Curcumin Ameliorates the Cd-Induced Anxiety-like Behavior in Mice by Regulating Oxidative Stress and Neuro-Inflammatory Proteins in the Prefrontal Cortex Region of the Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Biochemical Reagents
2.2. Experimental Animals
- 1.
- Vehicle control = (1% carboxy methyl cellulose)
- 2.
- Cd control = (2.5 mg/kg of Cd for 60 days)
- 3.
- Cd + Cur20 = (2.5 mg/kg of Cd for 60 days and 20 mg/kg of curcumin for 30 days)
- 4.
- Cd + Cur40 = (2.5 mg/kg of Cd for 60 days and 40 mg/kg of curcumin for 30 days)
- 5.
- Cd + Cur80 = (2.5 mg/kg of Cd for 60 days and 80 mg/kg of curcumin for 30 days)
- 6.
- Cd + Cur160 = (2.5 mg/kg of Cd for 60 days and 160 mg/kg of curcumin for 30 days)
- 7.
- Cur160 = (160 mg/kg of curcumin for 30 days)
- 8.
- Cd + Pio30 = (2.5 mg/kg of Cd for 60 days and 30 mg/kg of pioglitazone for 30 days)
2.3. Behavior Studies
2.3.1. Y-Maze
2.3.2. Elevated Plus-Maze
2.4. Oxidative Stress Test
2.4.1. Lipid Peroxidation
2.4.2. SOD Activity
2.4.3. Catalase Activity
2.4.4. Glutathione Level
2.5. Analysis of Inflammatory Markers
2.6. Morphometric and Histopathological Analyses
2.7. Statistical Analysis
3. Results
3.1. Curcumin Increases the Body Weight in Cd Exposed Mice
3.2. Curcumin Improves Spatial Working Memory of Mice Exposed to Cd
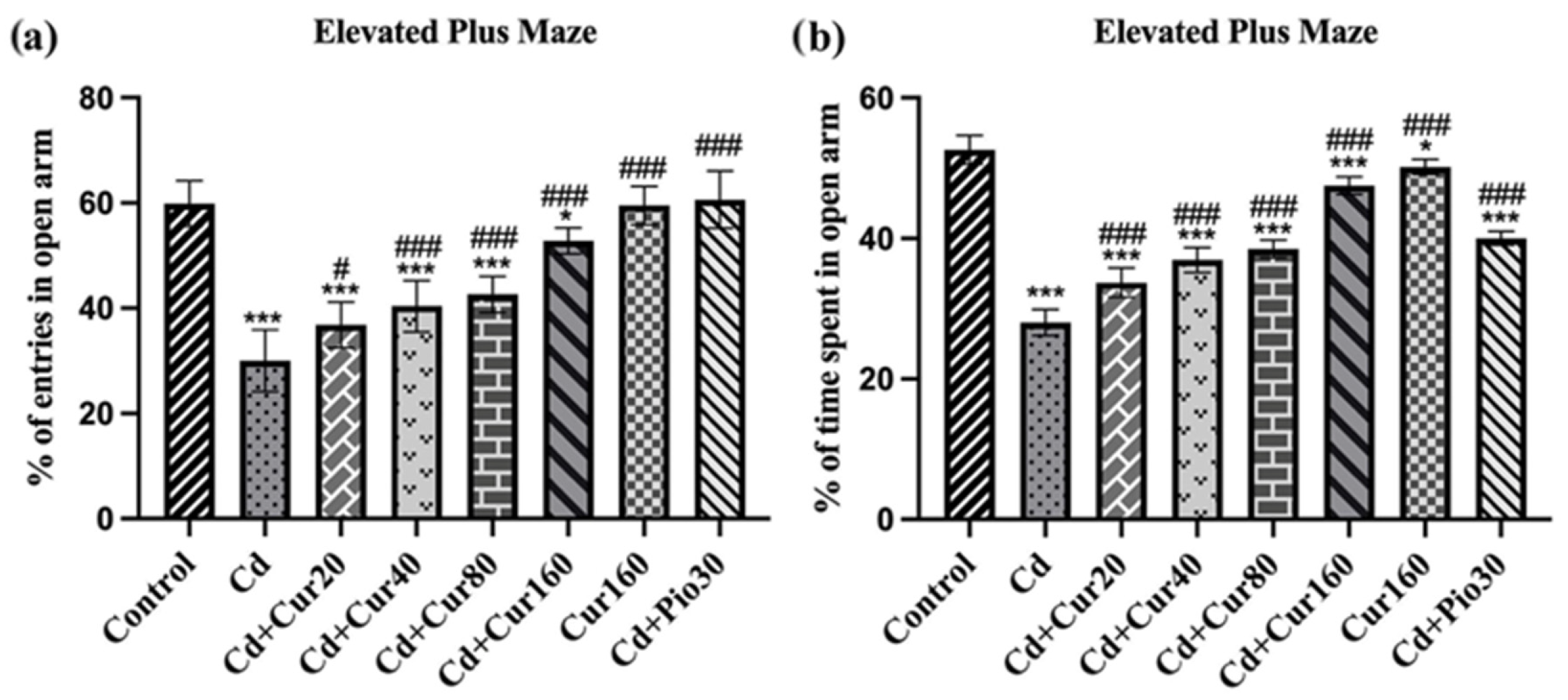
3.3. Curcumin Ameliorates Cd-Induced Anxiety-like Behavior
3.4. Curcumin Protects Cd-Induced Prefrontal Cortex Oxidative Stress
3.5. Curcumin Protects Cd-Induced Prefrontal Cortex Neuroinflammation
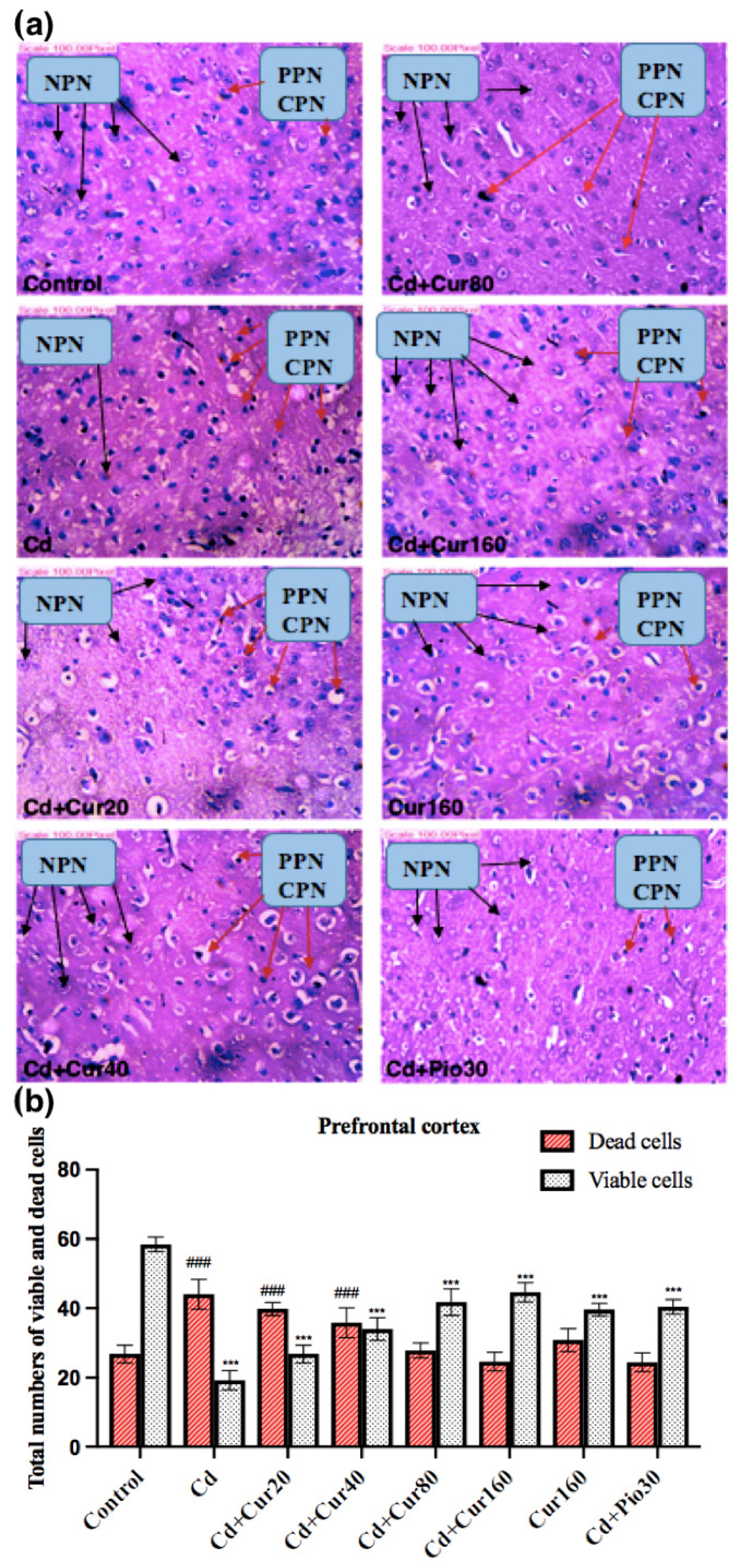
3.6. Curcumin Protects the Neurodegeneration Induced by Cd
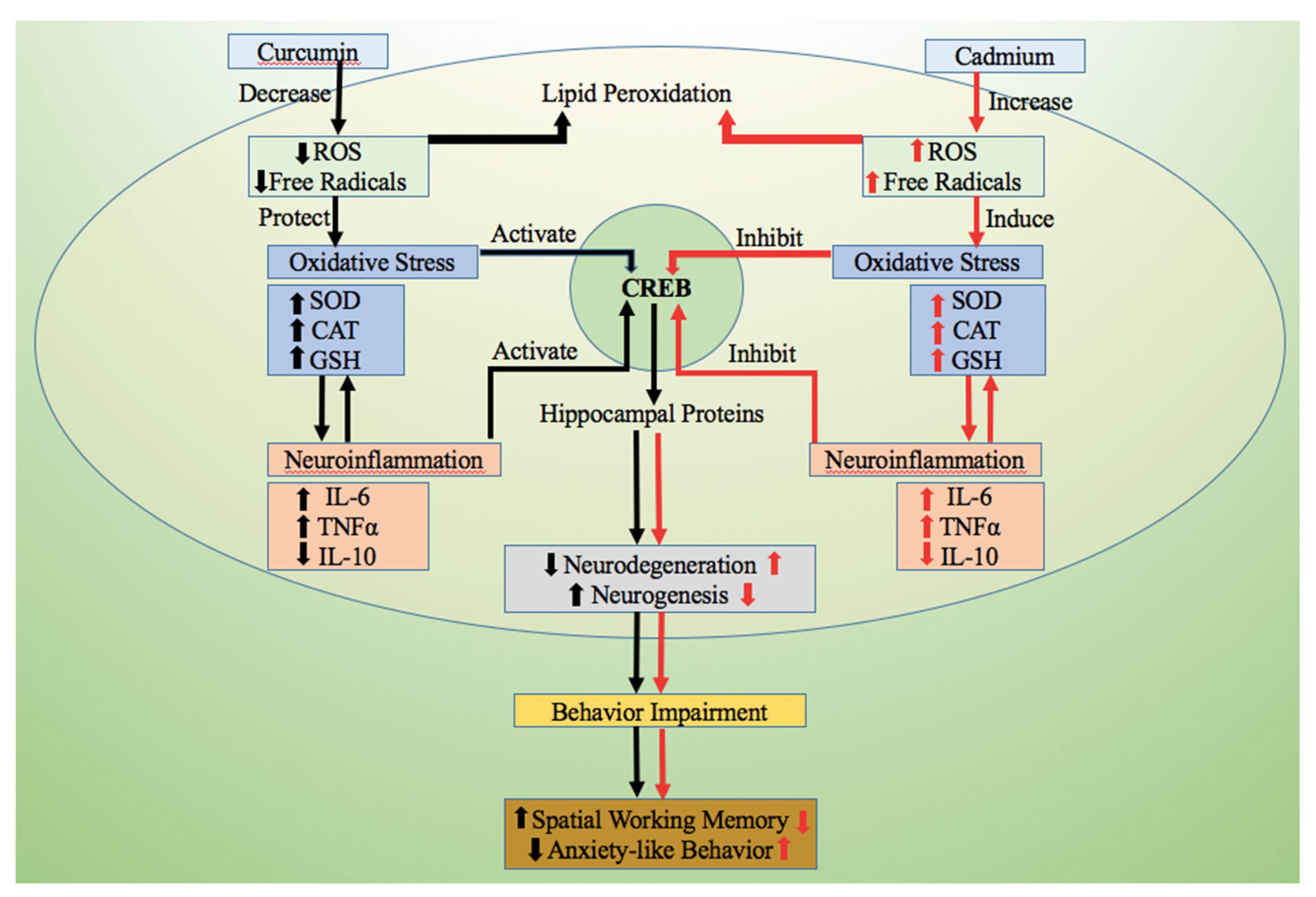
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.; Sharma, M.; Kumar, G.A.; Rao, N.G.; Prasad, K.; Mathur, P.; Dandona, L. The burden of neurological disorders across the states of India: The Global Burden of Disease Study 1990–2019. Lancet Glob. Health 2021, 9, e1129–e1144. [Google Scholar] [CrossRef]
- Sagar, R.; Dandona, R.; Gururaj, G.; Dhaliwal, R.S.; Singh, A.; Ferrari, A.; Dua, T.; Ganguli, A.; Varghese, M.; Chakma, J.K.; et al. The burden of mental disorders across the states of India: The global burden of disease study 1990–2017. Lancet Psychiatry 2020, 7, 148–161. [Google Scholar] [CrossRef] [Green Version]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sahebkar, A. The underlying role of oxidative stress in neurodegeneration: A mechanistic review. CNS Neurol. Disord. Drug Targets 2018, 17, 207–215. [Google Scholar] [CrossRef]
- Amraie, E.; Pouraboli, I.; Rajaei, Z. Neuroprotective effects of Levisticum officinale on LPS-induced spatial learning and memory impairments through neurotrophic, anti-inflammatory, and antioxidant properties. Food Funct. 2020, 11, 6608–6621. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef]
- Namgyal, D.; Sarwat, M. Saffron as a neuroprotective agent. In Saffron: The Age Old Panacea in New Light; Sarwat, M., Sumaiya, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–102. [Google Scholar]
- Fakhri, S.; Piri, S.; Moradi, S.Z.; Khan, H. Phytochemicals targeting oxidative stress, interconnected neuroinflammatory and neuroapoptotic pathways following radiation. Curr. Neuropharmacol. 2021, 8, 34370636. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chinnici, C.; Tang, H.; Trojanowski, J.Q.; Lee, V.M.; Praticò, D. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. J. Neuroinflamm. 2004, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 2020, 442, 152542. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Attaluri, S.; Kodali, M.; Shuai, B.; Shetty, G.A.; Upadhya, D.; Hattiangady, B.; Madhu, L.N.; Upadhya, R.; Bates, A.; et al. Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro-and systemic inflammation in a model of Gulf War Illness. Redox Biol. 2020, 28, 101389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Funahashi, S. Working memory in the prefrontal cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Penn, E.; Tracy, D.K. The drugs don’t work? Antidepressants and the current and future pharmacological management of depression. Ther. Adv. Psychopharmacol. 2012, 2, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.M.; Kesavapany, S. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Namgyal, D.; Chandan, K.; Ali, S.; Mehta, R.; Sarwat, M. Curcumin improves the behavior and memory in mice by modulating the core circadian genes and their associated micro-RNAs. J. Pharmacol. Pharmacother. 2020, 11, 44–52. [Google Scholar] [CrossRef]
- Namgyal, D.; Chandan, K.; Ali, S.; Ahmad, A.; Hashim, M.J.; Sarwat, M. Aberrant Lighting Causes Anxiety-like Behavior in Mice but Curcumin Ameliorates the Symptoms. Animals 2021, 11, 2590. [Google Scholar] [CrossRef]
- Namgyal, D.; Chandan, K.; Sultan, A.; Aftab, M.; Ali, S.; Mehta, R.; El-Serehy, H.A.; Al-Misned, F.A.; Sarwat, M. Dim light at night induced neurodegeneration and ameliorative effect of curcumin. Cells 2020, 9, 2093. [Google Scholar] [CrossRef]
- Santana-Martínez, R.A.; Silva-Islas, C.A.; Fernández-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. The therapeutic effect of curcumin in quinolinic acid-induced neurotoxicity in rats is associated with BDNF, ERK1/2, Nrf2, and antioxidant enzymes. Antioxidants 2019, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Prieur, E.A.; Jadavji, N.M. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio Protoc. 2019, 9, e3162. [Google Scholar] [CrossRef] [Green Version]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Wills, E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Luis, A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Wang, H.; Abel, G.M.; Storm, D.R.; Xia, Z. Cadmium exposure impairs adult hippocampal neurogenesis. Toxicol. Sci. 2019, 171, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Matsushita, M.T.; Zhang, L.; Abel, G.M.; Mommer, B.C.; Huddy, T.F.; Storm, D.R.; Xia, Z. Inducible and conditional stimulation of adult hippocampal neurogenesis rescues cadmium-induced impairments of adult hippocampal neurogenesis and hippocampus-dependent memory in mice. Toxicol. Sci. 2020, 177, 263–280. [Google Scholar] [CrossRef]
- Sant’Ana, M.G.; Moraes, R.; Bernardi, M.M. Toxicity of cadmium in Japanese quail: Evaluation of body weight, hepatic and renal function, and cellular immune response. Environ. Res. 2005, 99, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xum, Z.; Gaomm, Q.; Xu, Y.; Wang, B.; Dai, Y. Protective role of wogonin against cadmium induced testicular toxicity: Involvement of antioxidant, anti-inflammatory and anti-apoptotic pathways. Life Sci. 2020, 258, 118192. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Kedwany, F.S.; Baghdadi, H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and β-carotene. Food Chem. Toxicol. 2004, 42, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Hanai, H.; Tozawa, K.; Aoshi, T.; Uchijima, M.; Nagata, T.; Koide, Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid–induced colitis in mice. Gastroenterology 2002, 123, 1912–1922. [Google Scholar] [CrossRef] [Green Version]
- Billerey-Larmonier, C.; Uno, J.K.; Larmonier, N.; Midura, A.J.; Timmermann, B.; Ghishan, F.K.; Kiela, P.R. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm. Bowel Dis. 2008, 14, 780–793. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, L.; Abel, G.M.; Storm, D.R.; Xia, Z. Cadmium exposure impairs cognition and olfactory memory in male C57BL/6 mice. Toxicol. Sci. 2018, 161, 87–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Gao, X.; Luo, X.; Li, L.; Ma, M.; Zhu, Y.; Zhao, L.; Li, R. The effects of long-term exposure to low doses of cadmium on the health of the next generation of mice. Chem. Biol. Interact. 2019, 312, 108792. [Google Scholar] [CrossRef]
- Lamtai, M.; Azirar, S.; Zghari, O.; Ouakki, S.; El Hessni, A.; Mesfioui, A.; Ouichou, A. Melatonin ameliorates cadmium-induced affective and cognitive impairments and hippocampal oxidative stress in rat. Biol. Trace Elem. Res. 2021, 199, 1445–1455. [Google Scholar] [CrossRef]
- Shen, J.D.; Wei, Y.; Li, Y.J.; Qiao, J.Y.; Li, Y.C. Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress. Metab. Brain Dis. 2017, 32, 1163–1172. [Google Scholar] [CrossRef]
- Solanki, N.; Salvi, A.; Patki, G.; Salim, S. Modulating oxidative stress relieves stress-induced behavioral and cognitive impairments in rats. Int. J. Neuropsychopharmacol. 2017, 20, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Ju, B.; Zhang, Y.Z.; Yin, H.L.; Liu, Y.J.; Wang, S.S.; Zeng, Z.L.; Yang, X.P.; Wang, H.T.; Li, J.F. Protective effect of curcumin against oxidative stress-induced injury in rats with parkinson’s disease through the Wnt/β-catenin signaling pathway. Cell. Physiol. Biochem. 2017, 43, 2226–2241. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Saeed, K.; Khan, A.; Muhammad, T.; Khan, M.S.; Jo, M.G.; Rehman, S.U.; Kim, M.O. Natural dietary supplementation of curcumin protects mice brains against ethanol-induced oxidative stress-mediated neurodegeneration and memory impairment via Nrf2/TLR4/RAGE signaling. Nutrients 2019, 11, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, S.; Longobardim, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative effects of curcumin on the hepatotoxicity induced by Ochratoxin A in rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef]
- McManus, R.M.; Heneka, M.T. Role of neuroinflammation in neurodegeneration: New insights. Alzheimer’s Res. Ther. 2017, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Hao, R.; Song, X.; Li, F.; Tan, X.; Sun-Waterhouse, D.; Li, D. Caffeic acid phenethyl ester reversed cadmium-induced cell death in hippocampus and cortex and subsequent cognitive disorders in mice: Involvements of AMPK/SIRT1 pathway and amyloid-tau-neuroinflammation axis. Food Chem. Toxicol. 2020, 144, 111636. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Wang, W.; Song, M.; Sanidad, K.; Sukamtoh, E.; Zheng, J.; Tian, L.; Xiao, H.; Liu, Z.; et al. Structure–activity relationship of curcumin: Role of the methoxy group in anti-inflammatory and anticolitis effects of curcumin. J. Agric. Food Chem. 2017, 65, 4509–4515. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci. 2018, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H.; et al. Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Afifi, O.K.; Embaby, A.S. Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. J. Microsc. Ultrastruct. 2016, 4, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namgyal, D.; Ali, S.; Hussain, M.D.; Kazi, M.; Ahmad, A.; Sarwat, M. Curcumin Ameliorates the Cd-Induced Anxiety-like Behavior in Mice by Regulating Oxidative Stress and Neuro-Inflammatory Proteins in the Prefrontal Cortex Region of the Brain. Antioxidants 2021, 10, 1710. https://doi.org/10.3390/antiox10111710
Namgyal D, Ali S, Hussain MD, Kazi M, Ahmad A, Sarwat M. Curcumin Ameliorates the Cd-Induced Anxiety-like Behavior in Mice by Regulating Oxidative Stress and Neuro-Inflammatory Proteins in the Prefrontal Cortex Region of the Brain. Antioxidants. 2021; 10(11):1710. https://doi.org/10.3390/antiox10111710
Chicago/Turabian StyleNamgyal, Dhondup, Sher Ali, Muhammad Delwar Hussain, Mohsin Kazi, Ajaz Ahmad, and Maryam Sarwat. 2021. "Curcumin Ameliorates the Cd-Induced Anxiety-like Behavior in Mice by Regulating Oxidative Stress and Neuro-Inflammatory Proteins in the Prefrontal Cortex Region of the Brain" Antioxidants 10, no. 11: 1710. https://doi.org/10.3390/antiox10111710






