Positive Association between Indoor Gaseous Air Pollution and Obesity: An Observational Study in 60 Households
Abstract
1. Introduction
2. Materials and Methods
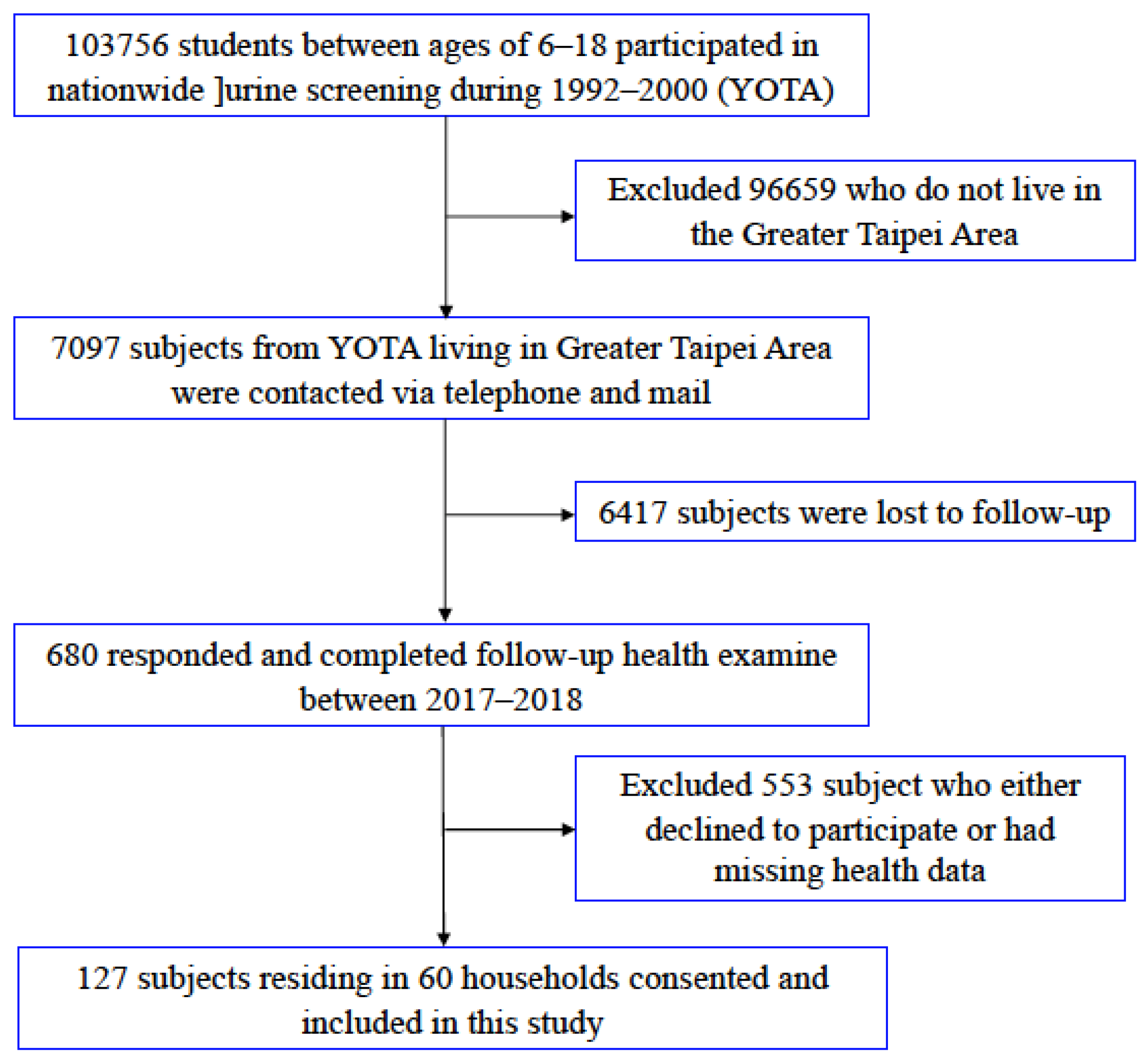
2.1. Study Subjects
2.2. Cardiovascular Risk Factors Assessment and Serum Analysis
2.3. 24-h Continuous Indoor Air Quality Monitoring
2.4. Statistical Analyses
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 September 2021).
- Null, N. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Lavie, C.J.; De Schutter, A.; Parto, P.; Jahangir, E.; Kokkinos, P.; Ortega, F.B.; Arena, R.; Milani, R.V. Obesity and preva-lence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog. Cardiovasc. Dis. 2016, 58, 537–547. [Google Scholar] [CrossRef]
- Storheim, K.; Zwart, J.-A. Musculoskeletal disorders and the Global Burden of Disease study. Ann. Rheum. Dis. 2014, 73, 949–950. [Google Scholar] [CrossRef]
- Garg, S.K.; Maurer, H.; Reed, K.; Selagamsetty, R. Diabetes and cancer: Two diseases with obesity as a common risk factor. Diabetes Obes. Metab. 2014, 16, 97–110. [Google Scholar] [CrossRef]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Cuervo, M.; Goni, L.; Martinez, J.A. Genetic and nongenetic factors ex-plaining metabolically healthy and unhealthy phenotypes in participants with excessive adiposity: Relevance for person-alized nutrition. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819877303. [Google Scholar] [CrossRef]
- Sly, P.D.; Carpenter, D.O.; Van den Berg, M.; Stein, R.T.; Landrigan, P.J.; Brune-Drisse, M.-N.; Suk, W. Health conse-quences of environmental exposures: Causal thinking in global environmental epidemiology. Annals Glob. Health 2016, 82, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Holtcamp, W. Obesogens: An Environmental Link to Obesity. Environ. Health Perspect. 2012, 120, a62–a68. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- McConnell, R.; Gilliland, F.; Goran, M.; Allayee, H.; Hricko, A.; Mittelman, S. Does near-roadway air pollution contribute to childhood obesity? Pediatr. Obes. 2016, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.J.; Li, Z.; Gow, A.; Chung, K.F.; Hu, M.; Sun, Z.; Zeng, L.; Zhu, T.; Jia, G.; et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016, 30, 2115–2122. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Chuang, H.-C.; Liu, I.-J.; Chen, H.-W.; Chuang, K.-J. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci. Total Environ. 2013, 463–464, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.-J.; Chan, C.-C.; Su, T.-C.; Lee, C.-T.; Tang, C.-S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Dysfunction in Young Adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Zhong, M.; Hotchkiss, I.P.; Lewandowski, R.P.; Wagner, J.G.; Bramble, L.A.; Yang, Y.; Wang, A.; Harkema, J.R.; et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 2011, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Deiuliis, J.; Lumeng, C.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.; Lu, B.; Parthasarathy, S.; et al. Ambient Air Pollution Exaggerates Adipose Inflammation and Insulin Resistance in a Mouse Model of Diet-Induced Obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Voss, J.; Knight, B. The Association of Ambient Air Pollution and Physical Inactivity in the United States. PLoS ONE 2014, 9, e90143. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Ji, M.; Yan, H.; Guan, C. Impact of ambient air pollution on obesity: A systematic review. Int. J. Obes. 2018, 42, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; McConnell, R.; Wolch, J.; Chang, R.; Lam, C.; Dunton, G.; Gilliland, F.; Lurmann, F.; Islam, T.; Berhane, K. Traffic-related air pollution and obesity formation in children: A longitudinal, multilevel analysis. Environ. Health 2014, 13, 49. [Google Scholar] [CrossRef]
- McConnell, R.; Shen, E.; Gilliland, F.D.; Jerrett, M.; Wolch, J.; Chang, C.-C.; Lurmann, F.; Berhane, K. A Longitudinal Cohort Study of Body Mass Index and Childhood Exposure to Secondhand Tobacco Smoke and Air Pollution: The Southern California Children’s Health Study. Environ. Health Perspect. 2015, 123, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Ponticiello, B.G.; Capozzella, A.; Di Giorgio, V.; Casale, T.; Giubilati, R.; Tomei, G.; Tomei, F.; Rosati, M.V.; Sancini, A. Overweight and urban pollution: Preliminary results. Sci. Total Environ. 2015, 518–519, 61–64. [Google Scholar] [CrossRef]
- Shin, J.; Choi, J.; Kim, K. Association between long-term exposure of ambient air pollutants and cardiometabolic diseases: A 2012 Korean Community Health Survey. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 144–151. [Google Scholar] [CrossRef]
- Dong, G.-H.; Qian, Z.M.; Liu, M.-M.; Wang, D.; Ren, W.-H.; Flick, L.H.; Fu, J.; Wang, J.; Chen, W.; Simckes, M.; et al. Ambient air pollution and the prevalence of obesity in chinese children: The seven northeastern cities study. Obesity 2012, 22, 795–800. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Development of WHO Guidelines for Indoor Air Quality. Available online: http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/development-of-who-guidelines-for-indoor-air-quality (accessed on 25 September 2021).
- De Gennaro, G.; Farella, G.; Marzocca, A.; Mazzone, A.; Tutino, M. Indoor and Outdoor Monitoring of Volatile Organic Compounds in School Buildings: Indicators Based on Health Risk Assessment to Single out Critical Issues. Int. J. Environ. Res. Public Health 2013, 10, 6273–6291. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Lin, C.-Y.; Lin, Y.-C.; Chuang, K.-J. The effects of indoor particles on blood pressure and heart rate among young adults in Taipei, Taiwan. Indoor Air 2009, 19, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.-J.; Chuang, H.-C.; Lin, L.-Y. Indoor Air Pollution, Nighttime Heart Rate Variability and Coffee Consumption among Convenient Store Workers. PLoS ONE 2013, 8, e63320. [Google Scholar] [CrossRef]
- Kajbafzadeh, M.; Brauer, M.; Karlen, B.; Carlsten, C.; Van Eeden, S.; Allen, R.W. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: A HEPA filter intervention study. Occup. Environ. Med. 2015, 72, 394–400. [Google Scholar] [CrossRef]
- Kermah, D.; Shaheen, M.; Pan, D.; Friedman, T.C. Association between secondhand smoke and obesity and glucose ab-normalities: Data from the National Health and Nutrition Examination Survey (NHANES 1999–2010). BMJ Open Diabetes Res. Care 2017, 5, e000324. [Google Scholar] [CrossRef]
- Rajkumar, S.; Young, B.N.; Clark, M.L.; Benka-Coker, M.L.; Bachand, A.M.; Brook, R.D.; Nelson, T.L.; Volckens, J.; Reynolds, S.J.; L’Orange, C.; et al. Household air pollution from biomass-burning cookstoves and metabolic syndrome, blood lipid concentrations, and waist circumference in Honduran women: A cross-sectional study. Environ. Res. 2019, 170, 46–55. [Google Scholar] [CrossRef]
- Su, T.-C.; Liao, C.-C.; Chien, K.-L.; Hsu, S.H.-J.; Sung, F.-C. An Overweight or Obese Status in Childhood Predicts Subclinical Atherosclerosis and Prehypertension/Hypertension in Young Adults. J. Atheroscler. Thromb. 2014, 21, 1170–1182. [Google Scholar] [CrossRef][Green Version]
- Day, D.B.; Xiang, J.; Mo, J.; Li, F.; Chung, M.K.; Gong, J.; Weschler, C.J.; Ohman-Strickland, P.A.; Sundell, J.; Weng, W.; et al. Association of Ozone Exposure with Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern. Med. 2017, 177, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human health risks of formaldehyde indoor levels: An issue of concern. J. Environ. Sci. Health Part A 2016, 51, 357–363. [Google Scholar] [CrossRef]
- White, L.F.; Jerrett, M.; Yu, J.; Marshall, J.D.; Rosenberg, L.; Coogan, P.F. Ambient Air Pollution and 16-Year Weight Change in African-American Women. Am. J. Prev. Med. 2016, 51, e99–e105. [Google Scholar] [CrossRef]
- Wallwork, R.S.; Colicino, E.; Zhong, J.; Kloog, I.; Coull, B.A.; Vokonas, P.; Schwartz, J.D.; Baccarelli, A.A. Ambient Fine Particulate Matter, Outdoor Temperature, and Risk of Metabolic Syndrome. Am. J. Epidemiol. 2017, 185, 30–39. [Google Scholar] [CrossRef]
- de Bont, J.; Casas, M.; Barrera-Gómez, J.; Cirach, M.; Rivas, I.; Valvi, D.; Álvarez, M.; Dadvand, P.; Sunyer, J.; Vrijheid, M. Ambient air pollution and overweight and obesity in school-aged children in Barcelona, Spain. Environ. Int. 2019, 125, 58–64. [Google Scholar] [CrossRef]
- Hersoug, L.-G.; Sjödin, A.; Astrup, A. A proposed potential role for increasing atmospheric CO2 as a promoter of weight gain and obesity. Nutr. Diabetes 2012, 2, e31. [Google Scholar] [CrossRef] [PubMed]
- Zheutlin, A.R.; Adar, S.D.; Park, S.K. Carbon dioxide emissions and change in prevalence of obesity and diabetes in the United States: An ecological study. Environ. Int. 2014, 73, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-building syndrome. The Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Kim, C.; Lim, Y.; Yang, J.; Hong, C.; Shin, D. Effects of indoor CO2 concentrations on wheezing attacks in children. Indoor Air 2002, 2, 583–584. [Google Scholar]
- Kalay, N.; Ozdogru, I.; Cetinkaya, Y.; Eryol, N.K.; Dogan, A.; Gul, I.; Inanc, T.; Ikizceli, I.; Oguzhan, A.; Abaci, A. Car-diovascular effects of carbon monoxide poisoning. Am. J. Cardiol. 2007, 99, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Szponar, J.; Kołodziej, M.; Majewska, M.; Zaleski, K.; Lewandowska-Stanek, H. Myocardial injury in the course of carbon monoxide poisoning. Przeglad Lek. 2012, 69, 528–534. [Google Scholar]
- Garg, J.; Krishnamoorthy, P.; Palaniswamy, C.; Khera, S.; Ahmad, H.; Jain, D.; Aronow, W.S.; Frishman, W.H. Cardio-vascular abnormalities in carbon monoxide poisoning. Am. J. Ther. 2018, 25, e339–e348. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Chungag, A.; Engwa, G.A.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. Effect of Seasonal Variation on the Relationship of Indoor Air Particulate Matter with Measures of Obesity and Blood Pressure in Children. J. Health Pollut. 2021, 11, 210610. [Google Scholar] [CrossRef]
- Vrijheid, M.; Fossati, S.; Maitre, L.; Márquez, S.; Roumeliotaki, T.; Agier, L.; Andrusaityte, S.; Cadiou, S.; Casas, M.; De Castro, M.; et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ. Health Perspect. 2020, 128, 067009. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-C.; Su, H.-J.; Liang, H.-H. Association between indoor air pollutant exposure and blood pressure and heart rate in subjects according to body mass index. Sci. Total Environ. 2016, 539, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hormigos-Jimenez, S.; Padilla-Marcos, M.A.; Meiss, A.; Gonzalez-Lezcano, R.A.; Feijó-Muñoz, J. Experimental validation of the age-of-the-air CFD analysis: A case study. Sci. Technol. Built Environ. 2018, 24, 994–1003. [Google Scholar] [CrossRef]

| BMI ≥ 25 (N = 38) | BMI < 25 (N = 89) | p-Value | |
|---|---|---|---|
| Mean ± SD or n (%) | |||
| Age (years) | 41.84 ± 14.13 | 43.92 ± 15.89 | 0.4869 |
| Male gender; no. (%) | 22 (57.89) | 29 (32.58) | 0.0077 |
| Marital status; no. (%) Married Single | 20 (52.63) 18 (47.37) | 57 (64.04) 32 (35.96) | 0.2280 |
| Employment type, % | 0.0873 | ||
| Blue-collar worker | 20 (52.63) | 45 (50.56) | |
| White-collar worker | 7 (18.42) | 6 (6.74) | |
| BMI (kg/m2) | 28.61 ± 2.89 | 21.65 ± 2.27 | <0.0001 |
| Waistline (cm) | 95.58 ± 8.40 | 77.64 ± 8.18 | <0.0001 |
| Systolic BP (mmHg) | 124.38 ± 13.20 | 118.34 ± 14.77 | 0.0314 |
| Diastolic BP(mmHg) | 72.19 ± 9.33 | 66.50 ± 9.10 | 0.0017 |
| Hypertension; no. (%) | 8 (21.05) | 11 (12.36) | 0.2085 |
| Cholesterol (mg/dL) | 173.47 ± 33.31 | 199.93 ± 44.9 | 0.0004 |
| LDL (mg/dL) | 109.11 ± 31.92 | 129.55 ± 72.69 | 0.0296 |
| HDL (mg/dL) | 46.42 ± 9.99 | 61.95 ± 18.89 | <0.0001 |
| Triglyceride (mg/dL) | 134 ± 83.28 | 105.63 ± 102.22 | 0.1337 |
| Glucose AC (mg/dL) | 96.11 ± 32.22 | 93.08 ± 34.65 | 0.6462 |
| Diabetes; no. (%) | 11 (28.95) | 12 (13.48) | 0.0382 |
| Smoking habit, no. (%) | 0.0500 | ||
| Never | 26 (68.42) | 76 (85.39) | |
| Former | 4 (10.53) | 7 (7.87) | |
| Current | 8 (21.05) | 6 (6.74) | |
| Alcohol consumption, no. (%) | 0.6306 | ||
| Never | 26 (68.42) | 63 (70.79) | |
| Former | 5 (13.16) | 7 (7.87) | |
| Current | 7 (18.42) | 19 (21.35) | |
| Exercise habit, % | 12 (31.58) | 38 (42.70) | 0.2403 |
| Incense burning, % | 0.0353 | ||
| Everyday | 19 (50.00) | 24 (27.27) | |
| Not Everyday | 2 (5.26) | 12 (13.64) | |
| Never | 17 (44.74) | 52 (59.09) | |
| Education level, % | 0.9396 | ||
| College or graduate school | 10 (26.32) | 24 (26.97) | |
| High school or below | 28 (73.68) | 65 (73.03) | |
| Sleep deprivation; no. (%) | 15 (39.47) | 33 (37.08) | 0.0873 |
| Diet supplement; no. (%) | 17 (44.74) | 48 (53.93) | 0.3424 |
| Cook at home; no. (%) | 36 (94.74) | 85 (95.51) | - |
| Mean ± SD | Median | IQR | Range (Min to Max) | ||
|---|---|---|---|---|---|
| PM2.5, μg/m3 | 12 h | 27.61 ± 16.42 | 23.40 | 22.41 | 86.06 (8.44–94.50) |
| 24 h | 31.30 ± 16.50 | 27.72 | 24.03 | 92.38 (8.72–101.10) | |
| PM10, μg/m3 | 12 h | 28.74 ± 16.81 | 23.80 | 22.83 | 94.93 (0.04–94.97) |
| 24 h | 33.06 ± 16.74 | 29.76 | 23.27 | 92.41 (10.44–102.85) | |
| CO, ppm | 12 h | 0.34 ± 0.30 | 0.31 | 0.42 | 1.25 (0.00–1.25) |
| 24 h | 0.37 ± 0.30 | 0.31 | 0.46 | 1.12 (0.00–1.12) | |
| CO2, ppm | 12 h | 544.95 ± 174.63 | 493.98 | 188.97 | 723.92 (332.64–1056.56) |
| 24 h | 533.54 ± 155.48 | 487.82 | 169.71 | 750.76 (359.12–1109.88) | |
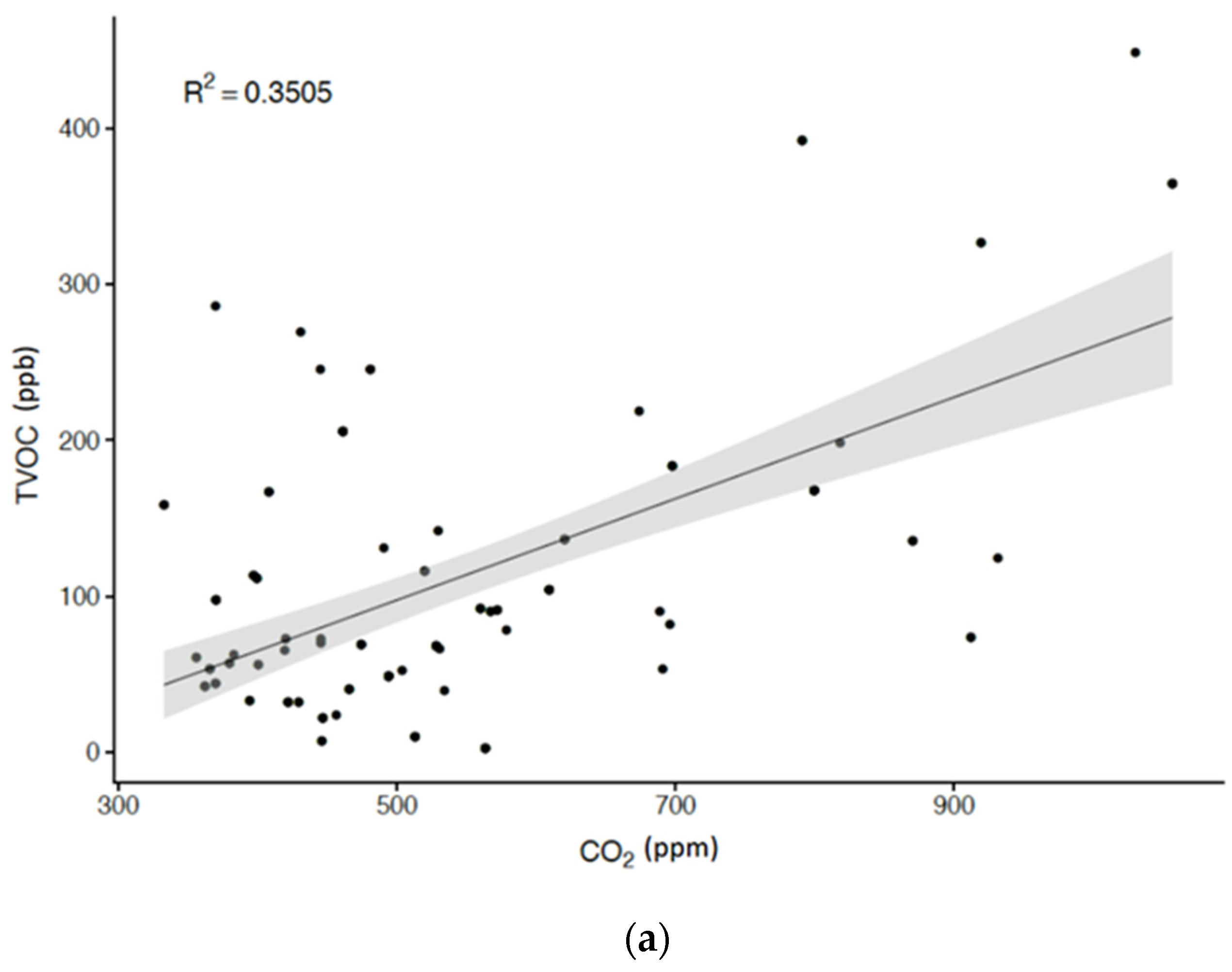
| TVOC, ppb | 12 h | 112.48 ± 96.50 | 78.13 | 109.91 | 445.51 (2.86–448.37) |
| 24 h | 99.79 ± 84.22 | 71.11 | 76.06 | 425.92 (2.09–428.01) | |
| Formaldehyde, ppb | 12 h | 4.47 ± 2.82 | 4.53 | 5.06 | 10.28 (0.09–10.37) |
| 24 h | 4.57 ± 2.62 | 4.95 | 5.02 | 9.63 (0.37–10.00) |
| Obesity Indicators (Unit) | TVOC (ppb) | Formaldehyde (ppm) | CO (ppm) | CO2 (ppm) |
|---|---|---|---|---|
| Body Mass Index (kg/m2) | 0.3925 ‡ | 0.1947 * | 0.2249 * | 0.2941 † |
| Waistline (cm) | 0.3434 ‡ | 0.1897 * | 0.1272 | 0.2290 * |
| Fat Percentage (%) | 0.3267 ‡ | 0.2571 † | 0.2087 * | 0.2833 † |
| Fat Mass (kg) | 0.3354 ‡ | 0.2084 * | 0.1798 | 0.2614 † |
| Fat Free Mass (kg) | 0.2852 † | 0.1498 | 0.1109 | 0.1811 |
| Muscle Mass (kg) | 0.2867 † | 0.1492 | 0.1080 | 0.1811 |
| Visceral Fat Rating | 0.3353 ‡ | 0.2239 * | 0.2036 * | 0.2498 † |
| Overweight (BMI ≥ 25 kg/m3) | Abdominal Obesity (Male ≥ 90 cm, Female ≥ 80 cm) | Fat Percentage ≥ 32% | ||||
|---|---|---|---|---|---|---|
| Model 1 | OR (95%CI) | p-value | OR (95%CI) | p-value | OR (95%CI) | p-value |
| PM2.5 | 0.87 (0.50, 1.53) | 0.6396 | 0.80 (0.47, 1.35) | 0.3993 | 1.13 (0.66, 1.94) | 0.6538 |
| PM10 | 0.91 (0.52, 1.57) | 0.7285 | 0.75 (0.44, 1.28) | 0.2976 | 1.16 (0.68, 1.98) | 0.5913 |
| CO | 1.71 (1.00, 2.91) | 0.0482 | 1.14 (0.68, 1.88) | 0.6231 | 1.92 (1.11, 3.35) | 0.0206 |
| CO2 | 1.77 (1.17, 2.66) | 0.0065 | 1.61 (1.08, 2.40) | 0.0197 | 1.51 (1.00, 2.27) | 0.0491 |
| TVOC | 2.05 (1.30, 3.22) | 0.0020 | 1.95 (1.24, 3.06) | 0.0039 | 1.45 (0.94, 2.23) | 0.0929 |
| Formaldehyde | 2.25 (1.09, 4.66) | 0.0291 | 1.74 (0.89, 3.42) | 0.1055 | 1.51 (0.73, 3.16) | 0.2693 |
| Model 2 | ||||||
| PM2.5 | 0.90 (0.52, 1.56) | 0.6976 | 0.80 (0.46, 1.37) | 0.4104 | 1.07 (0.57, 1.98) | 0.8374 |
| PM10 | 0.92 (0.53, 1.59) | 0.7596 | 0.75 (0.43, 1.30) | 0.3022 | 1.13 (0.62, 2.07) | 0.6861 |
| CO | 1.86 (1.06, 3.25) | 0.0297 | 1.12 (0.67, 1.86) | 0.6699 | 2.17 (1.15, 4.10) | 0.0170 |
| CO2 | 1.90 (1.23, 1.23) | 0.0036 | 1.62 (1.08, 2.43) | 0.0186 | 1.55 (0.98, 2.43) | 0.0586 |
| TVOC | 2.14 (1.33, 3.44) | 0.0016 | 1.97 (1.25, 3.11) | 0.0036 | 1.59 (0.97, 2.61) | 0.0665 |
| Formaldehyde | 2.20 (1.03, 4.69) | 0.0405 | 1.82 (0.92, 3.59) | 0.0863 | 1.85 (0.83, 4.12) | 0.1342 |
| Model 3 | ||||||
| PM2.5 | 0.87 (0.44, 1.71) | 0.6806 | 0.97 (0.50, 1.92) | 0.9402 | 1.18 (0.53, 2.64) | 0.6858 |
| PM10 | 0.90 (0.46, 1.74) | 0.7463 | 0.92 (0.47, 1.78) | 0.7956 | 1.28 (0.59, 2.81) | 0.5344 |
| CO | 2.25 (1.14, 4.44) | 0.0191 | 1.20 (0.65, 2.21) | 0.5639 | 3.39 (1.34, 8.57) | 0.0098 |
| CO2 | 2.46 (1.41, 4.29) | 0.0015 | 1.67 (1.01, 2.76) | 0.0460 | 1.88 (1.06, 3.33) | 0.0310 |
| TVOC | 2.70 (1.48, 4.94) | 0.0012 | 2.38 (1.28, 4.40) | 0.0059 | 2.09 (1.09, 3.99) | 0.0231 |
| Formaldehyde | 2.69 (1.05, 6.85) | 0.0386 | 1.48 (0.64, 3.40) | 0.3565 | 1.48 (0.56, 3.94) | 0.4335 |
| Overweight (BMI ≥ 25 kg/m3) | Abdominal Obesity (Male ≥ 90 cm, Female ≥ 80 cm) | Fat Percentage ≥ 32% (≥75th Percentile) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pollutants (IQR) | aOR (95%CI) | p-Value | VIF | aOR (95%CI) | p-Value | VIF | aOR (95%CI) | p-Value | VIF | |
| (1) | CO2 | 1.69 (0.84, 3.37) | 0.1385 | 1.80 | 1.08 (0.58, 2.02) | 0.8079 | 1.79 | 1.38 (0.68, 2.79) | 0.3841 | 1.80 |
| TVOC | 1.95 (0.94, 4.07) | 0.0731 | 1.73 | 2.28 (1.12, 4.63) | 0.0232 | 1.72 | 1.69 (0.76, 3.76) | 0.1989 | 1.73 | |
| (2) | CO2 | 2.37 (1.24, 4.54) | 0.0090 | 1.70 | 1.73 (0.95, 3.16) | 0.0729 | 1.70 | 2.21 (1.09, 4.47) | 0.0301 | 1.70 |
| Formaldehyde | 1.17 (0.38, 3.63) | 0.7858 | 1.67 | 0.86 (0.31, 2.42) | 0.7796 | 1.66 | 0.67 (0.19, 2.31) | 0.5237 | 1.67 | |
| (3) | CO2 | 1.86 (1.01, 3.44) | 0.0480 | 1.50 | 1.59 (0.89, 2.86) | 0.1177 | 1.50 | 1.63 (0.81, 3.30) | 0.1805 | 1.50 |
| CO | 1.68 (0.81, 3.50) | 0.1621 | 1.29 | 0.94 (0.47, 1.87) | 0.8623 | 1.29 | 2.75 (1.04, 7.29) | 0.0418 | 1.29 | |
| (4) | TVOC | 2.60 (1.32, 5.13) | 0.0057 | 1.48 | 2.59 (1.31, 5.11) | 0.0062 | 1.48 | 2.60 (1.12, 6.03) | 0.0235 | 1.48 |
| Formaldehyde | 1.25 (0.42, 3.70) | 0.6841 | 1.50 | 0.76 (0.29, 2.03) | 0.5878 | 1.50 | 0.59 (0.17, 2.09) | 0.4164 | 1.50 | |
| (5) | TVOC | 2.22 (1.13, 4.35) | 0.0206 | 1.43 | 2.32 (1.13, 4.79) | 0.0222 | 1.43 | 1.68 (0.77, 3.64) | 0.1928 | 1.43 |
| CO | 1.45 (0.69, 3.03) | 0.3308 | 1.31 | 0.87 (0.42, 1.80) | 0.7125 | 1.31 | 2.65 (1.00, 7.00) | 0.0494 | 1.31 | |
| (6) | Formaldehyde | 1.47 (0.47, 4.58) | 0.5104 | 1.57 | 1.07 (0.38, 2.98) | 0.9012 | 1.58 | 0.62 (0.17, 2.33) | 0.4845 | 1.57 |
| CO | 2.01 (0.93, 4.34) | 0.0754 | 1.38 | 1.12 (0.56, 2.23) | 0.7553 | 1.39 | 4.46 (1.50, 13.21) | 0.0071 | 1.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-K.; Wu, C.; Su, T.-C. Positive Association between Indoor Gaseous Air Pollution and Obesity: An Observational Study in 60 Households. Int. J. Environ. Res. Public Health 2021, 18, 11447. https://doi.org/10.3390/ijerph182111447
Chen J-K, Wu C, Su T-C. Positive Association between Indoor Gaseous Air Pollution and Obesity: An Observational Study in 60 Households. International Journal of Environmental Research and Public Health. 2021; 18(21):11447. https://doi.org/10.3390/ijerph182111447
Chicago/Turabian StyleChen, Jia-Kun, Charlene Wu, and Ta-Chen Su. 2021. "Positive Association between Indoor Gaseous Air Pollution and Obesity: An Observational Study in 60 Households" International Journal of Environmental Research and Public Health 18, no. 21: 11447. https://doi.org/10.3390/ijerph182111447
APA StyleChen, J.-K., Wu, C., & Su, T.-C. (2021). Positive Association between Indoor Gaseous Air Pollution and Obesity: An Observational Study in 60 Households. International Journal of Environmental Research and Public Health, 18(21), 11447. https://doi.org/10.3390/ijerph182111447







