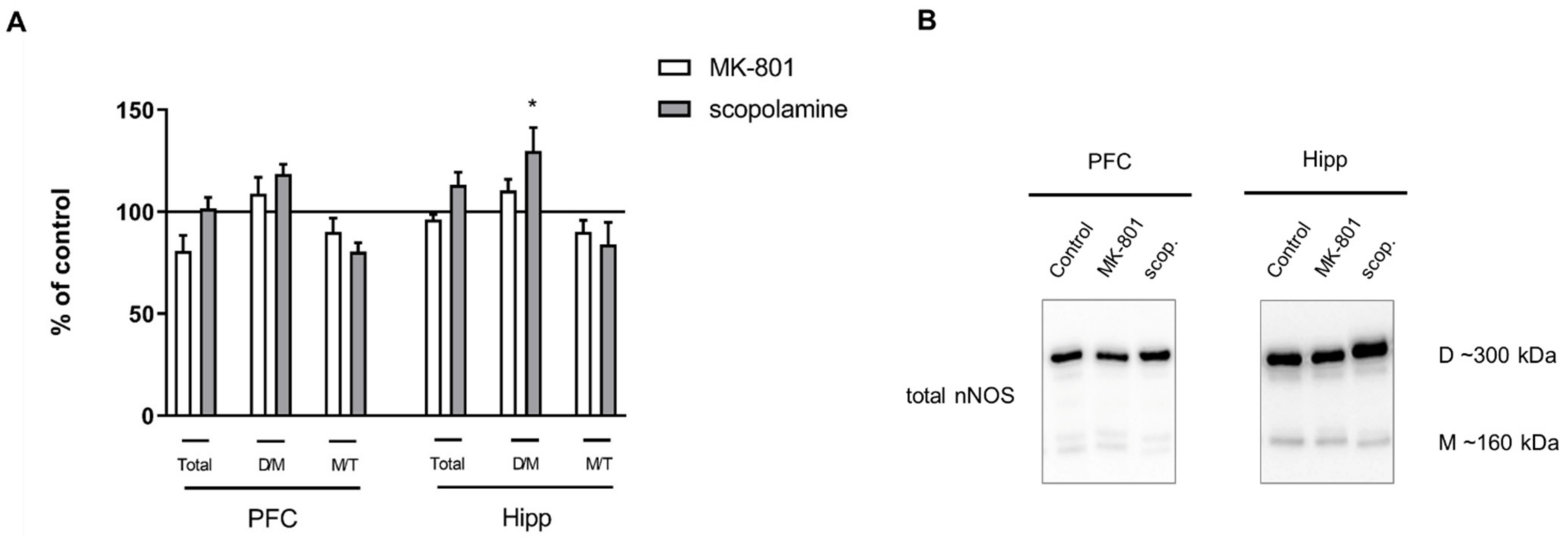
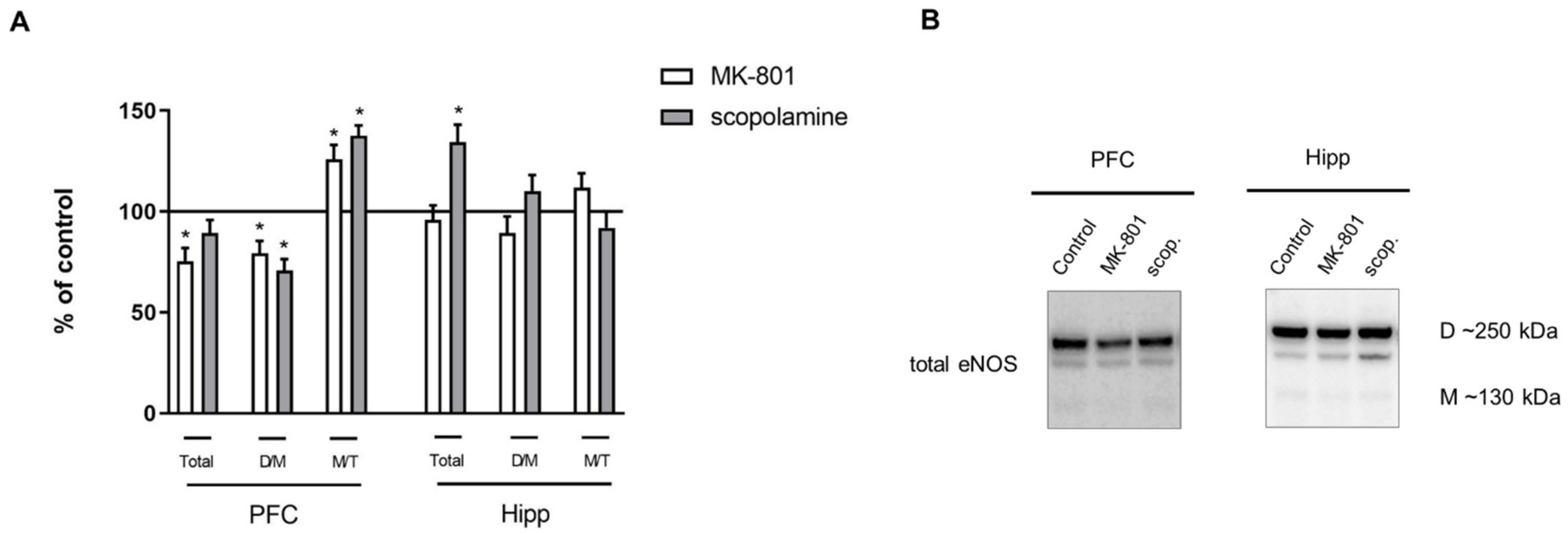
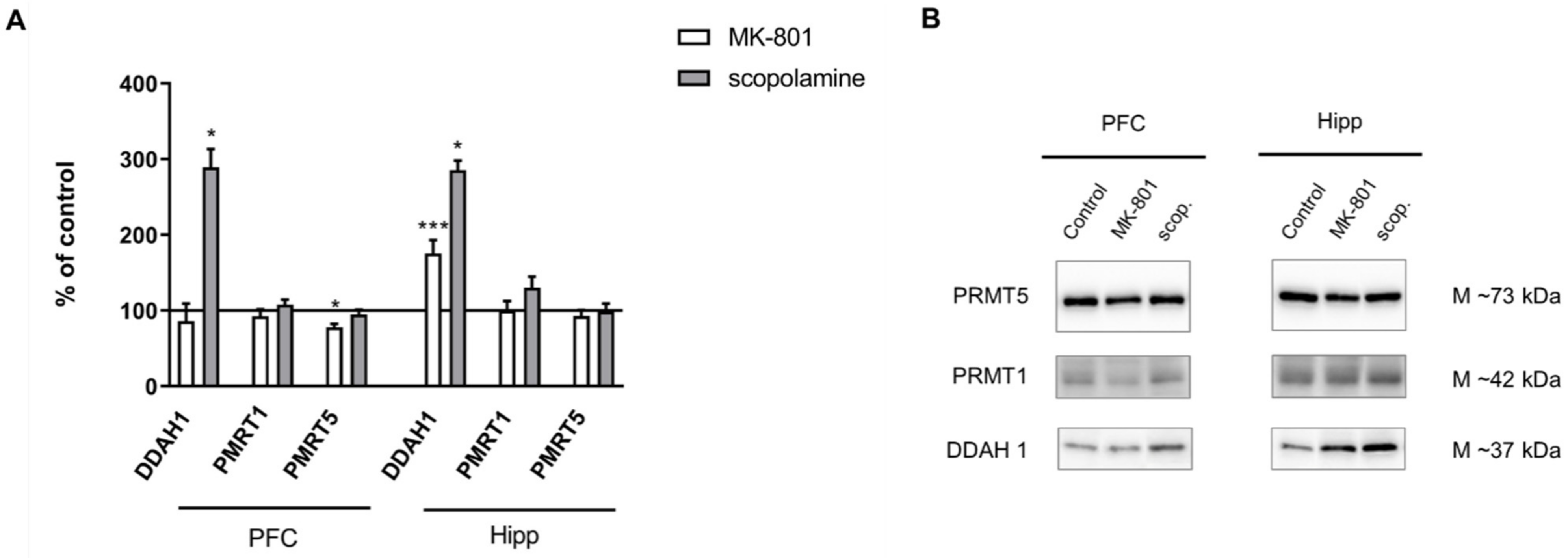
2.2.3. The Levels of eNOS, nNOS, DDAH1, PMRT1 and PMRT5
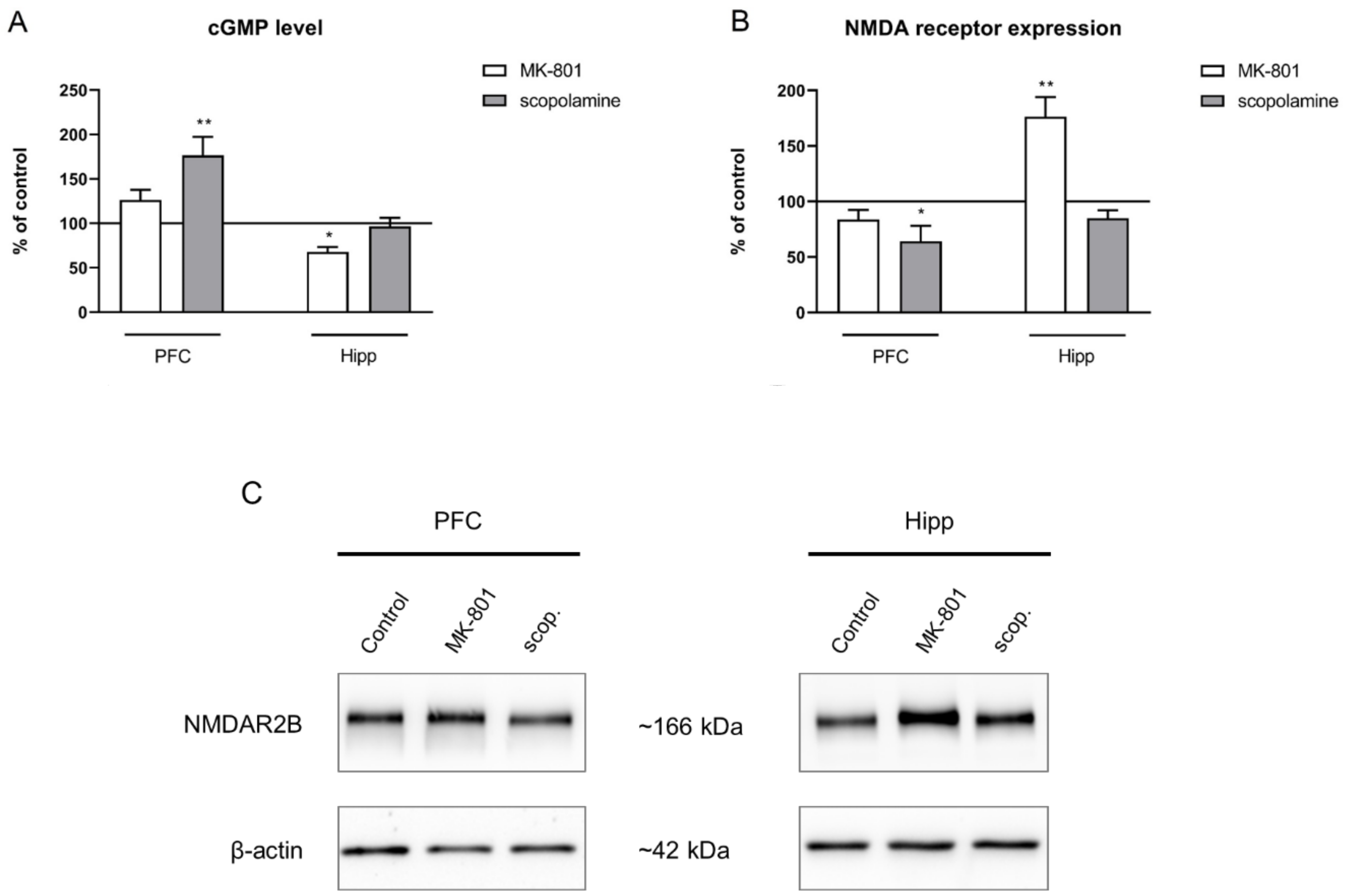
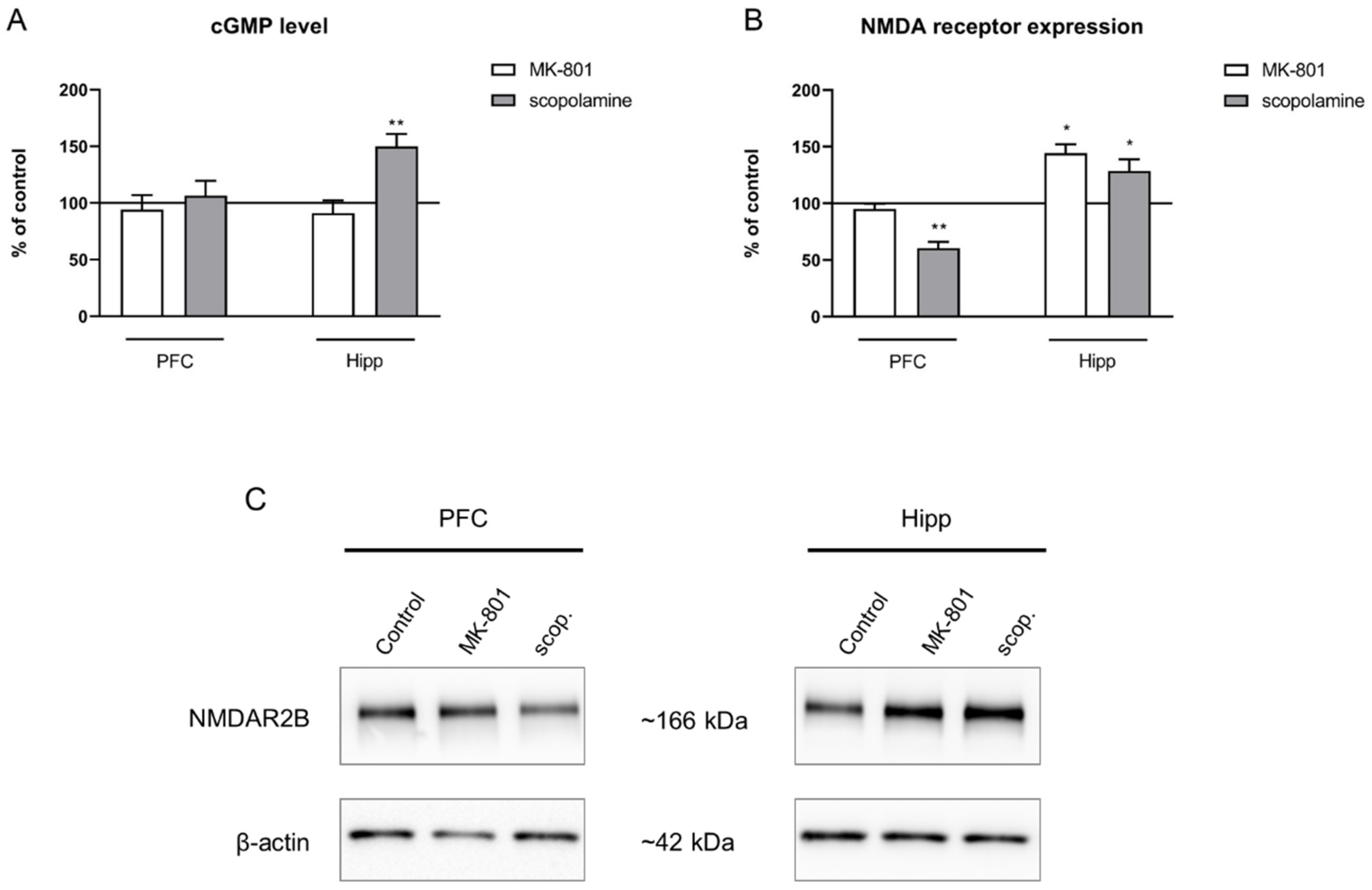
2.2.4. cGMP and NMDA Receptor Expression
2.3. Chronic MK-801 or Scopolamine Administration
2.3.1. Amino Acids in PFC and Hippocampus
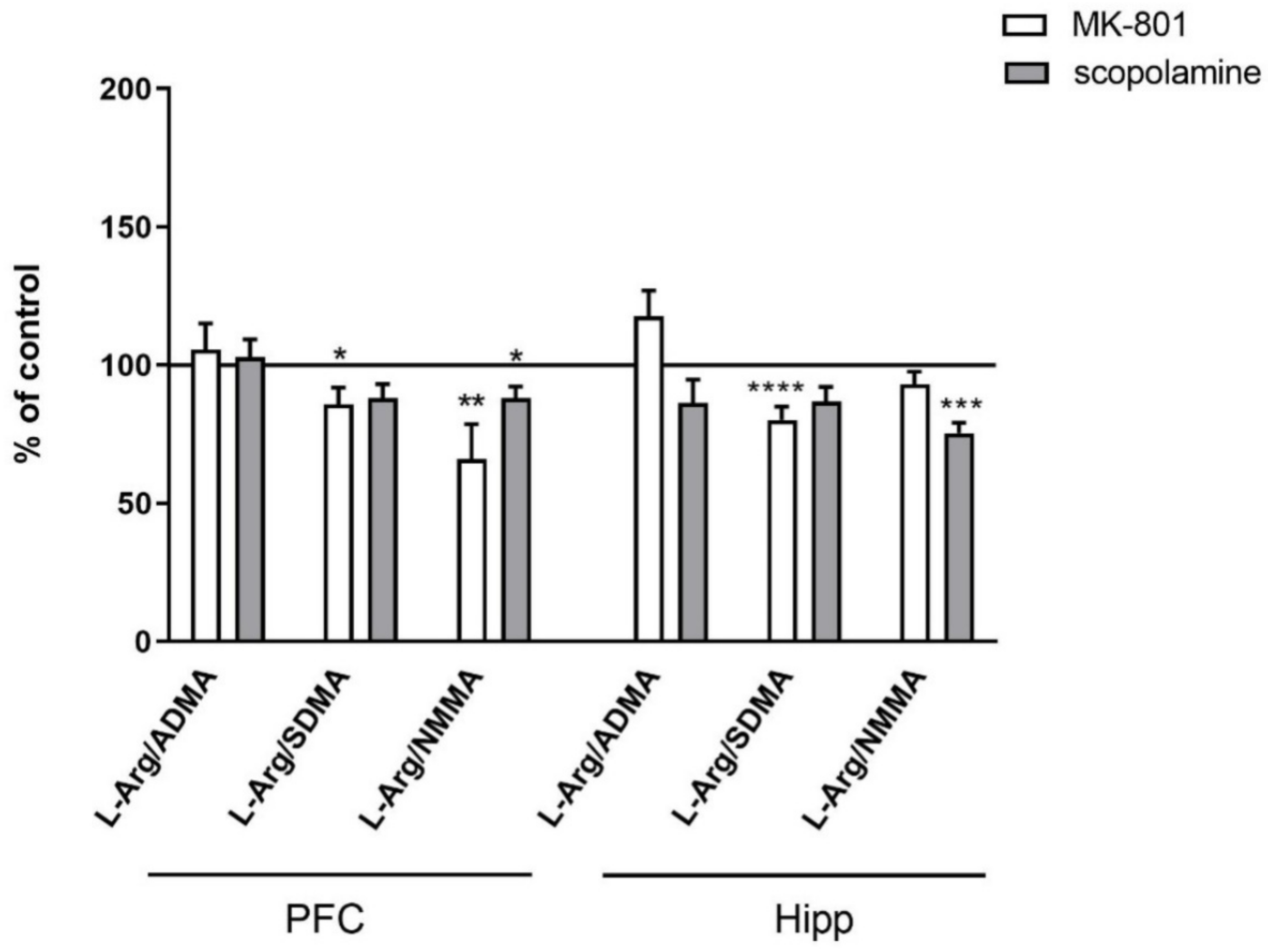
2.3.2. L-Arginine Derivatives in PFC and Hippocampus
2.3.3. The Levels of nNOS, eNOS, DDAH1, PMRT1 and PMRT5
2.3.4. cGMP and NMDA Receptor Expression
2.4. Amino Acid Levels and L-Arginine Derivatives in Plasma after MK-801 and Scopolamine Administration
2.4.1. Acute Administration
2.4.2. Chronic Administration
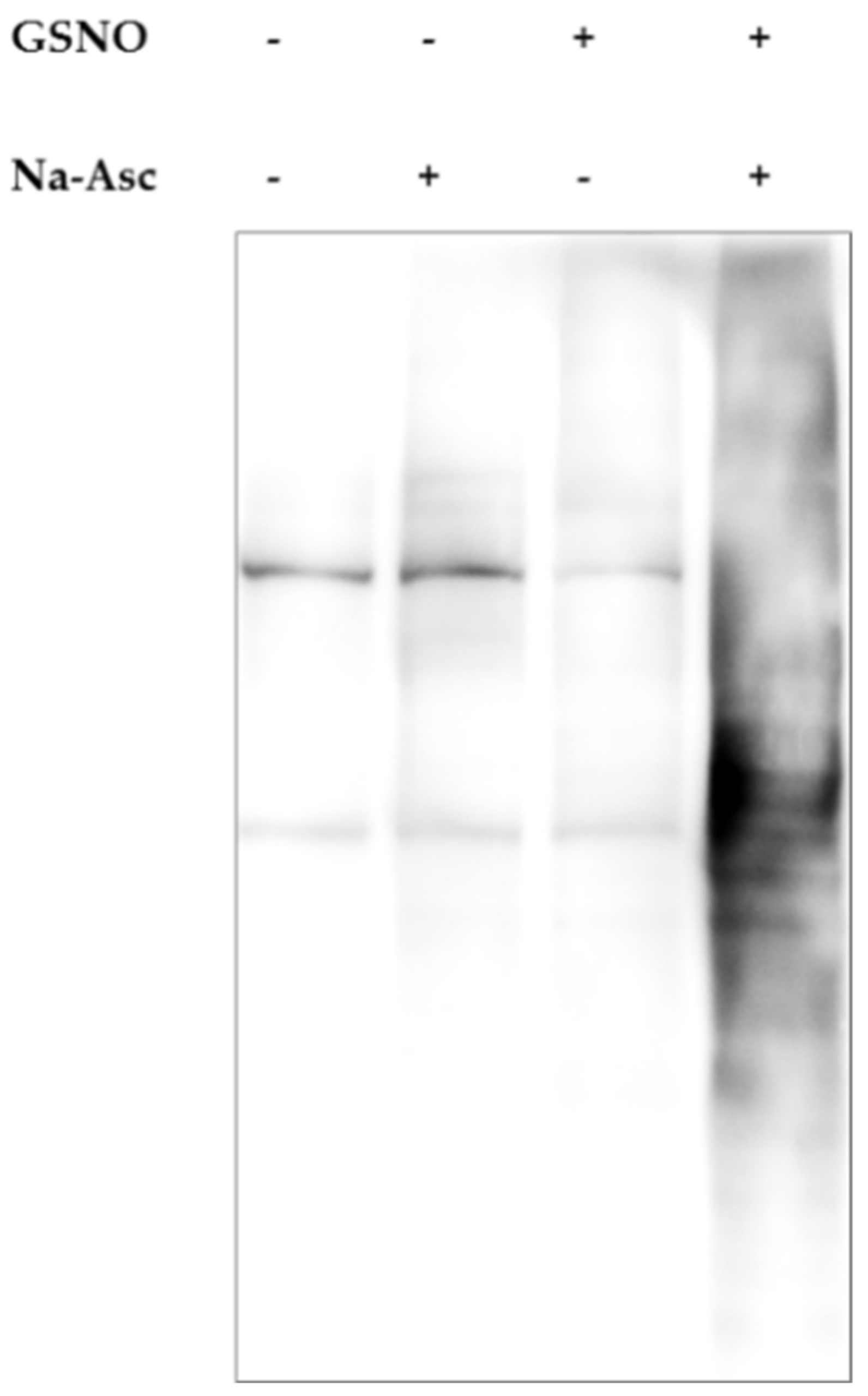
2.5. S-Nitrosylation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. L-Arginine Levels and Its Derivatives
4.4. Western Blotting
4.5. Detection of Protein S-Nitrosylation Using Biotin Switch Assay
Western Blot Assessment of S-Nitrosylation
4.6. cGMP ELISA
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camina, E.; Güell, F. The Neuroanatomical, Neurophysiological and Psychological Basis of Memory: Current Models and Their Origins. Front. Pharmacol. 2017, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Nursey, J.; Phelps, A.J. Stress, Trauma, and Memory in PTSD. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 169–176. ISBN 9780128009512. [Google Scholar]
- Eichenbaum, H. The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav. Brain Res. 2001, 127, 199–207. [Google Scholar] [CrossRef]
- Warden, M.R.; Miller, E.K. Task-Dependent Changes in Short-Term Memory in the Prefrontal Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15801–15810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funahashi, S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef]
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory Consolidation. Cold Springs Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glutamatergic theories of schizophrenia. Isr. J. Psychiatry Relat. Sci. 2010, 47, 4–16. [Google Scholar] [PubMed]
- Neill, J.C.; Barnes, S.; Cook, S.; Grayson, B.; Idris, N.F.; McLean, S.L.; Snigdha, S.; Rajagopal, L.; Harte, M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol. Ther. 2010, 128, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Kiebler, M.; Lee, C.J.; Lev-Ram, V.; Tsien, R.Y.; Kandel, E.R.; Hawkins, R.D. Nitric Oxide Acts Directly in the Presynaptic Neuron to Produce Long-Term Potentiationin Cultured Hippocampal Neurons. Cell 1996, 87, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Pigott, B.M.; Garthwaite, J. Nitric oxide is required for L-type Ca2+ channel-dependent long-term potentiation in the hippocampus. Front. Synaptic Neurosci. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.-B.; Tenneti, L.; Le, D.A.; Ortiz, J.; Bai, G.; Chen, H.-S.V.; Lipton, S.A. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000, 3, 15–21. [Google Scholar] [CrossRef]
- Ozdemir, H.; Ertugrul, A.; Basar, K.; Saka, E. Differential effects of antipsychotics on hippocampal presynaptic protein expressions and recognition memory in a schizophrenia model in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 62–68. [Google Scholar] [CrossRef]
- Cieślik, P.; Woźniak, M.; Tokarski, K.; Kusek, M.; Pilc, A.; Płoska, A.; Radulska, A.; Pelikant-Małecka, I.; Żołnowska, B.; Sławiński, J.; et al. Simultaneous activation of muscarinic and GABA B receptors as a bidirectional target for novel antipsychotics. Behav. Brain Res. 2019, 359, 671–685. [Google Scholar] [CrossRef]
- Cieślik, P.; Radulska, A.; Burnat, G.; Kalinowski, L.; Wierońska, J.M. Serotonergic–muscarinic interaction within the prefrontal cortex as a novel target to reverse schizophrenia-related cognitive symptoms. Int. J. Mol. Sci. 2021, 22, 8612. [Google Scholar] [CrossRef]
- Cieślik, P.; Domin, H.; Chocyk, A.; Gruca, P.; Litwa, E.; Płoska, A.; Radulska, A.; Pelikant-Małecka, I.; Brański, P.; Kalinowski, L.; et al. Simultaneous activation of mGlu2 and muscarinic receptors reverses MK-801-induced cognitive decline in rodents. Neuropharmacology 2020, 174, 107866. [Google Scholar] [CrossRef]
- Cieślik, P.; Woźniak, M.; Rook, J.M.; Tantawy, M.N.; Conn, P.J.; Acher, F.; Tokarski, K.; Kusek, M.; Pilc, A.; Wierońska, J.M. Mutual activation of glutamatergic mGlu4 and muscarinic M4 receptors reverses schizophrenia-related changes in rodents. Psychopharmacology 2018, 235, 2897–2913. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K.; Podkowa, A.; Mogilski, S.; Zaręba, P.; Kulig, K.; Sałat, R.; Malikowska, N.; Filipek, B. The effect of GABA transporter 1 (GAT1) inhibitor, tiagabine, on scopolamine-induced memory impairments in mice. Pharmacol. Rep. 2015, 67, 1155–1162. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Saigusa, D.; Takahashi, M.; Kanemitsu, Y.; Ishida, A.; Abe, T.; Yamakuni, T.; Suzuki, N.; Tomioka, Y. Determination of Asymmetric Dimethylarginine and Symmetric Dimethylarginine in Biological Samples of Mice Using LC/MS/MS. Am. J. Anal. Chem. 2011, 2, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Atzler, D.; Xu, X.; Zhang, P.; Guo, H.; Lu, Z.; Fassett, J.; Schwedhelm, E.; Böger, R.H.; Bache, R.J.; et al. Dimethylarginine Dimethylaminohydrolase-1 Is the Critical Enzyme for Degrading the Cardiovascular Risk Factor Asymmetrical Dimethylarginine. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1540–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.J.; Hodson, H.F.; Moncada, S. A specific inhibitor of nitric oxide formation from l-arginine attenuates endothelium-dependent relaxation. Br. J. Pharmacol. 1989, 96, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danysz, W.; Zajaczkowski, W.; Parsons, C.G. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav. Pharmacol. 1995, 6, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Piedrafita, B.; Cauli, O.; Montoliu, C.; Felipo, V. The function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and learning ability decrease in parallel in mature compared with young rats. Learn. Mem. 2007, 14, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Neitz, A.; Mergia, E.; Imbrosci, B.; Petrasch-Parwez, E.; Eysel, U.T.; Koesling, D.; Mittmann, T. Postsynaptic NO/cGMP increases NMDA receptor currents via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Cereb. Cortex 2014, 24, 1923–1936. [Google Scholar] [CrossRef]
- Baek, K.J.; Thiel, B.A.; Lucas, S.; Stuehr, D.J. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J. Biol. Chem. 1993, 268, 21120–21129. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, H.; Rizzo, A.N.; Zhang, W.; Mai, Y.; Ye, M. Endothelial Nitric Oxide Synthase Dimerization Is Regulated by Heat Shock Protein 90 Rather than by Phosphorylation. PLoS ONE 2014, 9, e105479. [Google Scholar] [CrossRef] [Green Version]
- Frankiewicz, T.; Potier, B.; Bashir, Z.I.; Collingridge, G.L.; Parsons, C.G. Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. Br. J. Pharmacol. 1996, 117, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirotsu, I.; Hori, N.; Katsuda, N.; Ishihara, T. Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices. Brain Res. 1989, 482, 194–197. [Google Scholar] [CrossRef]
- Leung, L.S.; Shen, B.; Rajakumar, N.; Ma, J. Cholinergic Activity Enhances Hippocampal Long-Term Potentiation in CA1 during Walking in Rats. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 9297–9304. [Google Scholar] [CrossRef]
- Knox, L.T.; Jing, Y.; Fleete, M.S.; Collie, N.D.; Zhang, H.; Liu, P. Scopolamine impairs behavioural function and arginine metabolism in the rat dentate gyrus. Neuropharmacology 2011, 61, 1452–1462. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Ivashchenko, D.V.; Ivanov, M.V.; Neznanov, N.G. Role of nitric oxide and related molecules in schizophrenia pathogenesis: Biochemical, genetic and clinical aspects. Front. Physiol. 2015, 6, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüth, H.-J.; Münch, G.; Arendt, T. Aberrant expression of NOS isoforms in Alzheimer’s disease is structurally related to nitrotyrosine formation. Brain Res. 2002, 53, 135–143. [Google Scholar] [CrossRef]
- Balez, R.; Ooi, L. Getting to NO Alzheimer’s Disease: Neuroprotection versus Neurotoxicity Mediated by Nitric Oxide. Oxid. Med. Cell. Longev. 2016, 2016, 3806157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conn, P.J.; Lindsley, C.W.; Jones, C.K. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009, 30, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Javitt, D.C. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine-Glutamate Interactions. Int. Rev. Neurobiol. 2007, 78, 69–108. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Hasselmo, M.E.; Wyble, B.P. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behav. Brain Res. 1997, 89, 1–34. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef] [Green Version]
- Tamagnini, F.; Barker, G.; Warburton, E.C.; Burattini, C.; Aicardi, G.; Bashir, Z.I. Nitric oxide-dependent long-term depression but not endocannabinoid-mediated long-term potentiation is crucial for visual recognition memory. J. Physiol. 2013, 591, 3963–3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, H.; Hemart, N.; Jaillard, D.; Crepel, F. Long-term depression requires nitric oxide and guanosine 3′:5′ cyclic monophosphate production in rat cerebellar Purkinje cells. Eur. J. Neurosci. 1993, 5, 1079–1082. [Google Scholar] [CrossRef]
- Calabresi, P.; Gubellini, P.; Centonze, D.; Sancesario, G.; Morello, M.; Giorgi, M.; Pisani, A.; Bernardi, G. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J. Neurosci. 1999, 19, 2489–2499. [Google Scholar] [CrossRef] [Green Version]
- Rozov, A.V.; Valiullina, F.F.; Bolshakov, A.P. Mechanisms of Long-Term Plasticity of Hippocampal GABAergic Synapses. Biochemistry 2017, 82, 257–263. [Google Scholar] [CrossRef]
- Babiec, W.E.; Guglietta, R.; Jami, S.A.; Morishita, W.; Malenka, R.C.; O’Dell, T.J. Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J. Neurosci. 2014, 34, 5285–5290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scullion, S.E.; Barker, G.R.I.; Warburton, E.C.; Randall, A.D.; Brown, J.T. Muscarinic Receptor-Dependent Long Term Depression in the Perirhinal Cortex and Recognition Memory are Impaired in the rTg4510 Mouse Model of Tauopathy. Neurochem. Res. 2019, 44, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Fish, J.E.; Marsden, P.A. Endothelial nitric oxide synthase: Insight into cell-specific gene regulation in the vascular endothelium. Cell. Mol. Life Sci. 2006, 63, 144–162. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Li, C.; Arrick, D.M.; Yang, S.; Baluna, A.E.; Sun, H. Role of nitric oxide synthases in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS ONE 2014, 9, e93134. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, A.V.R.; D’Uscio, L.V.; He, T.; Das, P.; Younkin, S.G.; Katusic, Z.S. Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice. J. Neurochem. 2015, 134, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Austin, S.A.; Katusic, Z.S. Loss of Endothelial Nitric Oxide Synthase Promotes p25 Generation and Tau Phosphorylation in a Murine Model of Alzheimer’s Disease. Circ. Res. 2016, 119, 1128–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, G.S. Amyloid-β and Tau The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.-S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated with Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2008, 10, 129. [Google Scholar] [CrossRef]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers. Dement. 2018, 14, 1640–1650. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The blood–brain barrier in psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Nakamura, T.; Tu, S.; Akhtar, M.W.; Sunico, C.R.; Okamoto, S.; Lipton, S.A. Aberrant Protein S-nitrosylation in neurodegenerative diseases. Neuron 2013, 78, 596–614. [Google Scholar] [CrossRef] [Green Version]
- Raju, K.; Doulias, P.-T.; Evans, P.; Krizman, E.N.; Jackson, J.G.; Horyn, O.; Daikhin, Y.; Nissim, I.; Yudkoff, M.; Nissim, I.; et al. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci. Signal. 2015, 8, ra68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.R.; Binder, D.K. Post-translational Regulation of GLT-1 in Neurological Diseases and Its Potential as an Effective Therapeutic Target. Front. Mol. Neurosci. 2019, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Sibson, N.R.; Dhankhar, A.; Mason, G.F.; Behar, K.L.; Rothman, D.L.; Shulman, R.G. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc. Natl. Acad. Sci. USA 1997, 94, 2699–2704. [Google Scholar] [CrossRef] [Green Version]
- Gruetter, R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem. Int. 2002, 41, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Gropman, A.L.; Summar, M.; Leonard, J.V. Neurological implications of urea cycle disorder. J. Inherit. Metab. Dis. 2007, 30, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Khan, N.S.; Puri, B.K.; Hirsch, S.R. Elevated endogenous nitric oxide synthase inhibitor in schizophrenic plasma may reflect abnormalities in brain nitric oxide production. Neurosci. Lett. 1996, 215, 209–211. [Google Scholar] [CrossRef]
- Gubandru, M.; Margina, D.; Tsitsimpikou, C.; Goutzourelas, N.; Tsarouhas, K.; Ilie, M.; Tsatsakis, A.M.; Kouretas, D. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem. Toxicol. 2013, 61, 209–214. [Google Scholar] [CrossRef]
- Selly, M.L. Increased concentrations of homocysteine and asymmetric dimethylarginine and decreased concentrations of nitric oxide in the plasma of patients with Alzheimer’s disease. Neurobiol. Aging 2003, 24, 903–907. [Google Scholar] [CrossRef]
- Mulder, C.; Wahlund, L.-O.; Blomberg, M.; de Jong, S.; van Kamp, G.J.; Scheltens, P.; Teerlink, T. Alzheimer’s disease is not associated with altered concentrations of the nitric oxide synthase inhibitor asymmetric dimethylarginine in cerebrospinal fluid. J. Neural Transm. 2002, 109, 1203–1208. [Google Scholar] [CrossRef]
- Chang, F.; Flavahan, S.; Flavahan, N.A. Potential pitfalls in analyzing structural uncoupling of enos: Aging is not associated with increased enzyme monomerization. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H80–H88. [Google Scholar] [CrossRef]
- Forrester, M.T.; Foster, M.W.; Benhar, M.; Stamler, J.S. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 2009, 46, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the ⋅NO/cGMP signaling pathway. Biochim. Biophys. Acta-Bioenerg. 1999, 1411, 334–350. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Sun, J.; Druhan, L.J.; Zweier, J.L. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch. Biochem. Biophys. 2010, 494, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Pou, S.; Pou, W.S.; Bredt, D.S.; Snyder, S.H.; Rosen, G.M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992, 267, 24173–24176. [Google Scholar] [CrossRef]
- Malinski, T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimer’s Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Boll, K.M.; Noto, C.; Bonifácio, K.L.; Bortolasci, C.C.; Gadelha, A.; Bressan, R.A.; Barbosa, D.S.; Maes, M.; Moreira, E.G. Oxidative and nitrosative stress biomarkers in chronic schizophrenia. Psychiatry Res. 2017, 253, 43–48. [Google Scholar] [CrossRef]
- Thomas, D.D.; Miranda, K.M.; Colton, C.A.; Citrin, D.; Espey, M.G.; Wink, D.A. Heme proteins and nitric oxide (NO): The neglected, eloquent chemistry in NO redox signaling and regulation. Antioxid. Redox Signal. 2003, 5, 307–317. [Google Scholar] [CrossRef]

| Compound | Q1 Mass (Da) Precursor Ion | Q3 Mass (Da) Product Ion | DP (Volts) | CE (Volts) |
|---|---|---|---|---|
| SDMA | 203.30 | 172.10 | 25 | 19 |
| SDMA | 203.30 | 133.10 | 25 | 19 |
| ADMA | 203.30 | 45.90 | 15 | 37 |
| ADMA | 203.30 | 42.90 | 15 | 67 |
| NMMA | 189.08 | 69.90 | 10 | 33 |
| NMMA | 189.08 | 116.00 | 10 | 21 |
| L-Arginine | 175.07 | 70.10 | 30 | 30 |
| L-Arginine | 175.07 | 116.00 | 30 | 20 |
| L-Citrulline | 176.06 | 159.10 | 15 | 13 |
| L-Citrulline | 176.06 | 69.90 | 15 | 32 |
| L-Glutamate | 148.12 | 84.0 | 10 | 21 |
| L-Glutamate | 148.12 | 130.0 | 10 | 13 |
| L-Glutamine | 147.12 | 84.0 | 10.0 | 23 |
| L-Glutamine | 147.12 | 130.0 | 10.0 | 13 |
| L-Ornithine | 133.01 | 70.0 | 10.0 | 23 |
| L-Ornithine | 133.01 | 116.0 | 10.0 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślik, P.; Siekierzycka, A.; Radulska, A.; Płoska, A.; Burnat, G.; Brański, P.; Kalinowski, L.; Wierońska, J.M. Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies. Int. J. Mol. Sci. 2021, 22, 12282. https://doi.org/10.3390/ijms222212282
Cieślik P, Siekierzycka A, Radulska A, Płoska A, Burnat G, Brański P, Kalinowski L, Wierońska JM. Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies. International Journal of Molecular Sciences. 2021; 22(22):12282. https://doi.org/10.3390/ijms222212282
Chicago/Turabian StyleCieślik, Paulina, Anna Siekierzycka, Adrianna Radulska, Agata Płoska, Grzegorz Burnat, Piotr Brański, Leszek Kalinowski, and Joanna M. Wierońska. 2021. "Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies" International Journal of Molecular Sciences 22, no. 22: 12282. https://doi.org/10.3390/ijms222212282
APA StyleCieślik, P., Siekierzycka, A., Radulska, A., Płoska, A., Burnat, G., Brański, P., Kalinowski, L., & Wierońska, J. M. (2021). Nitric Oxide-Dependent Mechanisms Underlying MK-801- or Scopolamine-Induced Memory Dysfunction in Animals: Mechanistic Studies. International Journal of Molecular Sciences, 22(22), 12282. https://doi.org/10.3390/ijms222212282







