Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications
Abstract
1. Introduction
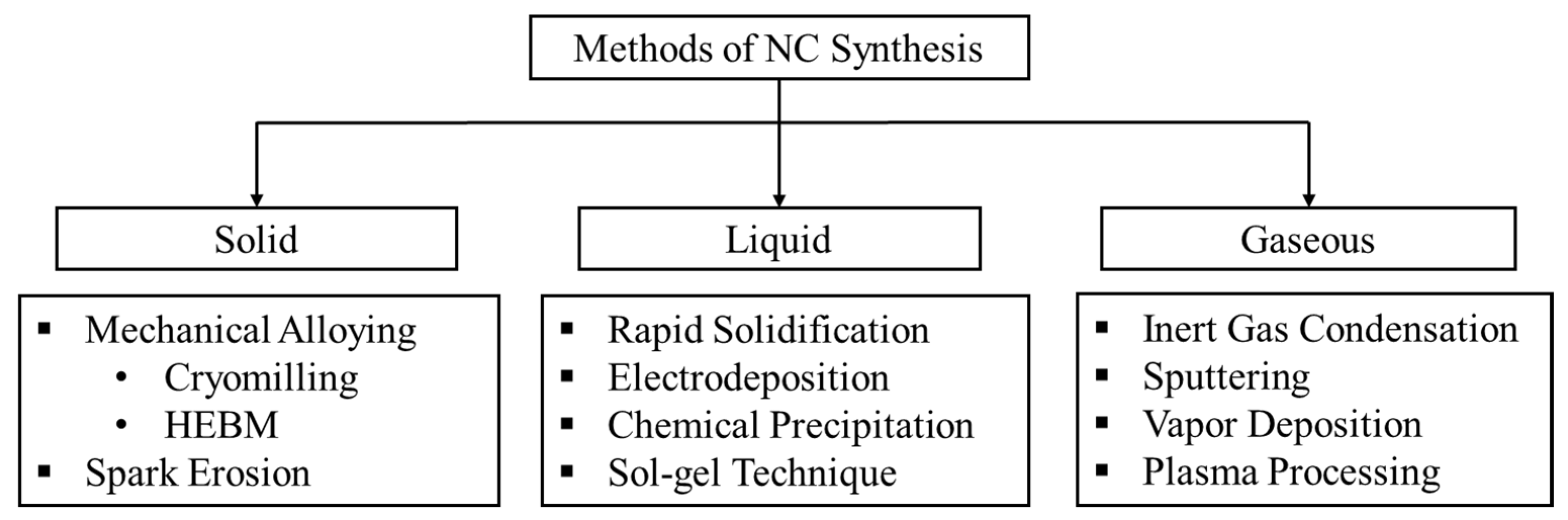
2. Processing of NC Materials
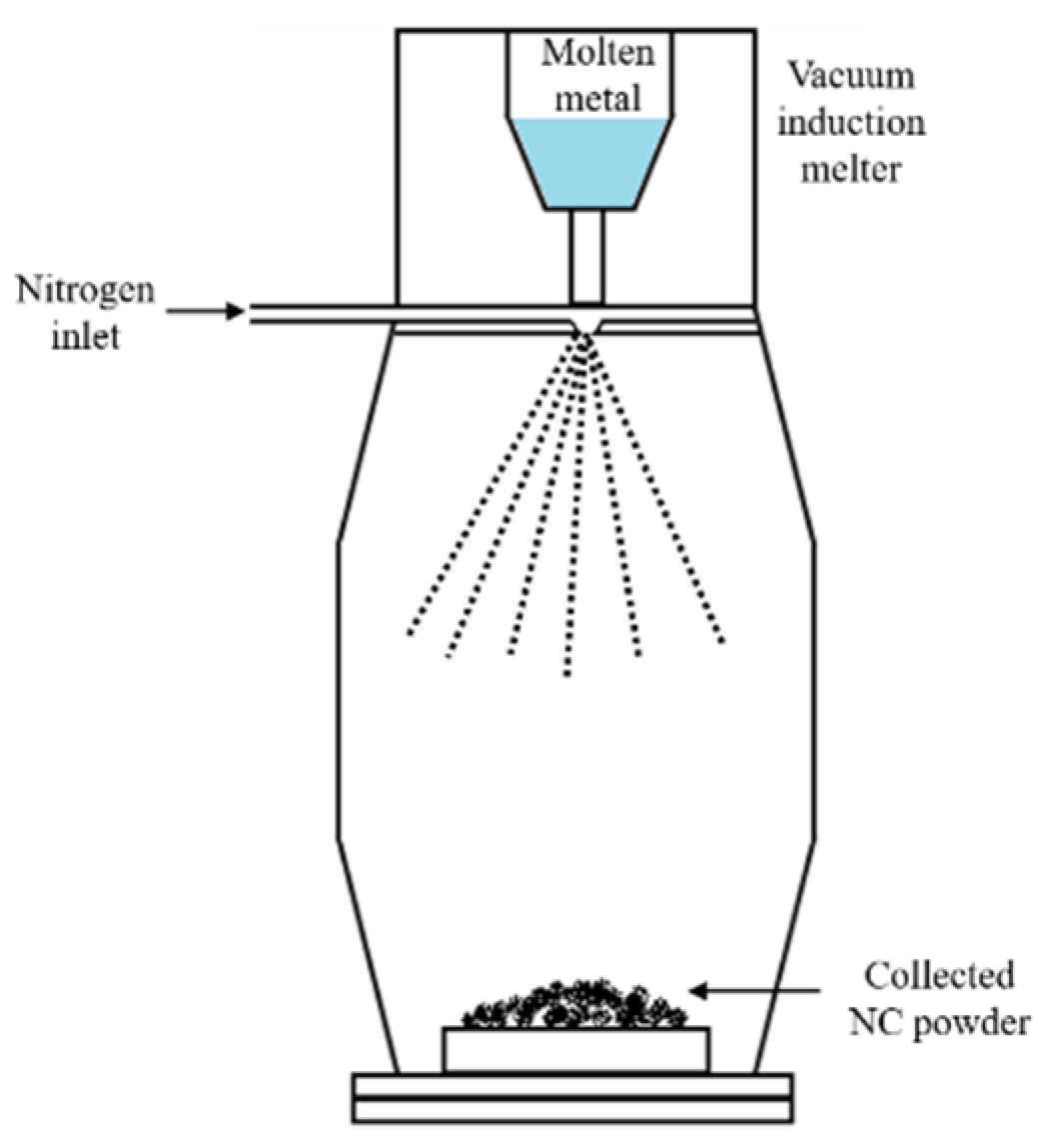
2.1. Rapid Solidification
2.2. Chemical Precipitation
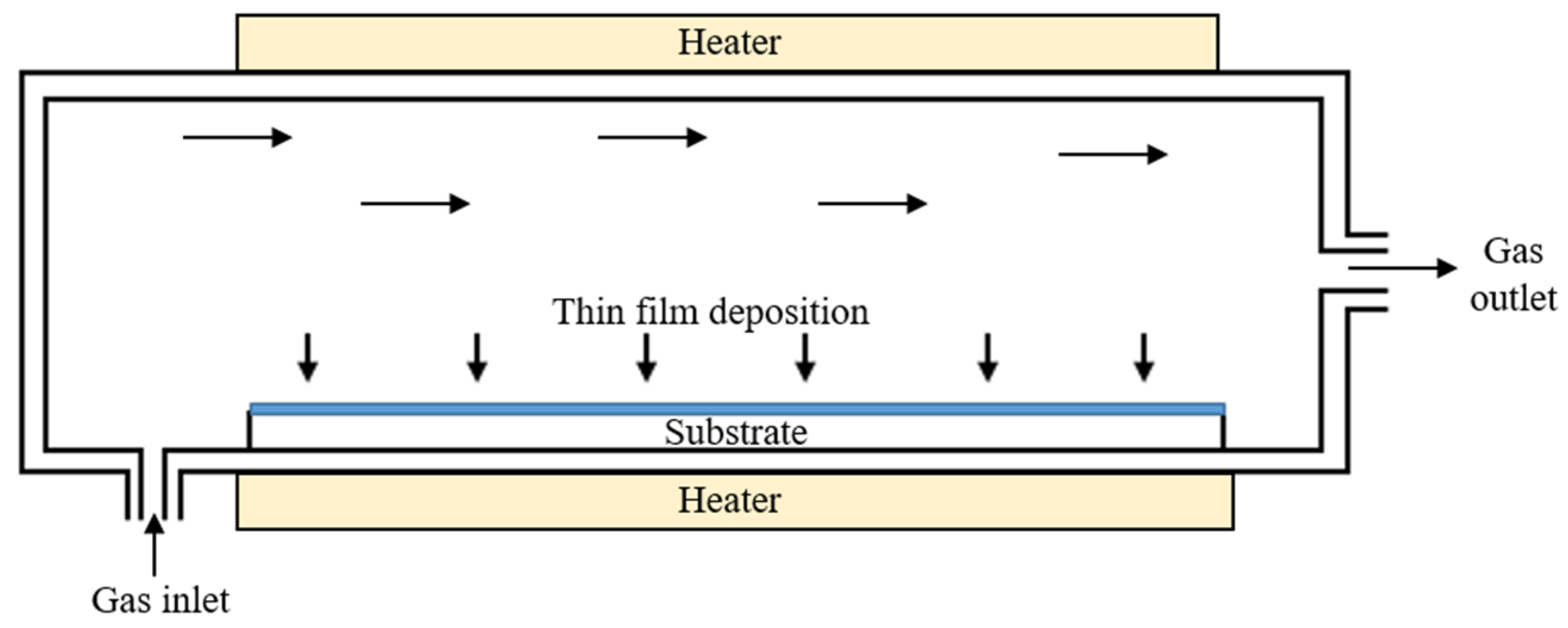
2.3. Chemical Vapor Deposition
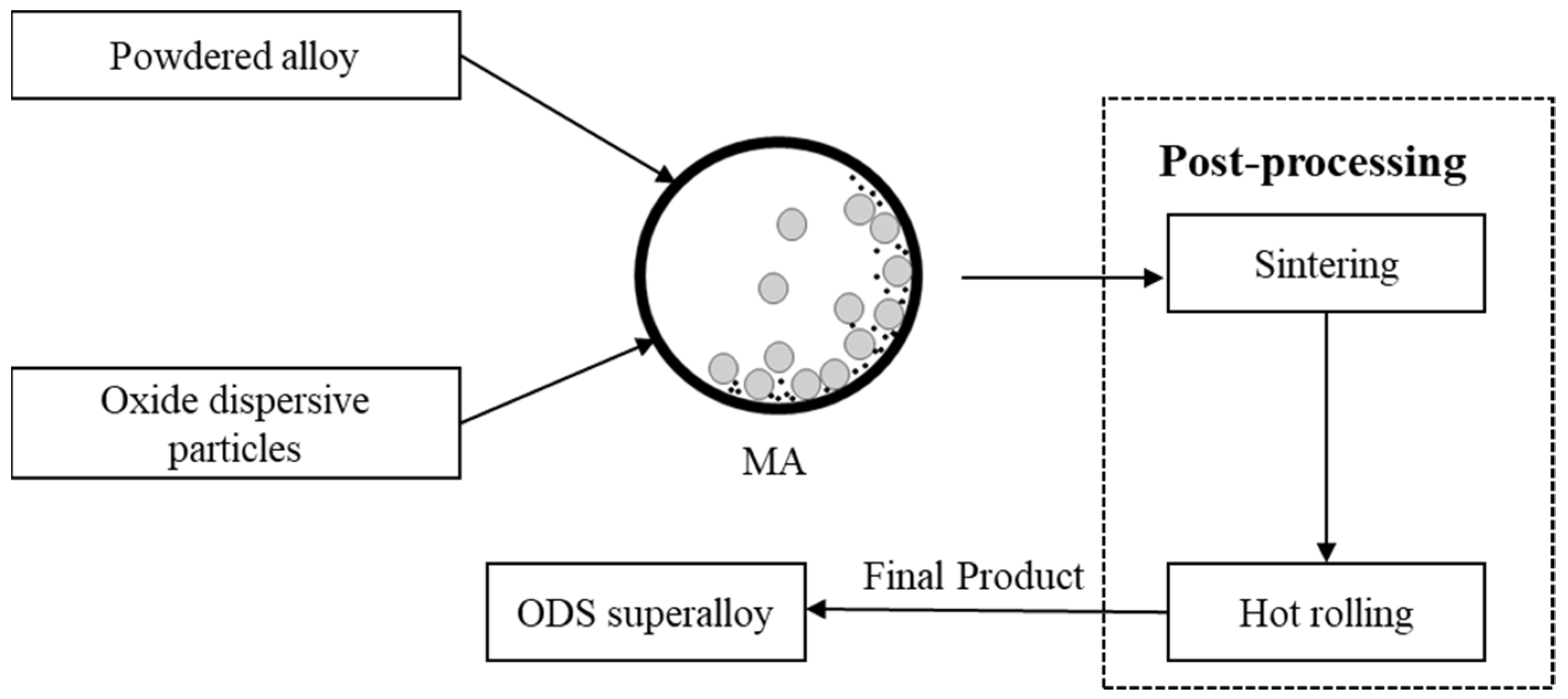
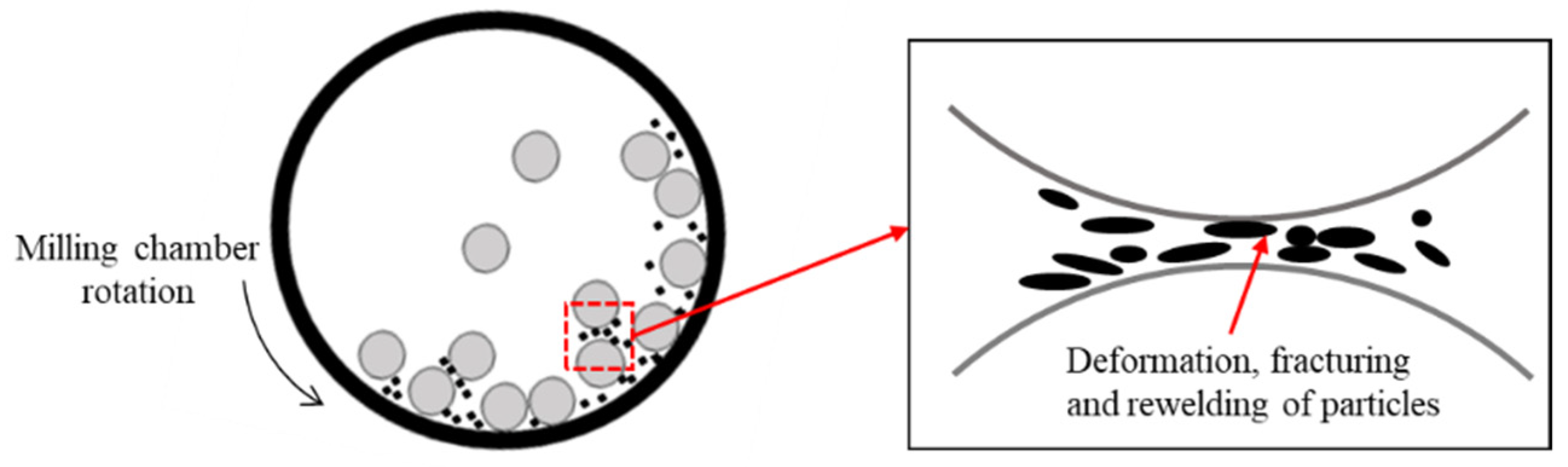
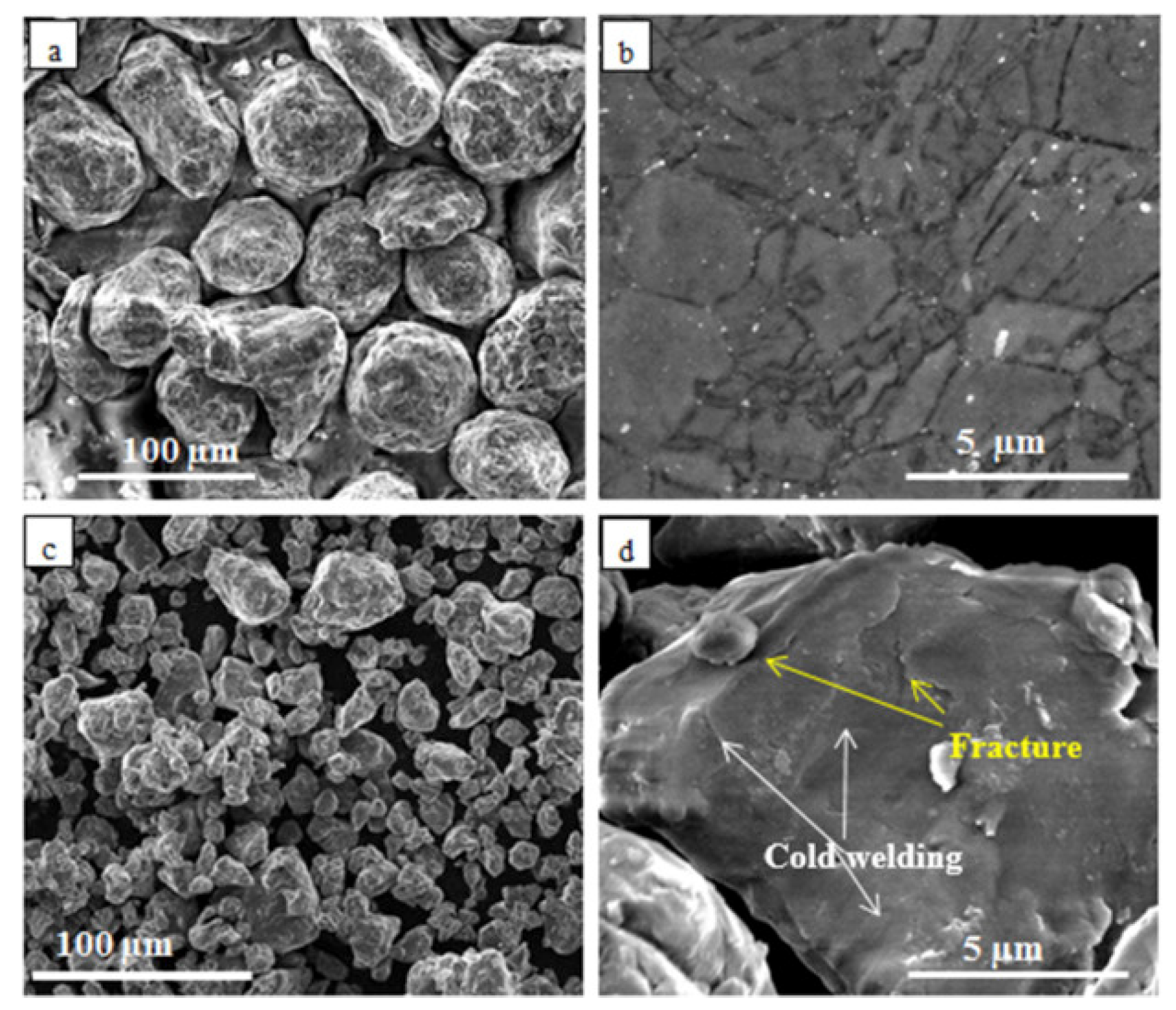
2.4. Mechanical Alloying
2.4.1. High Energy Ball Milling
2.4.2. Cryomilling
3. Microstructural Features of NC Materials
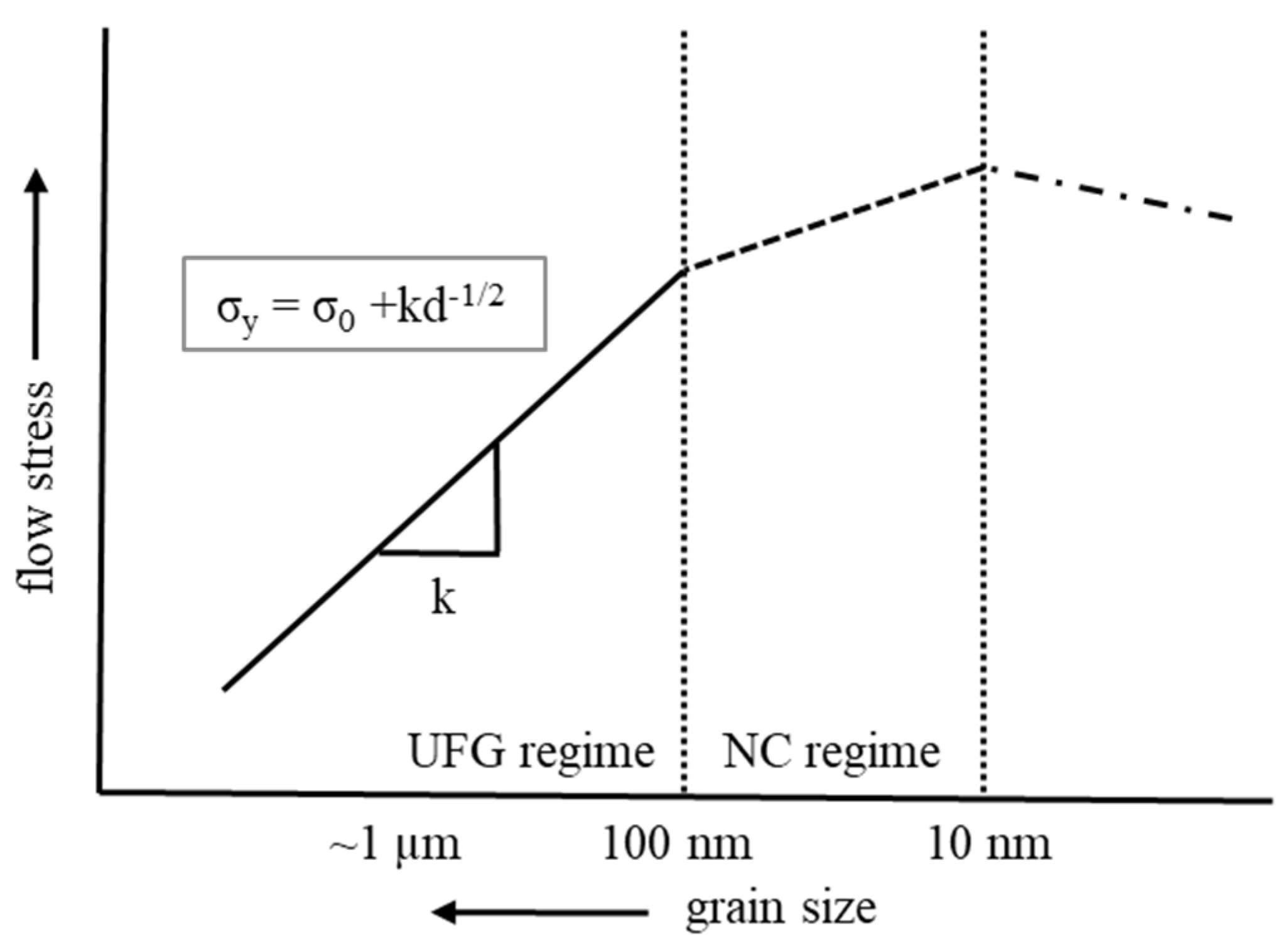
4. Mechanical Properties of NC Materials
5. Applications, Challenges, and Future Scope
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCM | Biomorphic carbon materials |
| CC | Cold compaction |
| CCVD | Catalytic chemical vapor deposition |
| CNT | Carbon nanotubes |
| CoF | Coefficient of friction |
| CPP | Cyclic potentiodynamic polarization |
| EPD | Electrophoretic deposition |
| FCC | Face centered cubic |
| HA | Hydroxyapatite |
| HF | Hydrofluoric acid |
| HIP | Hot isostatic pressing |
| HEBM | High energy ball milling |
| HNO3 | Nitric acid |
| HT | High temperature |
| MA | Mechanical alloying |
| MEMS | Microelectromechanical systems |
| MC | Microcrystalline |
| NC | Nanocrystalline |
| PECVD | Plasma enhanced chemical vapor deposition |
| RE | Rare earth |
| RPM | Rotations per minute |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| SPS | Spark plasma sintering |
| SLM | Selective laser melting |
| SPD | Severe plastic deformation |
| SS | Stainless steel |
| TEM | Transmission electron microscopy |
| UTS | Ultimate tensile stress |
| UFC | Ultra-fine crystalline |
| UFG | Ultra-fine grains |
References
- Zhu, Y.T.; Langdon, T.G. Influence of grain size on deformation mechanisms: An extension to nanocrystalline materials. Mater. Sci. Eng. A 2005, 409, 234–242. [Google Scholar] [CrossRef]
- Whang, S.H. Microstructure, Mechanical Properties and Applications. In Nanostructured Metals and Alloys; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.D.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef]
- Lowe, T.C.; Valiev, R.Z. The use of severe plastic deformation techniques in grain refinement. JOM 2004, 56, 64–68. [Google Scholar] [CrossRef]
- Witkin, D.B.; Lavernia, E.J. Synthesis and mechanical behavior of nanostructured materials via cryomilling. Prog. Mater. Sci. 2006, 51, 1–60. [Google Scholar] [CrossRef]
- Nagarajan, B.; Kumar, D.; Fan, Z.; Castagne, S. Effect of deep cold rolling on mechanical properties and microstructure of nickel-based superalloys. Mater. Sci. Eng. A 2018, 728, 196–207. [Google Scholar] [CrossRef]
- John, M.; Kalvala, P.R.; Misra, M.; Menezes, P.L. Peening Techniques for Surface Modification: Processes, Properties, and Applications. Materials 2021, 14, 3841. [Google Scholar] [CrossRef] [PubMed]
- Huot, J. Nanocrystalline metal hydrides obtained by severe plastic deformations. Metals 2012, 2, 22–40. [Google Scholar] [CrossRef]
- Birringer, R.; Gleiter, H.; Klein, H.P.; Marquardt, P. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder? Phys. Lett. A 1984, 102, 365–369. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Li, J.; Weng, G.J. A secant-viscosity composite model for the strain-rate sensitivity of nanocrystalline materials. Int. J. Plast. 2007, 23, 2115–2133. [Google Scholar] [CrossRef]
- Pun, S.C.; Wang, W.; Khalajhedayati, A.; Schuler, J.D.; Trelewicz, J.R.; Rupert, T.J. Nanocrystalline Al-Mg with extreme strength due to grain boundary doping. Mater. Sci. Eng. A 2017, 64, 400–406. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E. Micromechanical modelling of nanocrystalline and ultrafine grained metals: A short overview. Comput. Mater. Sci. 2015, 96, 365–373. [Google Scholar] [CrossRef]
- Wei, Y.; Bower, A.F.; Gao, H. Enhanced strain-rate sensitivity in fcc nanocrystals due to grain-boundary diffusion and sliding. Acta Mater. 2008, 56, 1741–1752. [Google Scholar] [CrossRef]
- Henderson, H.B.; Hammons, J.A.; Baker, A.A.; McCall, S.K.; Li, T.T.; Perron, A.; Sims, Z.C.; Ott, R.T.; Meng, F.; Thompson, M.J.; et al. Enhanced thermal coarsening resistance in a nanostructured aluminum-cerium alloy produced by additive manufacturing. Mater. Des. 2021, 209, 109988. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Swihart, M.T. Vapor-phase production of nanomaterials. Chem. Soc. Rev. 2021, 50, 7132–7249. [Google Scholar] [CrossRef]
- Ibrahim, R.S.; Azab, A.A.; Mansour, A.M. Synthesis and structural, optical, and magnetic properties of Mn-doped CdS quantum dots prepared by chemical precipitation method. J. Mater. Sci. Mater. Electron. 2021, 32, 19980–19990. [Google Scholar] [CrossRef]
- Khan, M.U.F.; Mirza, F.; Gupta, R.K. High Hardness and Thermal Stability of Nanocrystalline Mg–Al Alloys Synthesized by the High-Energy Ball Milling; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 4, ISBN 1330972783. [Google Scholar]
- Tian, L. A Short Review on Mechanical Behavior of Nanocrystalline Materials. Int. J. Metall. Met. Phys. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Liu, J.; Khan, A.S.; Takacs, L.; Meredith, C.S. Mechanical behavior of ultrafine-grained/nanocrystalline titanium synthesized by mechanical milling plus consolidation: Experiments, modeling and simulation. Int. J. Plast. 2015, 64, 151–163. [Google Scholar] [CrossRef]
- Deng, H.; Chen, A.; Chen, L.; Wei, Y.; Xia, Z.; Tang, J. Bulk nanostructured Ti-45Al-8Nb alloy fabricated by cryomilling and Spark Plasma Sintering. J. Alloys Compd. 2019, 772, 140–149. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.; Li, Y.; Moss, W.; Lavernia, E.J. Enhanced tensile strength and high ductility in cryomilled commercially pure titanium. Scr. Mater. 2009, 60, 586–589. [Google Scholar] [CrossRef]
- Lu, L.; Pan, Q.; Hattar, K.; Boyce, B.L. Fatigue and fracture of nanostructured metals and alloys. MRS Bull. 2021, 46, 258–264. [Google Scholar] [CrossRef]
- An, X.; Lin, Q.; Wu, S.; Zhang, Z. Improved Fatigue Strengths of Nanocrystalline Cu and Cu–Al Alloys. Mater. Res. Lett. 2015, 3, 135–141. [Google Scholar] [CrossRef]
- Schuler, J.D.; Barr, C.M.; Heckman, N.M.; Copeland, G.; Boyce, B.L.; Hattar, K.; Rupert, T.J. In Situ High-Cycle Fatigue Reveals Importance of Grain Boundary Structure in Nanocrystalline Cu-Zr. JOM 2019, 71, 1221–1232. [Google Scholar] [CrossRef]
- Peng, R.; Fu, L.; Zhou, L. Improved wear resistance by phase transformation of surface nanocrystalline 1090 steel prepared by sandblasting technique. Appl. Surf. Sci. 2016, 388, 406–411. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Peng, S. Synthesis and evaluation of TaC nanocrystalline coating with excellent wear resistance, corrosion resistance, and biocompatibility. Ceram. Int. 2021, 47, 20032–20044. [Google Scholar] [CrossRef]
- Roy, D.; Mahesh, B.V.; Atwater, M.A.; Chan, T.E.; Scattergood, R.O.; Koch, C.C. Grain size stability and hardness in nanocrystalline Cu-Al-Zr and Cu-Al-Y alloys. Mater. Sci. Eng. A 2014, 598, 217–223. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, B.; Wang, Z.; Shi, J.; Yang, C.; Dong, D.; Wang, X. Thermal stabilization of nanocrystalline promoting conductive corrosion resistance of TiN–Ag films for metal bipolar plates. Vacuum 2021, 195, 110631. [Google Scholar] [CrossRef]
- Rojas, D.P.; Espeso, J.I.; Fernández, L.R.; Barquín, L.F. Heat capacity of nanocrystalline Yb2O3. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Hohl, J.; Kumar, P.; Misra, M.; Menezes, P.; Mushongera, L.T. Thermodynamic stabilization of nanocrystalline aluminum. J. Mater. Sci. 2021, 56, 14611–14623. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Divinski, S.; Rösner, H.; Wilde, G. Functional Nanostructured Materials-Microstructure, Thermodynamic Stability and Atomic Mobility; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; Volume 1, ISBN 9780080449654. [Google Scholar]
- Zhu, Y.T.; Wu, X.L. Ductility and plasticity of nanostructured metals: Differences and issues. Mater. Today Nano 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Froes, F.H.; Suryanarayana, C. Nanocrystalline metals for structural applications. JOM 1989, 41, 12–17. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abd El-Hameed, A.M. Characterization of CdSe-nanocrystals used in semiconductors for aerospace applications: Production and optical properties. NRIAG J. Astron. Geophys. 2014, 3, 82–87. [Google Scholar] [CrossRef]
- Kumar, H.H.; Lonkar, C.M.; Balasubramanian, K. Structure–Property Correlation and Harvesting Power from Vibrations of Aerospace Vehicles by Nanocrystalline La–Pb(Ni1/3Sb2/3)–PbZrTiO3 Ferroelectric Ceramics Synthesized by Mechanical Activation. J. Am. Ceram. Soc. 2017, 100, 215–223. [Google Scholar] [CrossRef]
- Sevillano, J.G.; Aldazabal, J. Ductilization of nanocrystalline materials for structural applications. Scr. Mater. 2004, 51, 795–800. [Google Scholar] [CrossRef]
- Hoefler, H.J.; Averback, R.S. Grain growth in nanocrystalline TiO2 and its relation to vickers hardness and fracture toughness. Scr. Metall. 1990, 24, 2401–2406. [Google Scholar] [CrossRef]
- Baghbanan, M.; Erb, U.; Palumbo, G. Towards the application of nanocrystalline metals in MEMS. Phys. Status Solidi Appl. Mater. Sci. 2006, 203, 1259–1264. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Nanocrystalline SrMnO3 perovskite prepared by sol–gel self-combustion method for sensor applications. J. Sol-Gel Sci. Technol. 2021, 97, 146–154. [Google Scholar] [CrossRef]
- Bauer, J.C.; Chen, X.; Liu, Q.; Phan, T.H.; Schaak, R.E. Converting nanocrystalline metals into alloys and intermetallic compounds for applications in catalysis. J. Mater. Chem. 2008, 18, 275–282. [Google Scholar] [CrossRef]
- Grössinger, R.; Sato, R.; Holzer, D.; Dahlgren, M. Properties, benefits, and application of nanocrystalline structures in magnetic materials. Adv. Eng. Mater. 2003, 5, 285–290. [Google Scholar] [CrossRef]
- Cunningham, W.S.; Hattar, K.; Zhu, Y.; Edwards, D.J.; Trelewicz, J.R. Suppressing irradiation induced grain growth and defect accumulation in nanocrystalline tungsten through grain boundary doping. Acta Mater. 2021, 206, 116629. [Google Scholar] [CrossRef]
- Kellogg, F.; Demaree, J.D. Exploring the Feasibility of Using Cryomilled Aluminum Powder as Feedstock for Laser Powder Bed Fusion Additive Manufacturing; CCDC Army Research Laboratory: Harford County, MD, USA, 2020; ISBN 0000000330480. [Google Scholar]
- Elangovan, H.; Sengupta, S.; Narayanan, R.; Chattopadhyay, K. Silicon nanoparticles with UV range photoluminescence synthesized through cryomilling induced phase transformation and etching. J. Mater. Sci. 2021, 56, 1515–1526. [Google Scholar] [CrossRef]
- Mahrous, F.; Koshani, R.; Tavakolian, M.; Conley, K.; van de Ven, T.G.M. Formation of hairy cellulose nanocrystals by cryogrinding. Cellulose 2021, 28, 8387–8403. [Google Scholar] [CrossRef]
- Roy, D.; Chakraborty, S.; Gupta, A.K.; BasuMallick, A.; Scattergood, R.O.; Koch, C.C. Synergistic effect of Nb and Zr additions on the structure-property relationships of nanocrystalline Cu processed by mechanical alloying and hot pressing. J. Alloys Compd. 2021, 854, 157174. [Google Scholar] [CrossRef]
- Youssef, K.M.; Scattergood, R.O.; Murty, K.L.; Koch, C.C. Nanocrystalline Al-Mg alloy with ultrahigh strength and good ductility. Scr. Mater. 2006, 54, 251–256. [Google Scholar] [CrossRef]
- Renkó, J.B.; Károly, D.; Bonyár, A. Design, manufacture, and characterization of a novel Ti-based nanocrystalline alloy. J. Mater. Res. Technol. 2021, 11, 498–506. [Google Scholar] [CrossRef]
- Baláž, M.; Dobrozhan, O.; Tešinský, M.; Zhang, R.Z.; Džunda, R.; Dutková, E.; Rajňák, M.; Chen, K.; Reece, M.J.; Baláž, P. Scalable and environmentally friendly mechanochemical synthesis of nanocrystalline rhodostannite (Cu2FeSn3S8). Powder Technol. 2021, 388, 192–200. [Google Scholar] [CrossRef]
- Esteves, L.; Christudasjustus, J.; O’Brien, S.P.; Witharamage, C.S.; Darwish, A.A.; Walunj, G.; Stack, P.; Borkar, T.; Akans, R.E.; Gupta, R.K. Effect of V content on corrosion behavior of high-energy ball milled AA5083. Corros. Sci. 2021, 186, 109465. [Google Scholar] [CrossRef]
- Singh, P.K.; Mondal, S.; Das, A.K.; Mishra, S.K.; Singh, D.K.; Singh, S.K. Production of W-based nanoparticles via spark erosion process along with their characterization and optimization for practical application in gas sensor. Appl. Phys. A 2020, 126, 77. [Google Scholar] [CrossRef]
- Efimov, A.A.; Lizunova, A.A.; Volkov, I.A.; Mylnikov, D.A.; Arsenov, P.V.; Ivanov, V.V. A new approach to the high-yield synthesis of nanoparticles by spark discharge. J. Phys. Conf. Ser. 2016, 741, 012035. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Wang, W.; Zhang, Q. High effective preparation of amorphous-like si nanoparticles using spark erosion followed by bead milling. Nanomaterials 2021, 11, 594. [Google Scholar] [CrossRef]
- Nishimoto, S.; Yamasaki, M.; Inoue, S.; Kawamura, Y. Investigation of Microstructural Factors Affecting the Plane-Strain Fracture Toughness of Mg–Zn–Y–Al Alloys Processed by Consolidation of Rapidly Solidified Ribbons. In Proceedings of the Magnesium 2021; Luo, A., Pekguleryuz, M., Agnew, S., Allison, J., Kainer, K., Nyberg, E., Poole, W., Sadayappan, K., Williams, B., Yue, S., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 71–77. [Google Scholar]
- Lee, K.; Ahn, J.; Kim, J. Compositional effect on the magnetic and microstructural properties of Fe-based nano-crystalline alloys. IEEE Trans. Magn. 2021, 9464, 1. [Google Scholar] [CrossRef]
- Ipatov, M.; Zhukova, V.; Dominguez, L.; Alvarez, K.L.; Chizhik, A.; Zhukov, A.; Gonzalez, J. Structural and low-temperature magnetic properties of as-quenched and annealed Ni–Si–B alloys produced by rapid solidification. Intermetallics 2021, 132, 107140. [Google Scholar] [CrossRef]
- Ohodnicki, P.; Kautz, E.J.; Devaraj, A.; Yu, Y.; Aronhime, N.; Krimer, Y.; McHenry, M.E.; Leary, A. Nanostructure and compositional segregation of soft magnetic FeNi-based nanocomposites with multiple nanocrystalline phases. J. Mater. Res. 2021, 36, 105–113. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Tailoring and patterning the grain size of nanocrystalline alloys. Acta Mater. 2007, 55, 371–379. [Google Scholar] [CrossRef]
- Giallonardo, J.D.; Erb, U.; Aust, K.T.; Palumbo, G. The influence of grain size and texture on the Young’s modulus of nanocrystalline nickel and nickel-iron alloys. Philos. Mag. 2011, 91, 4594–4605. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Microstructural evolution during the heat treatment of nanocrystalline alloys. J. Mater. Res. 2007, 22, 3233–3248. [Google Scholar] [CrossRef]
- Priya, S.D.; Nesaraj, A.S.; Selvakumar, A.I. Facile wet-chemical synthesis and evaluation of physico-chemical characteristics of novel nanocrystalline NdCoO3-based perovskite oxide as cathode for LT-SOFC applications. Bull. Mater. Sci. 2021, 44, 2–8. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Drozdov, K.A.; Krylov, I.V.; Batuk, M.; Hadermann, J.; Filatova, D.G.; Khmelevsky, N.O.; Kozlovsky, V.F.; Maltseva, L.N.; et al. Sensors and Actuators: B. Chemical Photoresistive gas sensor based on nanocrystalline ZnO sensitized with colloidal perovskite CsPbBr 3 nanocrystals. Sens. Actuators B Chem. 2021, 329, 129035. [Google Scholar] [CrossRef]
- Ermokhina, N.I.; Shvalagin, V.V.; Romanovska, N.I.; Manoryk, P.A.; Barakov, R.Y.; Kompanets, M.O.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Synthesis and characterization of different binary and ternary phase mixtures of mesoporous nanocrystalline titanium dioxide. SN Appl. Sci. 2021, 3, 491. [Google Scholar] [CrossRef]
- Possato, L.G.; Gonçalves, R.G.L.; Santos, R.M.M.; Chaves, T.F.; Briois, V.; Pulcinelli, S.H.; Martins, L.; Santilli, C.V. Sol-gel synthesis of nanocrystalline MgO and its application as support in Ni/MgO catalysts for ethanol steam reforming. Appl. Surf. Sci. 2021, 542. [Google Scholar] [CrossRef]
- Choudhary, B.L.; Kumari, N.; Kumari, J.; Kumar, A.; Dolia, S.N. Relaxation mechanism in Ni0.5Zn0.5Fe2O4 Nanocrystalline ferrite at a lower temperature. Mater. Lett. 2021, 304, 130731. [Google Scholar] [CrossRef]
- Hemben, A.; Chianella, I.; Leighton, G.J.T. Surface engineered iron oxide nanoparticles generated by inert gas condensation for biomedical applications. Bioengineering 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Fu, S.; Liu, S.; Ren, Z.; Yan, M.; Chen, S.; Lan, S.; Hahn, H.; Feng, T. Nanocrystalline CoCrFeNiMn high-entropy alloy with tunable ferromagnetic properties. J. Mater. Sci. Technol. 2021, 77, 126–130. [Google Scholar] [CrossRef]
- Xia, Y.; Nelli, D.; Ferrando, R.; Yuan, J.; Li, Z.Y. Shape control of size-selected naked platinum nanocrystals. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Balbus, G.H.; Kappacher, J.; Sprouster, D.J.; Wang, F.; Shin, J.; Eggeler, Y.M.; Rupert, T.J.; Trelewicz, J.R.; Kiener, D.; Maier-Kiener, V.; et al. Disordered interfaces enable high temperature thermal stability and strength in a nanocrystalline aluminum alloy. Acta Mater. 2021, 215, 116973. [Google Scholar] [CrossRef]
- Koch, C.C. Nanocrystalline high-entropy alloys. J. Mater. Res. 2017, 32, 3435–3444. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Wu, Y.; Zeng, Y.; He, H.; Lin, J.; Ye, Z. Effects of phosphorus doping in ZnO nanocrystals by metal organic chemical vapor deposition. Mater. Lett. 2012, 68, 258–260. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.Y.; Li, J.; Liu, G.; Wu, K.; Wang, Y.Q.; Sun, J. Microstructural evolution, mechanical properties and deformation mechanisms of nanocrystalline Cu thin films alloyed with Zr. Acta Mater. 2014, 76, 221–237. [Google Scholar] [CrossRef]
- Shi, X.; Yu, X.; Nie, C.; Li, F.; Zhang, S. Controlled growth of nanocrystalline aluminum nitride films for full color range. Ceram. Int. 2021, 47, 21546–21553. [Google Scholar] [CrossRef]
- Weerasinghe, J.; Scott, J.; Deshan, A.D.K.; Chen, D.; Singh, A.; Sen, S.; Sonar, P.; Vasilev, K.; Li, Q.; Ostrikov, K. Monochromatic Blue and Switchable Blue-Green Carbon Quantum Dots by Room-Temperature Air Plasma Processing. Adv. Mater. Technol. 2021. [Google Scholar] [CrossRef]
- Krishna, B.G.; Tiwari, S. Optical properties of CsPbBr3 perovskite nanocrystals with silver nanoparticles using a room-temperature synthesis process. Opt. Mater. 2021, 119, 111357. [Google Scholar] [CrossRef]
- Dorin, T.; Vahid, A.; Lamb, J. Chapter 11—Aluminium Lithium Alloys. In Fundamentals of Aluminium Metallurgy; Lumley, R.N., Ed.; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing Limited: Cambridge, UK, 2018; pp. 387–438. ISBN 978-0-08-102063-0. [Google Scholar]
- El-Eskandarany, M.S. 1—Introduction. In Mechanical Alloying, 2nd ed.; El-Eskandarany, M.S., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 1–12. ISBN 978-1-4557-7752-5. [Google Scholar]
- Smallman, R.E.; Bishop, R.J. Chapter 9—Modern alloy developments. In Modern Physical Metallurgy and Materials Engineering, 6th ed.; Smallman, R.E., Bishop, R.J., Eds.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 297–319. ISBN 978-0-7506-4564-5. [Google Scholar]
- Jun, F.A.N.G.; Zhong, Y.H.; Xia, M.K.; Zhang, F.W. Mechanical and thermo-physical properties of rapidly solidified Al−50Si−Cu(Mg) alloys for thermal management application. Trans. Nonferrous Met. Soc. China 2021, 31, 586–594. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Lin, G.; Wang, X.; Sun, Y.; Wang, X.; Ye, J.; Sun, Y.; Yu, Y.; Jiang, F.; et al. Microstructure and properties of novel Al-Ce-Sc, Al-Ce-Y, Al-Ce-Zr and Al-Ce-Sc-Y alloy conductors processed by die casting, hot extrusion and cold drawing. J. Mater. Sci. Technol. 2020, 58, 155–170. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, D.F.; Ma, C.H.; Guo, S.F. Improving mechanical properties and corrosion resistance of Mg☐6Zn☐Mn magnesium alloy by rapid solidification. Mater. Lett. 2013, 92, 45–48. [Google Scholar] [CrossRef]
- Qiu, X. Microstructure, hardness and corrosion resistance of Al2CoCrCuFeNiTix high-entropy alloy coatings prepared by rapid solidification. J. Alloys Compd. 2018, 735, 359–364. [Google Scholar] [CrossRef]
- Afonso, C.R.M.; Spinelli, J.E.; Bolfarini, C.; Botta, W.J.; Kiminami, C.S.; Garcia, A. Rapid solidification of an Al-5Ni alloy processed by spray forming. Mater. Res. 2012, 15, 779–785. [Google Scholar] [CrossRef]
- Li, C.; Jin, S.; Guan, W.; Tsang, C.W.; Chu, W.K.; Lau, W.K.; Liang, C. Chemical Precipitation Method for the Synthesis of Nb2O5 Modified Bulk Nickel Catalysts with High Specific Surface Area. J. Vis. Exp. 2017, 2018, e56987. [Google Scholar] [CrossRef]
- Sivasankari, S.; Kalaivizhi, R.; Gowriboy, N.; Ganesh, M.R.; Shazia Anjum, M. Hydroxyapatite integrated with cellulose acetate/polyetherimide composite membrane for biomedical applications. Polym. Compos. 2021, 42, 5512–5526. [Google Scholar] [CrossRef]
- Luna, I.Z.; Hilary, L.N.; Chowdhury, A.M.S.; Gafur, M.A.; Khan, N.; Khan, R.A. Preparation and Characterization of Copper Oxide Nanoparticles Synthesized via Chemical Precipitation Method. OALib 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Arunadevi, R.; Latha, M.; Velumani, S.; Oza, G.; Reyes-Figueroa, P.; Rohini, M.; Becerril-Juarez, I.G.; Lee, J.H.; Yi, J. Synthesis and characterization of cadmium sulfide nanoparticles by chemical precipitation method. J. Nanosci. Nanotechnol. 2015, 15, 8434–8439. [Google Scholar] [CrossRef]
- Lang, J.; Wang, J.; Zhang, Q.; Li, X.; Han, Q.; Wei, M.; Sui, Y.; Wang, D.; Yang, J. Chemical precipitation synthesis and significant enhancement in photocatalytic activity of Ce-doped ZnO nanoparticles. Ceram. Int. 2016, 42, 14175–14181. [Google Scholar] [CrossRef]
- Tahir, M.B.; Rafique, M.; Rafique, M.S.; Nawaz, T.; Rizwan, M.; Tanveer, M. Chapter 8—Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. In Nanotechnology and Photocatalysis for Environmental Applications; Tahir, M.B., Rafique, M., Rafique, M.S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–138. ISBN 978-0-12-821192-2. [Google Scholar]
- Kim, I.J.; Zhao, W.; Park, J.G.; Meng, Z. Carbon nanotube filter for heavy metal ion adsorption. Ceram. Int. 2021, 47, 33280–33285. [Google Scholar] [CrossRef]
- Juggernauth, K.A.; Kim, M.; Kim, K.; Li, J.; McLane, A.A.; Lee, J.; Hart, A.J.; Ok, J.G. Carbon nanotube-mediated three-dimensional vanadium oxide nanoarchitectures with tunable morphology and translatable functionality. Ceram. Int. 2021, 47, 32342–32348. [Google Scholar] [CrossRef]
- Zainal Ariffin, N.H.; Mohammad Haniff, M.A.S.; Syono, M.I.; Ambri Mohamed, M.; Hamzah, A.A.; Hashim, A.M. Low-Temperature Nitrogen Doping of Nanocrystalline Graphene Films with Tunable Pyridinic-N and Pyrrolic-N by Cold-Wall Plasma-Assisted Chemical Vapor Deposition. ACS Omega 2021, 6, 23710. [Google Scholar] [CrossRef]
- Ascencio-Hurtado, C.R.; Torres, A.; Ambrosio, R.; Moreno, M.; Álvarez-Quintana, J.; Hurtado-Macías, A. N-type amorphous silicon-germanium thin films with embedded nanocrystals as a novel thermoelectric material of elevated ZT. J. Alloys Compd. 2021, 890, 161843. [Google Scholar] [CrossRef]
- Bogatov, A.; Yashin, M.; Viljus, M.; Menezes, P.; Podgursky, V. Comparative analysis of two methods for evaluating wear rate of nanocrystalline diamond films. Key Eng. Mater. 2017, 721, 345–350. [Google Scholar] [CrossRef]
- Yashin, M.; Baronins, J.; Menezes, P.L.; Viljus, M.; Raadik, T.; Bogatov, A.; Antonov, M.; Podgursky, V. Wear rate of nanocrystalline diamond coating under high temperature sliding conditions. Solid State Phenom. 2017, 267 SSP, 219–223. [Google Scholar] [CrossRef]
- Neikov, O.D. Chapter 3—Mechanical Alloying. In Handbook of Non-Ferrous Metal Powders, 2nd ed.; Neikov, O.D., Naboychenko, S.S., Yefimov, N.A., Eds.; Elsevier: Oxford, UK, 2019; pp. 91–124. ISBN 978-0-08-100543-9. [Google Scholar]
- Zhou, A. 2—Methods of MAX-phase synthesis and densification—II. In Advances in Science and Technology of Mn+1AXn Phases; Low, I.M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 21–46. ISBN 978-1-84569-991-8. [Google Scholar]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Street, P. Recrystallisation of Practical Mechanically Alloyed Iron—Base and and Nickel—Base Superalloys. Mater. Sci. Eng. A 1997, 223, 64–77. [Google Scholar] [CrossRef]
- Claudio, L.; De Castro, B.S.M. Nanoparticles from Mechanical Attrition. Synth. Funct. Surf. Treat. Nanoparticles 2002, 5, 1–14. [Google Scholar]
- Faraji, G.; Kim, H.S.; Kashi, H.T. Introduction. In Severe Plastic Deformation; Faraji, G., Kim, H.S., Kashi, H.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–17. ISBN 978-0-12-813518-1. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Demétrio, K.B.; Nogueira, A.P.G.; Menapace, C.; Bendo, T.; Molinari, A. Effect of nanostructure on phase transformations during heat treatment of 2024 aluminum alloy. J. Mater. Res. Technol. 2021, 14, 1800–1808. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Cryomilling: An environment friendly approach of preparation large quantity ultra refined pure aluminium nanoparticles. J. Mater. Res. Technol. 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Tellkamp, V.L.; Lavernia, E.J. Processing and mechanical properties of nanocrystalline 5083 Al alloy. Nanostructured Mater. 1999, 12, 249–252. [Google Scholar] [CrossRef]
- Guan, D.; Gao, J.; Rainforth, W.M. Effect of cryomilling time on microstructure evolution and hardness of cryomilled AZ31 powders. Mater. Charact. 2021, 178, 111311. [Google Scholar] [CrossRef]
- Ye, J.; Ajdelsztajn, L.; Schoenung, J.M. Bulk nanocrystalline aluminum 5083 alloy fabricated by a novel technique: Cryomilling and spark plasma sintering. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 2569–2579. [Google Scholar] [CrossRef]
- Menezes, P.L.; Lanka, S.; Maccione, R.; Vadlamani, B.; Misra, M. Development of Bulk Nanocrystalline Aluminum Materials with Enhanced Mechanical Properties. In Proceedings of the Materials Science and Technology (MST) Conference, Columbus, OH, USA, 19 October 2021. [Google Scholar]
- Lavernia, E.J.; Han, B.Q.; Schoenung, J.M. Cryomilled nanostructured materials: Processing and properties. Mater. Sci. Eng. A 2008, 493, 207–214. [Google Scholar] [CrossRef]
- Guan, D.; Rainforth, W.M.; Sharp, J.; Gao, J.; Todd, I. On the use of cryomilling and spark plasma sintering to achieve high strength in a magnesium alloy. J. Alloys Compd. 2016, 688, 1141–1150. [Google Scholar] [CrossRef]
- Park, Y.S.; Chung, K.H.; Kim, N.J.; Lavernia, E.J. Microstructural investigation of nanocrystalline bulk Al-Mg alloy fabricated by cryomilling and extrusion. Mater. Sci. Eng. A 2004, 374, 211–216. [Google Scholar] [CrossRef]
- Esquivel, J.; Wachowiak, M.G.; O’Brien, S.P.; Gupta, R.K. Thermal stability of nanocrystalline Al-5at.%Ni and Al-5at.%V alloys produced by high-energy ball milling. J. Alloys Compd. 2018, 744, 651–657. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Ohkubo, T.; Hono, K. Microstructure and mechanical properties of bulk nanocrystalline Al–Fe alloy processed by mechanical alloying and spark plasma sintering. Acta Mater. 2009, 57, 3529–3538. [Google Scholar] [CrossRef]
- Bouaziz, O.; Estrin, Y.; Bréchet, Y.; Embury, J.D. Critical grain size for dislocation storage and consequences for strain hardening of nanocrystalline materials. Scr. Mater. 2010, 63, 477–479. [Google Scholar] [CrossRef]
- Crocker, A.G.; Elvidge, A.M.; Flewitt, P.E.J. Structure and properties of polycrystalline materials. Phys. Scr. 1987, 1987, 344–349. [Google Scholar] [CrossRef]
- Hanlon, T.; Tabachnikova, E.D.; Suresh, S. Fatigue behavior of nanocrystalline metals and alloys. Int. J. Fatigue 2005, 27, 1147–1158. [Google Scholar] [CrossRef]
- Birringer, R. Nanocrystalline materials. Mater. Sci. Eng. A 1989, 117, 33–43. [Google Scholar] [CrossRef]
- Naik, S.N.; Walley, S.M. The Hall–Petch and inverse Hall–Petch relations and the hardness of nanocrystalline metals. J. Mater. Sci. 2020, 55, 2661–2681. [Google Scholar] [CrossRef]
- Dong, H.; Wen, B.; Melnik, R. Relative importance of grain boundaries and size effects in thermal conductivity of nanocrystalline materials. Sci. Rep. 2014, 4, 7037. [Google Scholar] [CrossRef] [PubMed]
- Esteves, L.; Witharamage, C.S.; Christudasjustus, J.; Walunj, G.; O’Brien, S.P.; Ryu, S.; Borkar, T.; Akans, R.E.; Gupta, R.K. Corrosion behavior of AA5083 produced by high-energy ball milling. J. Alloys Compd. 2021, 857, 158268. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, Y.; Zhang, J.; Kuang, C.; Yue, M.; Zhou, S. Grain size-dependent electrical resistivity of bulk nanocrystalline Gd metals. Prog. Nat. Sci. Mater. Int. 2013, 23, 18–22. [Google Scholar] [CrossRef]
- Jang, J.M.; Lee, W.; Ko, S.H. The effects of grain boundary structures on mechanical properties in nanocrystalline al alloy. Arch. Metall. Mater. 2021, 66, 971–975. [Google Scholar] [CrossRef]
- Qing, X.; Xingming, G. The scale effect on the yield strength of nanocrystalline materials. Int. J. Solids Struct. 2006, 43, 7793–7799. [Google Scholar] [CrossRef][Green Version]
- Gollapudi, S.; Rajulapati, K.V.; Charit, I.; Koch, C.C.; Scattergood, R.O.; Murty, K.L. Creep in nanocrystalline materials: Role of stress assisted grain growth. Mater. Sci. Eng. A 2010, 527, 5773–5781. [Google Scholar] [CrossRef]
- Kottada, R.S.; Chokshi, A.H. Low temperature compressive creep in electrodeposited nanocrystalline nickel. Scr. Mater. 2005, 53, 887–892. [Google Scholar] [CrossRef]
- Zhang, K.; Weertman, J.R.; Eastman, J.A. Rapid stress-driven grain coarsening in nanocrystalline Cu at ambient and cryogenic temperatures. Appl. Phys. Lett. 2005, 87, 1–4. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Lu, K. Hardness and strain rate sensitivity of nanocrystalline Cu. Scr. Mater. 2006, 54, 1913–1918. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Qiao, L. Tribocorrosion Behavior of Nanocrystalline Metals—A Review. Mater. Trans. 2015, 56, 1759–1763. [Google Scholar] [CrossRef]
- Yousefi, E.; Sharafi, S.; Irannejad, A. The structural, magnetic, and tribological properties of nanocrystalline Fe-Ni permalloy and Fe-Ni-TiO2 composite coatings produced by pulse electro co-deposition. J. Alloy. Compd. 2018, 753, 308–319. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Xu, R.; Mai, Y.; Zhang, L.; Jie, X. Microstructure and Properties of Nanocrystalline Ni-Mo Coatings Prepared by Ultrasound-Assisted Pulse Electrodeposition. J. Mater. Eng. Perform. 2021, 30, 1–12. [Google Scholar] [CrossRef]
- Druga, J.; Kašiarová, M.; Dobročka, E.; Zemanova, M. Corrosion and tribological properties of nanocrystalline pulse electrodeposited Ni–W alloy coatings. Trans. IMF 2017, 95, 39–45. [Google Scholar] [CrossRef]
- Hussein, M.; Ankah, N.; Kumar, A.M.; Azeem, M.; Saravanan, S.; Sorour, A.; Al Aqeeli, N. Mechanical, biocorrosion, and antibacterial properties of nanocrystalline TiN coating for orthopedic applications. Ceram. Int. 2020, 46, 18573–18583. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J.-M. Nanocrystalline TiC-reinforced H13 steel matrix nanocomposites fabricated by selective laser melting. Mater. Des. 2016, 96, 150–161. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska-Zemła, D.; Królikowski, A.; Garbacz, H. Enhancement of the corrosion resistance and mechanical properties of nanocrystalline titanium by low-temperature annealing. Corros. Sci. 2018, 147, 342–349. [Google Scholar] [CrossRef]
- Javadhesari, S.M.; Alipour, S.; Akbarpour, M. Biocompatibility, osseointegration, antibacterial and mechanical properties of nanocrystalline Ti-Cu alloy as a new orthopedic material. Colloids Surf. B Biointerfaces 2020, 189, 110889. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, W.; Shen, L.; Qiu, M.; Tian, Z.; Jiang, W. Effects of flexible friction on the properties of nanocrystalline nickel prepared by jet electrodeposition. Surf. Coat. Technol. 2018, 333, 87–95. [Google Scholar] [CrossRef]
- Firouzi-Nerbin, H.; Nasirpouri, F.; Moslehifard, E. Pulse electrodeposition and corrosion properties of nanocrystalline nickel-chromium alloy coatings on copper substrate. J. Alloy. Compd. 2020, 822, 153712. [Google Scholar] [CrossRef]
- Cheng, W.; Ge, W.; Yang, Q.; Qu, X. Study on the corrosion properties of nanocrystalline nickel electrodepositied by reverse pulse current. Appl. Surf. Sci. 2013, 276, 604–608. [Google Scholar] [CrossRef]
- Qin, L.; Lian, J.-S.; Jiang, Q. Effect of grain size on corrosion behavior of electrodeposited bulk nanocrystalline Ni. Trans. Nonferrous Met. Soc. China 2010, 20, 82–89. [Google Scholar] [CrossRef]
- Gupta, R.; Fabijanic, D.; Dorin, T.; Qiu, Y.; Wang, J.; Birbilis, N. Simultaneous improvement in the strength and corrosion resistance of Al via high-energy ball milling and Cr alloying. Mater. Des. 2015, 84, 270–276. [Google Scholar] [CrossRef]
- Koch, C.C.; Langdon, T.G.; Lavernia, E.J. Bulk Nanostructured Materials. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5181–5199. [Google Scholar] [CrossRef]
- Newbery, A.P.; Nutt, S.R.; Lavernia, E. Multi-scale Al 5083 for military vehicles with improved performance. JOM 2006, 58, 56–61. [Google Scholar] [CrossRef]
- Tschopp, M.A.; Murdoch, H.A.; Kecskes, L.J.; Darling, K.A. Bulk nanocrystalline metals: Review of the current state of the art and future opportunities for copper and copper alloys. JOM 2014, 66, 1000–1019. [Google Scholar] [CrossRef]
- Cao, C.; Yao, G.; Jiang, L.; Sokoluk, M.; Wang, X.; Ciston, J.; Javadi, A.; Guan, Z.; De Rosa, I.; Xie, W.; et al. Bulk ultrafine grained/nanocrystalline metals via slow cooling. Sci. Adv. 2019, 5, eaaw2398. [Google Scholar] [CrossRef] [PubMed]



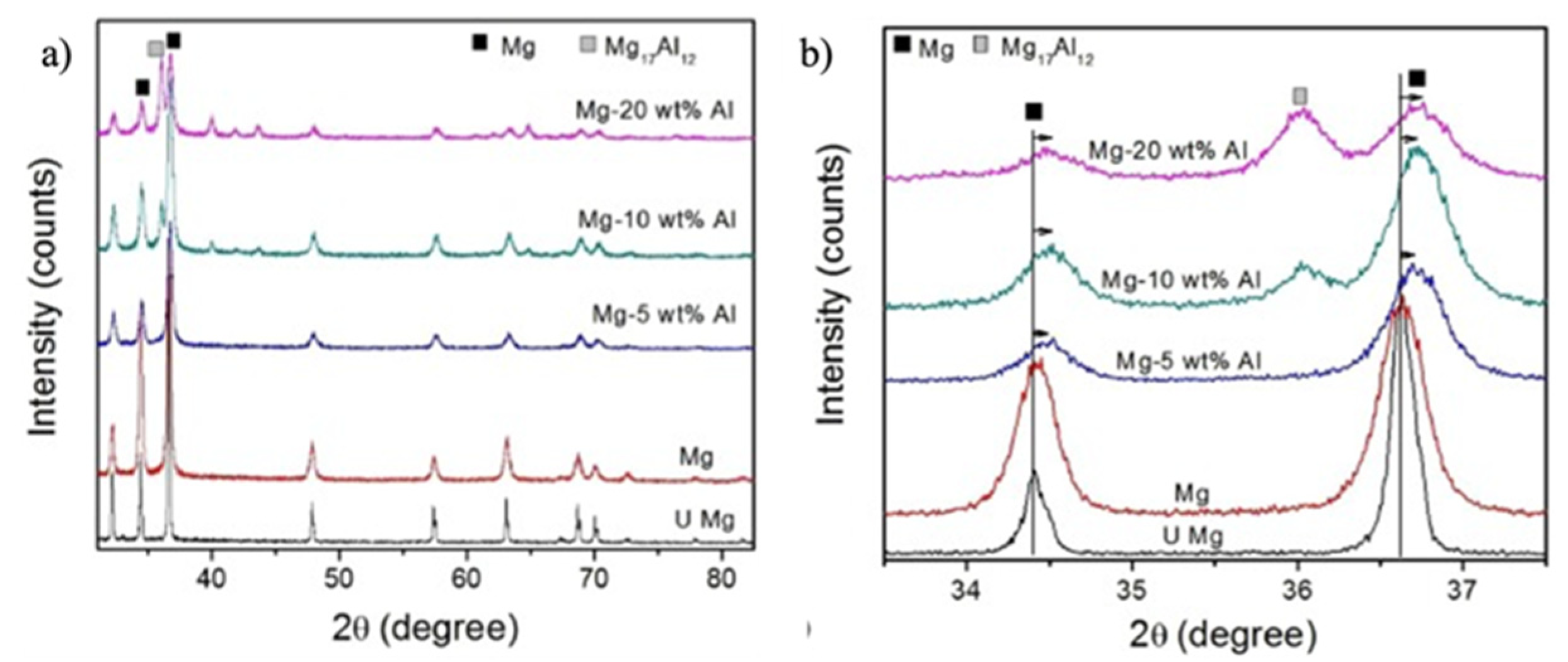
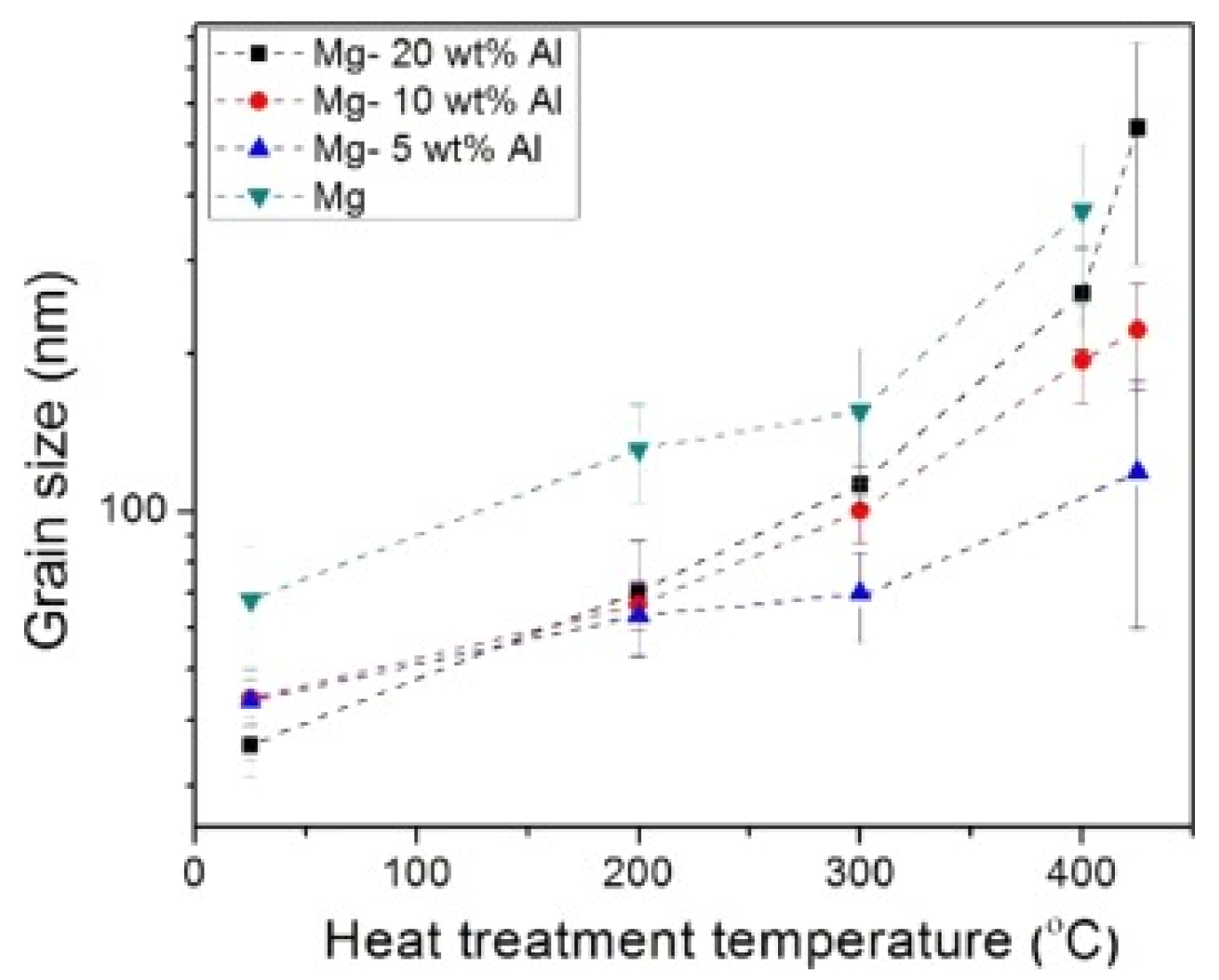


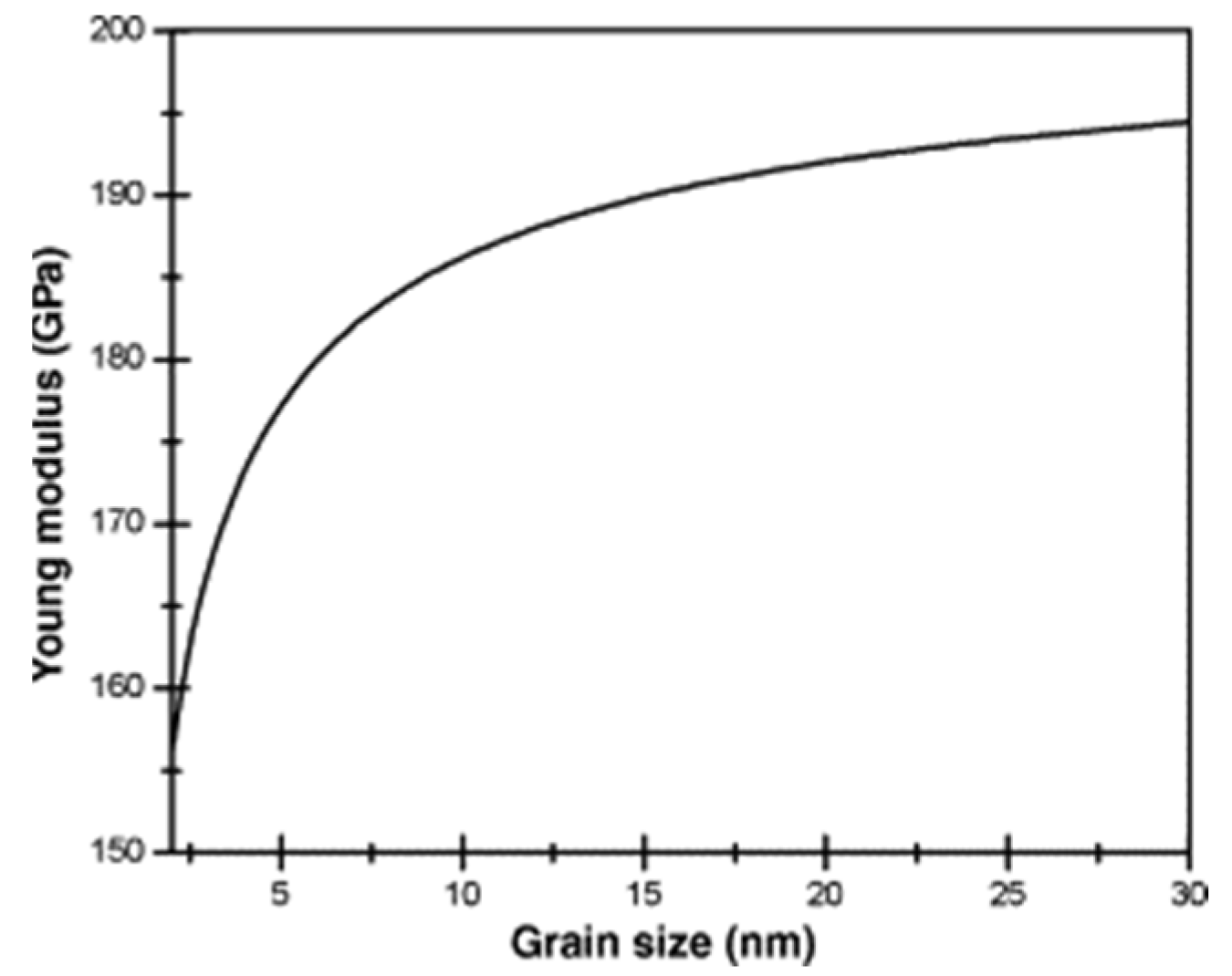
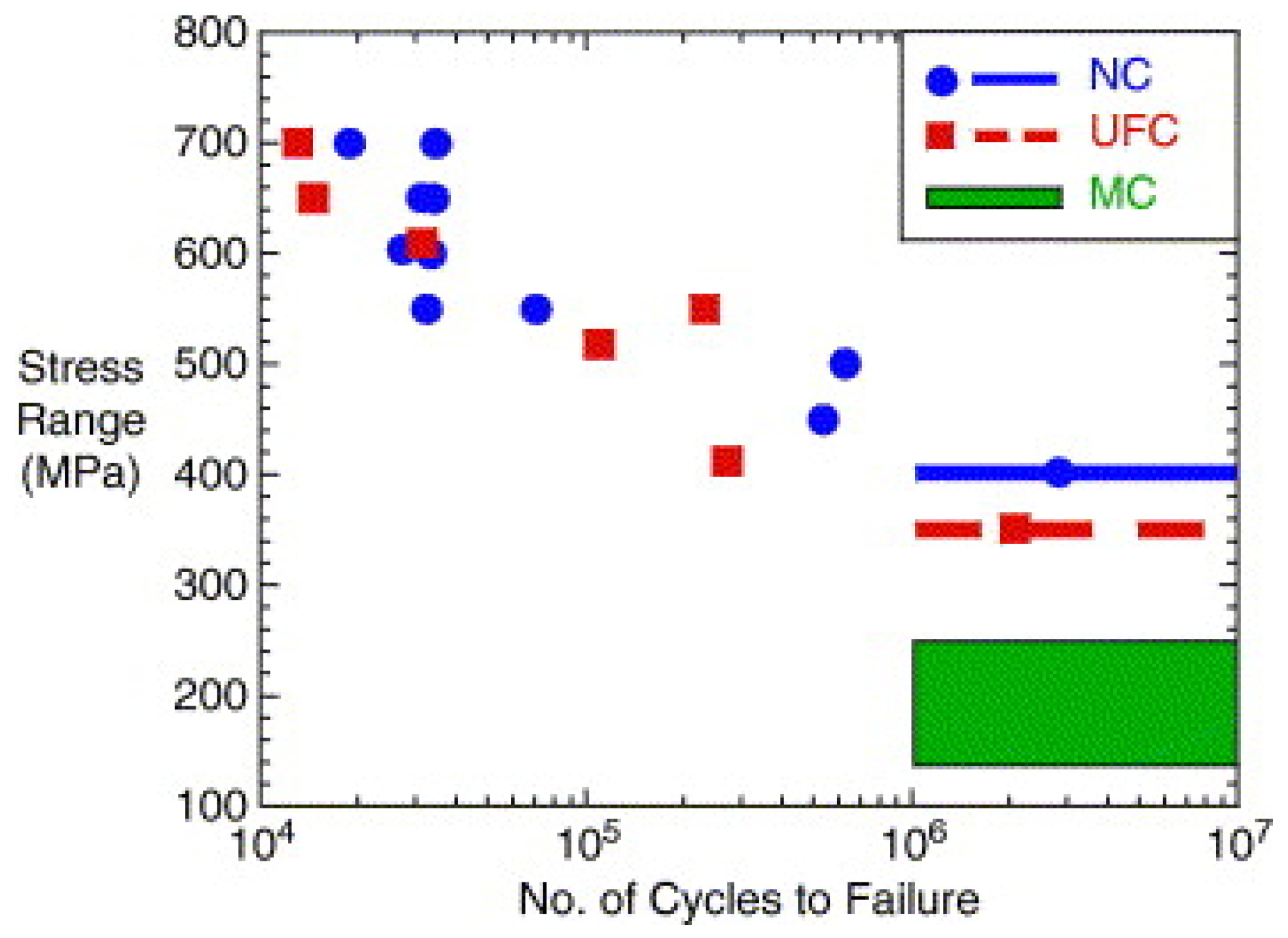

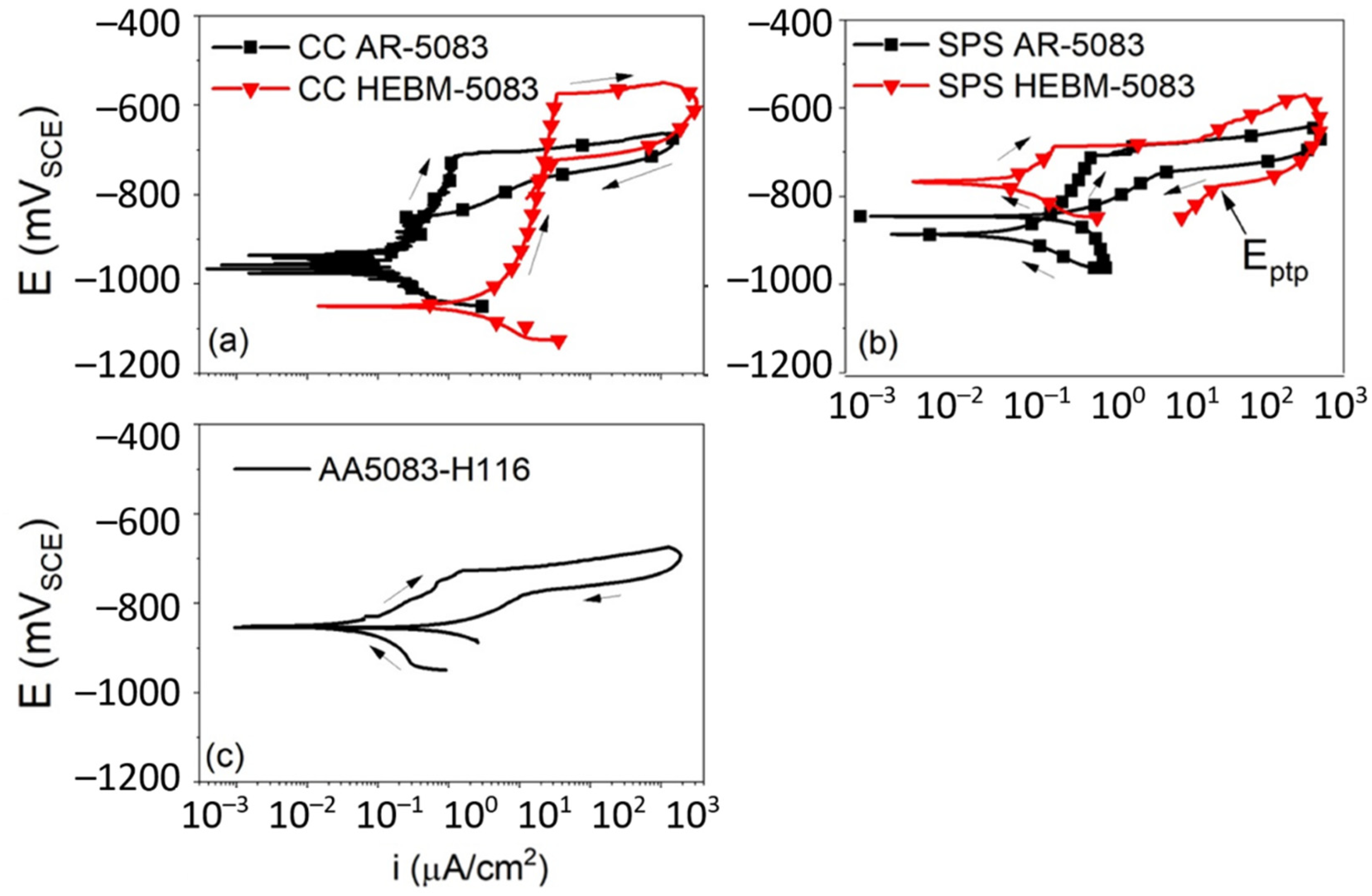
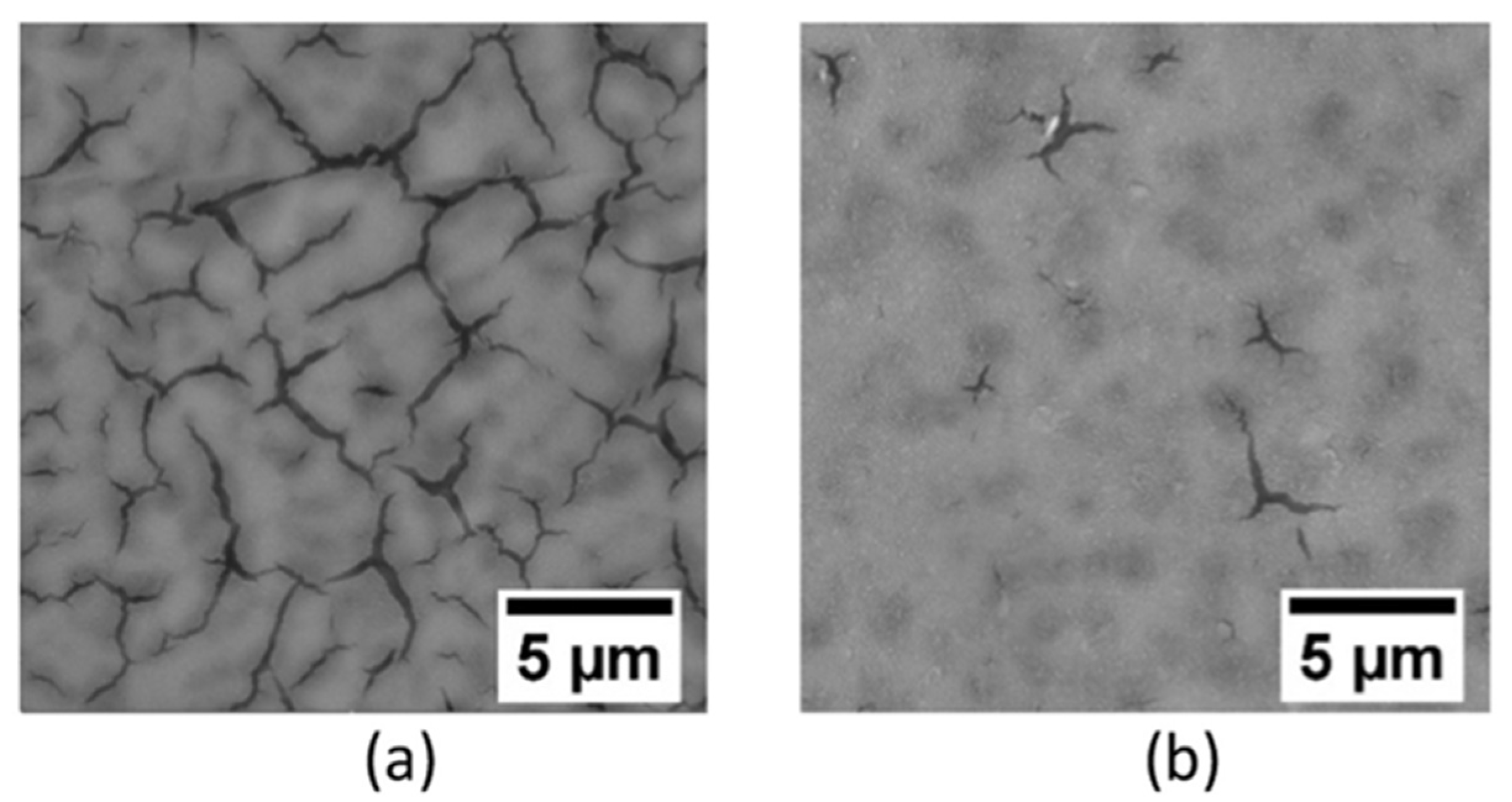

| Materials | Additives | Processing Route | Process Parameters | Outcomes |
|---|---|---|---|---|
| AA5083 [53] | Vanadium (0.5 and 5 wt. %) | HEBM followed by spark plasma sintering (SPS) or cold compaction (CC) |
SPS: 600 MPa pressure at 400 °C |
|
| Rhodostannite Cu2FeSn3S8 [52] | Ball milling, SPS |
|
| |
| Al 5083 [108] | Cryomilling, degassing, Hot isostatic pressing (HIP), and Extrusion |
|
| |
| AZ31 powders [109] | Cryomilling, |
|
| |
| 2024 Al alloy [106] | Spray atomization, Cryomilling, Sintering |
|
| |
| 5083 Al Alloy [110] | Gas Atomization, Cryomilling, CC, SPS |
|
| |
| Al [107] | Cryomilling |
|
| |
| AA5083 [46] | 0.15 wt. % graphite | Acoustic mixing, Cryomilling, Degassing, laser welding |
|
|
| Si [47] | Cryomilling, Etching, Functionalization |
|
| |
| Al [111] | 5 wt. % Mg | Cryomilling followed by SPS |
|
|
| Mechanical Properties | Increase/Decrease | Reference |
|---|---|---|
| Yield strength | Increases | [20,21,22] |
| Fracture strength | Increases | [20,21] |
| Ductility | Decreases | [35] |
| Specific heat | Increases | [30,31,120] |
| Hardness | Increases | [20,28,29,121] |
| Magnetic property | Increases | [58,59,60] |
| Thermal conductivity | Decreases | [122] |
| Fatigue life | Increases | [23,24,25,119] |
| Corrosion resistance | Increases | [53,123] |
| Electrical resistivity | Increases | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. https://doi.org/10.3390/cryst11111317
Kushwaha AK, John M, Misra M, Menezes PL. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals. 2021; 11(11):1317. https://doi.org/10.3390/cryst11111317
Chicago/Turabian StyleKushwaha, Amanendra K., Merbin John, Manoranjan Misra, and Pradeep L. Menezes. 2021. "Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications" Crystals 11, no. 11: 1317. https://doi.org/10.3390/cryst11111317
APA StyleKushwaha, A. K., John, M., Misra, M., & Menezes, P. L. (2021). Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals, 11(11), 1317. https://doi.org/10.3390/cryst11111317









