Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives
Abstract
:1. Introduction
2. Structure
3. Bioavailability Studies
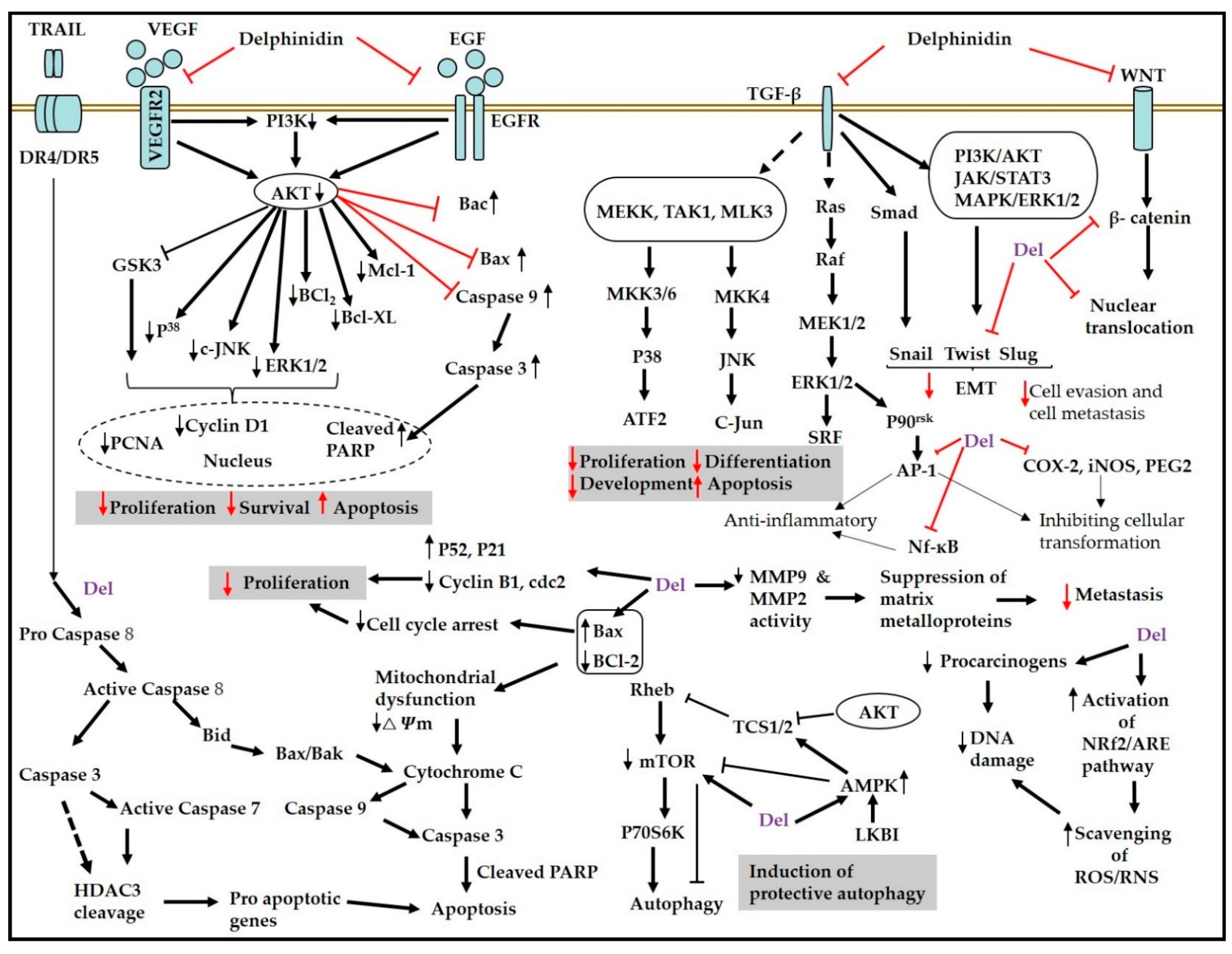
4. Delphinidin and Anticancer Activities
4.1. Effect of Delphinidin against Prostate Cancer
4.2. Effect of Delphinidin against Ovarian Cancer
4.3. Effect of Delphinidin against Colorectal Cancer
4.4. Effect of Delphinidin against Lung Cancer
4.5. Effect of Delphinidin against Skin Cancer
4.6. Effect of Delphinidin against Breast Cancer
4.7. Effect of Delphinidin against Hepatic Cancer
4.8. Effect of Delphinidin against Leukemia
4.9. Effect of Delphinidin against Bladder and Mesenchymal Tumors
4.10. Effect of Delphinidin against Glioma
4.11. Effect of Delphinidin against Osteosarcoma (OS)
5. Concluding Remarks, Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pieńkowska, N.; Bartosz, G.; Furdak, P.; Sadowska-Bartosz, I. Delphinidin Increases the Sensitivity of Ovarian Cancer Cell Lines to 3-Bromopyruvate. Int. J. Mol. Sci. 2021, 22, 709. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.; Chun, O. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Ozbay, T.; Nahta, R. Delphinidin inhibits HER2 and Erk1/2 signaling and suppresses growth of HER2-overexpressing and triple negative breast cancer cell lines. Breast Cancer Basic Clin. Res. 2011, 5, 143–154. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Park, M.; Sharma, A.; Lee, H.-J. Anti-adipogenic effects of delphinidin-3-O-β-glucoside in 3T3-L1 preadipocytes and primary white adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. GLOBOCAN 2018: Incidence and Mortality Worldwide: IARC CancerToday. Available online: https://gco.iarc.fr/today/home%202018 (accessed on 1 July 2021).
- Stein, C.J.; Colditz, G. Modifiable risk factors for cancer. Br. J. Cancer 2004, 90, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Mukhtar, H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010, 293, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bars-Cortina, D.; Sakhawat, A.; Piñol-Felis, C.; Motilva, M.-J. Chemopreventive effects of anthocyanins on colorectal and breast cancer: A review. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dharmawansa, K.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2016, 214, 119–128. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol. Nutr. Food Res. 2017, 61, 1700405. [Google Scholar] [CrossRef]
- Wang, X.; Kang, Y.; Li, J.; Jing, L.; Zhang, Y. Antiproliferative and apoptosis inducing effect of delphinidin against human bladder cancer cell line. Pharmacogn. Mag. 2021, 17, 101. [Google Scholar] [CrossRef]
- Kang, H.-M.; Park, B.-S.; Kang, H.-K.; Park, H.R.; Yu, S.-B.; Kim, I.-R. Delphinidin induces apoptosis and inhibits epithelial-to-mesenchymal transition via the ERK/p38 MAPK-signaling pathway in human osteosarcoma cell lines. Environ. Toxicol. 2018, 33, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Song, G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncol. Lett. 2017, 14, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Jeong, Y.-J.; Cho, H.-J.; Hoe, H.-S.; Park, K.-K.; Park, Y.-Y.; Choi, Y.H.; Kim, C.-H.; Chang, H.-W.; Park, Y.-J.; et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol. Rep. 2016, 37, 777–784. [Google Scholar] [CrossRef]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Agarwal, J.; Athar, M.; Elmets, C.A.; Afaq, F. Delphinidin Reduces Cell Proliferation and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells by Targeting EGFR/VEGFR2 Signaling Pathways. PLoS ONE 2013, 8, e77270. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Yun, J.-M. Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin. J. Cancer Prev. 2016, 21, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.-C.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.-N. Anthocyanin Delphinidin Prevents Neoplastic Transformation of Mouse Skin JB6 P+ Cells: Epigenetic Re-activation of Nrf2-ARE Pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Kwon, J.Y.; Hwang, M.K.; Rogozin, E.A.; Heo, Y.-S.; Bode, A.M.; Lee, H.J.; Dong, Z. Delphinidin Attenuates Neoplastic Transformation in JB6 Cl41 Mouse Epidermal Cells by Blocking Raf/Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase Signaling. Cancer Prev. Res. 2008, 1, 522–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.-H.; Ko, H.; Jeon, H.; Sung, G.-J.; Park, S.-Y.; Jun, W.J.; Lee, Y.-H.; Lee, J.; Lee, S.-W.; Yoon, H.-G.Y.; et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget 2016, 7, 56767–56780. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2016, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.; Jeong, M.-H.; Jeon, H.; Sung, G.-J.; So, Y.; Kim, I.; Son, J.; Lee, S.-W.; Yoon, H.-G.; Choi, K.-C. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget 2015, 6, 9970–9984. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. The diverse and complex roles of NF-κB subunits in cancer. Nat. Rev. Cancer 2012, 12, 121–132. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-κB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef] [Green Version]
- Koni, M.; Pinnarò, V.; Brizzi, M.F. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int. J. Mol. Sci. 2020, 21, 7697. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, W.; Zhang, Q.; Wu, Y.; Ni, S.; Tan, C.; Xu, M.; Sun, H.; Liu, C.; Wei, P.; et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J. Hematol. Oncol. 2018, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Tang, S. WNT/β-catenin signaling in the development of liver cancers. Biomed. Pharmacother. 2020, 132, 110851. [Google Scholar] [CrossRef]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, J.; Sanderson, J.T. Growth Inhibitory, Antiandrogenic, and Pro-apoptotic Effects of Punicic Acid in LNCaP Human Prostate Cancer Cells. J. Agric. Food Chem. 2010, 58, 12149–12156. [Google Scholar] [CrossRef]
- Au, C.W.; Siu, M.K.; Liao, X.; Wong, E.S.; Ngan, H.Y.; Tam, K.F.; Chan, D.C.; Chan, Q.K.; Cheung, A.N. Tyrosine kinase B receptor and BDNF expression in ovarian cancers–Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009, 281, 151–161. [Google Scholar] [CrossRef]
- Kuzniewska, B.; Rejmak, E.; Malik, A.R.; Jaworski, J.; Kaczmarek, L.; Kalita, K. Brain-Derived Neurotrophic Factor Induces Matrix Metalloproteinase 9 Expression in Neurons via the Serum Response Factor/c-Fos Pathway. Mol. Cell. Biol. 2013, 33, 2149–2162. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.-C.; Kim, H.; Kim, Y.-J.; Park, S.-H.; Song, J.-H.; Lee, K.H.; Lee, I.H.; Lee, Y.-K.; So, K.A.; Choi, K.-C.; et al. Delphinidin inhibits BDNF-induced migration and invasion in SKOV3 ovarian cancer cells. Bioorganic Med. Chem. Lett. 2017, 27, 5337–5343. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.; Jeong, W.; Song, G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol. Cell. Endocrinol. 2016, 422, 172–181. [Google Scholar] [CrossRef]
- Xie, H.; Tong, G.; Zhang, Y.; Liang, S.; Tang, K.; Yang, Q. PGK1 Drives Hepatocellular Carcinoma Metastasis by Enhancing Metabolic Process. Int. J. Mol. Sci. 2017, 18, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.H.; Lee, I.A.; Ha, Y.R.; Lim, J.; Sung, M.-K.; Lee, S.-J.; Kim, J.-S. PGK1 Induction by a Hydrogen Peroxide Treatment Is Suppressed by Antioxidants in Human Colon Carcinoma Cells. Biosci. Biotechnol. Biochem. 2008, 72, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.M.; Afaq, F.; Khan, N.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. 2009, 48, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Pan, Y.; Zhao, Y.; Ren, M.; Li, Y.; Lu, G.; Wu, K.; He, S. Delphinidin modulates JAK/STAT3 and MAPKinase signaling to induce apoptosis in HCT116 cells. Environ. Toxicol. 2021, 36, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Z.; Zhou, H.; Yue, J.; Mu, T.; Zhang, Q.; Bi, X. Upregulation of DKK3 by miR-483-3p plays an important role in the chemoprevention of colorectal cancer mediated by black raspberry anthocyanins. Mol. Carcinog. 2019, 59, 168–178. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, C.-H.; Hung, T.-W.; Lin, Y.-C.; Wang, C.-J.; Kao, S.-H. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Sci. Rep. 2019, 9, 18954. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazewski, C.; Liang, K.; de Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; de Mejia, E.G. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 2018, 242, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Kim, M.S.; De Mejia, E.G. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef] [Green Version]
- Cvorovic, J.; Tramer, F.; Granzotto, M.; Candussio, L.; Decorti, G.; Passamonti, S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch. Biochem. Biophys. 2010, 501, 151–157. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [Green Version]
- Oak, M.H.; Bedoui, J.; Madeira, S.F.; Chalupsky, K.; Schini-Kerth, V. Delphinidin and cyanidin inhibit PDGFAB-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br. J. Pharmacol. 2006, 149, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuyuki, S.; Fukui, S.; Watanabe, A.; Akune, S.; Tanabe, M.; Yoshida, K. Delphinidin Induces Autolysosome as well as Autophagosome Formation and Delphinidin-Induced Autophagy Exerts a Cell Protective Role. J. Biochem. Mol. Toxicol. 2012, 26, 445–453. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Zhang, W.; Peng, X.; Zhou, J.; Li, F.; Han, B.; Liu, X.; Ou, Y.; Yu, X. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer 2018, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Bak, D.-H.; Chung, B.Y.; Bai, H.-W.; Kang, B.S. Delphinidin enhances radio-therapeutic effects via autophagy induction and JNK/MAPK pathway activation in non-small cell lung cancer. Korean J. Physiol. Pharmacol. 2020, 24, 413–422. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Irrazabal, T.; Thakur, B.K.; Kang, M.; Malaise, Y.; Streutker, C.; Wong, E.O.Y.; Copeland, J.; Gryfe, R.; Guttman, D.S.; Navarre, W.W.; et al. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nat. Commun. 2020, 11, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Abu Zaid, M.; Mukhtar, H. Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Protects Human HaCaT Keratinocytes and Mouse Skin Against UVB-Mediated Oxidative Stress and Apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobiepanek, A.; Milner-Krawczyk, M.; Bobecka-Wesołowska, K.; Kobiela, T. The effect of delphinidin on the mechanical properties of keratinocytes exposed to UVB radiation. J. Photochem. Photobiol. B Biol. 2016, 164, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-G.; Jung, S.K.; Kim, J.-E.; Kim, Y.; Lee, H.J.; Jang, T.S.; Lee, K.W. NADPH oxidase is a novel target of delphinidin for the inhibition of UVB-induced MMP-1 expression in human dermal fibroblasts. Exp. Dermatol. 2013, 22, 428–430. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.-X.; Kai, K.; Li, J.-J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 2003, 25, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-Y.; Yun, S.-M.; Song, M.-Y.; Jung, K.; Kim, E.-H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef] [Green Version]
- Syed, D.N.; Afaq, F.; Sarfaraz, S.; Khan, N.; Kedlaya, R.; Setaluri, V.; Mukhtar, H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol. Appl. Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Mitri, Z.; Constantine, T.; O'Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef] [Green Version]
- Patidar, K.; Deshmukh, A.; Bandaru, S.; Lakkaraju, C.; Girdhar, A.; Gutlapalli, V.; Banerjee, T.; Nayarisseri, A.; Singh, S.K. Virtual Screening Approaches in Identification of Bioactive Compounds Akin to Delphinidin as Potential HER2 Inhibitors for the Treatment of Breast Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 2291–2295. [Google Scholar] [CrossRef] [Green Version]
- Zada, S.; Hwang, J.S.; Ahmed, M.; Lai, T.H.; Pham, T.M.; Elashkar, O.; Kim, D.R. Cross talk between autophagy and oncogenic signaling pathways and implications for cancer therapy. Biochim. Biophys. Acta (BBA) Bioenerg. 2021, 1876, 188565. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; Azevedo, J.; Soares, S.; Calhau, C.; Freitas, V.; Mateus, N. Influence of Anthocyanins, Derivative Pigments and Other Catechol and Pyrogallol-Type Phenolics on Breast Cancer Cell Proliferation. J. Agric. Food Chem. 2010, 58, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-Y.; Lee, S.-H.; An, I.-J.; Lee, H.-N.; Kim, H.-R.; Park, Y.-S.; Park, B.-K.; Kim, B.-S.; Kim, S.-K.; Cho, S.-D.; et al. Effects of Delphinidin in Anthocyanin on MDA-MB-231 Breast Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2014, 43, 231–237. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Im, N.-K.; Jang, W.J.; Jeong, C.-H.; Jeong, G.-S. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-κB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food 2014, 17, 855–861. [Google Scholar] [CrossRef]
- Hauptman, N.; Glavač, D. Long Non-Coding RNA in Cancer. Int. J. Mol. Sci. 2013, 14, 4655–4669. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Luo, E.; Liu, X.; Han, B.; Yu, X.; Peng, X. Delphinidin-3-glucoside suppresses breast carcinogenesis by inactivating the Akt/HOTAIR signaling pathway. BMC Cancer 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Peng, X.; Cheng, D.; Zhu, Y.; Du, J.; Li, J.; Yu, X. Delphinidin suppresses breast carcinogenesis through the HOTAIR /micro RNA -34a axis. Cancer Sci. 2019, 110, 3089–3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Yang, M.; Jiang, R.; An, N.; Wang, X.; Liu, B. Long Non-Coding RNA HOTAIR Regulates the Proliferation, Self-Renewal Capacity, Tumor Formation and Migration of the Cancer Stem-Like Cell (CSC) Subpopulation Enriched from Breast Cancer Cells. PLoS ONE 2017, 12, e0170860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, W.; Rothley, M.; Teller, N.; Jung, N.; Bulat, B.; Plaumann, D.; Vanderheiden, S.; Schmaus, A.; Cremers, N.; Göppert, B.; et al. Delphinidin is a novel inhibitor of lymphangiogenesis but promotes mammary tumor growth and metastasis formation in syngeneic experimental rats. Carcinogenesis 2013, 34, 2804–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-H.; Huang, C.-C.; Hung, C.-H.; Yao, F.-Y.; Wang, C.-J.; Chang, Y.-C. Delphinidin-rich extracts of Hibiscus sabdariffa L. trigger mitochondria-derived autophagy and necrosis through reactive oxygen species in human breast cancer cells. J. Funct. Foods 2016, 25, 279–290. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Yen, G.-C. Induction of Apoptosis by the Anthocyanidins through Regulation of Bcl-2 Gene and Activation of c-Jun N-Terminal Kinase Cascade in Hepatoma Cells. J. Agric. Food Chem. 2005, 53, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, S.Y.; Shi, Y.-H.; Fan, J.; Yin, X.-M. Delphinidin Induces Necrosis in Hepatocellular Carcinoma Cells in the Presence of 3-Methyladenine, an Autophagy Inhibitor. J. Agric. Food Chem. 2009, 58, 3957–3964. [Google Scholar] [CrossRef]
- Kimura, C.; Hayashi, M.; Mizuno, Y.; Oike, M. Endothelium-dependent epithelial–mesenchymal transition of tumor cells: Exclusive roles of transforming growth factor β1 and β2. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4470–4481. [Google Scholar] [CrossRef]
- Lim, W.C.; Kim, H.; Ko, H. Delphinidin inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma cells. J. Cell. Biochem. 2019, 120, 9887–9899. [Google Scholar] [CrossRef]
- Hou, D.-X.; Ose, T.; Lin, S.; Harazoro, K.; Imamura, I.; Kubo, M.; Uto, T.; Terahara, N.; Yoshimoto, M.; Fujii, M. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: Structure-activity relationship and mechanisms involved. Int. J. Oncol. 2003, 23, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, H.-P.; Hsu, J.-D.; Yang, S.-F.; Wang, C.-J. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol. Appl. Pharmacol. 2005, 205, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Tong, X.; Terahara, N.; Luo, D.; Fujii, M. Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces apoptosis in human leukemia cells through reactive oxygen species-mediated mitochondrial pathway. Arch. Biochem. Biophys. 2005, 440, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1·Cytochrome c Multimeric Complex Is a Functional Apoptosome That Activates Procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.C.; Huang, H.P.; Chang, K.T.; Wang, C.J.; Chang, Y.C. Anthocyanins from roselle extract arrest cell cycle G2/M phase transition via ATM/Chk pathway in p53-deficient leukemia HL-60 cells. Environ. Toxicol. 2017, 32, 1290–1304. [Google Scholar] [CrossRef]
- Takasawa, R.; Saeki, K.; Tao, A.; Yoshimori, A.; Uchiro, H.; Fujiwara, M.; Tanuma, S.-I. Delphinidin, a dietary anthocyanidin in berry fruits, inhibits human glyoxalase I. Bioorganic Med. Chem. 2010, 18, 7029–7033. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Sharif, T.; Kayali, A.; Abbas, M.; Dandache, I.; Etienne-Selloum, N.; Kevers, C.; Pincemail, J.; Auger, C.; Chabert, P.; et al. Delphinidin-3-O-glucoside and delphinidin-3-O-rutinoside mediate the redox-sensitive caspase 3-related pro-apoptotic effect of blackcurrant juice on leukaemia Jurkat cells. J. Funct. Foods 2015, 17, 847–856. [Google Scholar] [CrossRef]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. The Green Anti-Cancer Weapon. The Role of Natural Compounds in Bladder Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 7787. [Google Scholar] [CrossRef]
- Chamie, K.; Litwin, M.; Bassett, J.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S.; the Urologic Diseases in America Project. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [Green Version]
- Filipiak, K.; Hidalgo, M.; Silvan, J.M.; Fabre, B.; Carbajo, R.J.; Pineda-Lucena, A.; Ramos, A.; de Pascual-Teresa, B.; de Pascual-Teresa, S. Dietary gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct. 2013, 5, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bier, A.; Giladi, N.; Kronfeld, N.; Lee, H.K.; Cazacu, S.; Finniss, S.; Xiang, C.; Poisson, L.; Decarvalho, A.C.; Slavin, S.; et al. MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1. Oncotarget 2013, 4, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Wang, H.; Li, Y.; Yan, W.; Han, L.; Zhang, K.; Zhang, J.; Wang, Y.; Feng, Y.; et al. miR-137 is frequently down-regulated in glioblastoma and is a negative regulator of Cox-2. Eur. J. Cancer 2012, 48, 3104–3111. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Direct transfection of miR-137 mimics is more effective than DNA demethylation of miR-137 promoter to augment anti-tumor mechanisms of delphinidin in human glioblastoma U87MG and LN18 cells. Gene 2015, 573, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, L.M.; Roberts, A.B. TGF-β signaling: Positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002, 12, 22–29. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.-H.; Joo, C.-K. TGF-β1 regulates cell fate during epithelial–mesenchymal transition by upregulating survivin. Cell Death Dis. 2013, 4, e714. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, K. Transforming growth factor-β signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B 2009, 85, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouanouki, A.; Lamy, S.; Annabi, B. Anthocyanidins inhibit epithelial-mesenchymal transition through a TGFβ/Smad2 signaling pathway in glioblastoma cells. Mol. Carcinog. 2016, 56, 1088–1099. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, Y.-J.; Hwang, S.-C.; Kim, K.-D.; Moon, D.-K.; Kim, D.-H. Cytotoxic effects of delphinidin in human osteosarcoma cells. Acta Orthop. Traumatol. Turc. 2018, 52, 58–64. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F. Nanoparticle formulations to enhance tumor targeting of poorly soluble polyphenols with potential anticancer properties. Semin. Cancer Biol. 2017, 46, 205–214. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [Green Version]
- Assadpour, E.; Jafari, S.; Esfanjani, A. Protection of phenolic compounds within nanocarriers. CAB Rev. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC Adv. 2019, 9, 10842–10853. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Högger, P. Stability of Dietary Polyphenols under the Cell Culture Conditions: Avoiding Erroneous Conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A Randomized Clinical Trial Evaluating the Efficacy of an Anthocyanin–Maqui Berry Extract (Delphinol®) on Oxidative Stress Biomarkers. J. Am. Coll. Nutr. 2015, 34, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Portales, P.V. Delphinol® standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical trial. Panminerva Med. 2016, 58, 1–6. [Google Scholar] [PubMed]
- Shimizu, N.; Yamada, W.; Miyasaka, K.; Shimoda, H. Ameliorating Effects of Delphinol®, Anthocyanin Standardized Maqui Berry Extract, on Skin Brightness and Redness in Japanese Females: A Randomized Double-Blind Placebo-Controlled Pilot Study. J. Cosmet. Dermatol. Sci. Appl. 2020, 10, 149–162. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating Gut Microbiome with Bilberry Anthocyanin Combo to Enhance Anti-PD-L1 Efficiency against Murine Colon Cancer. Microorganisms 2020, 8, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ref. | Type | Cell/Animal | Intervention | Major Findings |
|---|---|---|---|---|
| Prostate Cancer | ||||
| [34] | In vitro | PC-3 | Del (30, 60, 90, 120, and 180 μmol/L), 48 h | ↓ Cell viability IC50 = 90 μmol/L, ↑ apoptosis, ↑ caspase-3, ↑ caspase-9, induction of PARP cleavage, ↑ BAX/Bcl-2 ratio, dose-dependent cell cycle arrest (G2/M phase), ↓ cyclin D1, ↓ cyclin A, ↓ cdk1, ↓ cdk2, and inhibited translocation as well as DNA binding of NF-κB and pIKKγ protein |
| In vivo | Athymic (nu/nu) male nude mice | Del (2 mg/animal) in 1:10 DMSO and normal saline, 3 times/week | No toxicity, inhibited tumorigenicity, ↓ Bcl-2 protein, ↑ BAX, ↓ cyclin D1 and NF-κB, and inhibited proliferation marker (Ki67 and PCNA) expression | |
| [25] | In vitro | PC-3 | Del (15, 30, 60, 120, 180, and 240 μM), 72 h | ↓ β-catenin and its target proteins—Axin2, LEF1, cyclin D1, c-myc, and TCF1—↑ E-cadherin |
| [32] | In vitro | LNCaP | Del (30, 60, and 90 μM) TRAIL (0, 50, 100, and 150 ng/mL), 12 h | Dose-dependent antiproliferative effect, altered cell and nucleus morphology, ↑ cleaved PARP, ↑ caspase-8, ↑ caspase-9, ↑ cleaved caspase-3, ↑ caspase-7, ↑ DR5, ↑ p21, ↑ BAX, ↓ Bcl-2, ↓ XIAP, ↓ MCL-1, ↓ cIAP-2, ↓ survival, and ↓HDAC3 |
| [29] | In vitro | LNCaP | Del (50, 100, and 150 μM), 24 h | ↑ Apoptosis, ↓ PARP-1, ↓HDAC3, ↑BAX, ↑PUMA, ↑p21, and ↑ p53 acetylation |
| [39] | In vitro | LNCaP | Del-3-glu (3, 10, 30, and 100 μM), 48 h | ↓ DHT, ↓AR, ↓PSA, and ↓SRD5A1 |
| Ovary Cancer | ||||
| [42] | In vitro | SKOV3 | BDNF (100 nM), 24 h Del (50 and 75 µΜ), 24 h | Dose-dependent inhibition of migration ability, ↓ MMP-2, ↓ MMP-9, and inhibited p-Akt and NF- κB translocation |
| [22] | In vitro | SKOV3 | Del (0.1, 1, and 10 µM), 48 h | ↓ p-Akt, ↓ pP70S6K, ↓ pS6, ↓ p-ERK1/2 MAPK, and ↓ p-P38 MAPK |
| [44] | In vitro | ES2 | Del (0.1, 1, 10, 50, and 100 µM), 48 h | ↓ Proliferation, ↓ metastasis, ↓ PI3K/Akt, and ↓ ERK1/2/JNK |
| Colorectal Cancer | ||||
| [46] | In vitro | HT-29 | H2O2 (50 μM) + Del 25 μg/mL, 24 h | ↓ PGK1 activity |
| [47] | In vitro | HCT-116 | Del (30, 60, 120, 180, and 240 μM), 48 h | Dose-dependent inhibition of viability, IC50 = 110 μM, ↑ apoptosis, ↑ cleaved PARP, ↓ procaspase-3, ↓ procaspase-8, ↓ procaspase-9, ↓ Bcl-2, ↑ BAX, ↑ G2/M phase arrest, ↑ p53, ↑ p21WAF1/Cip1, and ↓ NF-κB |
| [48] | In vitro | HCT-116 | Del (80, 100, and 120 μM), 48 h | Dose-dependent inhibition of viability, IC50 = 106 μM, ↑ BAX, ↑ caspase- 3, ↑ caspase-8, ↑ caspase- 9, ↑ cytochrome C, ↓ p-STAT-3, ↓ p-p38, and ↓ p-ERK1/2 |
| Colorectal Cancer | ||||
| [50] | In vitro | DLD-1, SW480, and SW620 | Del (20, 40, 60, 80, and 100 μM), 24 h | ↓ Migration, ↓ invasion, ↓ integrin αV/β3, ↓ FAK/Src/paxillin signaling, ↓ Snail, ↓ Twist, ↓ Slug, ↓ β-catenin, ↓ MMP-2, ↓ EMT, and ↑ E-cadherin |
| In vivo | Balb/c nude mice | DLD-1 implantation + Del 100 μM, 24 h | Reduced metastasis No change in liver weight | |
| [55] | In vitro | CRC cells (HCT-116, HT29); PBMCs pre-treated with anthocyanins and co-cultured with HCT-116 and HT-29 | D3G, cyanidin-3-O-glucoside and its metabolites (delphinidin chloride and GA) (100–600 μg/mL) | ↓ PD-L1 expression, ↓ PD-1 expression, and ↓ binding of PD-L1 to PD-1 |
| Lung Cancer | ||||
| [23] | In vitro | A549 | CoCl2 (200 µM) and EGF (20 ng/mL), Del (10, 20, and 40 µM), 24 h | ↓ Angiogenesis, ↓ HIF-1α, ↓VEGF, and ↓ PI3K/Akt/mTOR/p70S6K |
| [24] | In vitro | NSCLC | Del (5, 10, 20, 40, and 60 μM), 3 h | ↑ Caspase-3/9 and ↓ anti-apoptotic proteins (Bcl-2, Bcl-xL, and Mcl-1), ↑ Pro-apoptotic proteins (BAX and BAK) and ↓ EGFR as well as VEGFR2 |
| In vivo | Female athymic (nu/nu) nude mice | Del (1, 2 mg/animal) three times/week | Inhibited tumor growth, ↓ Ki67, ↓ PCNA, ↓CD31, ↓ VEGF, and apoptosis induction | |
| [63] | In vitro | NSCLC | Del (1, 5, 10, and 20 μM), 24 h + γ ray irradiation | ↑ Apoptosis, ↑ JNK/MAPK pathway, ↓ mTOR pathway, ↑ LC3-II, ↑ ATG5, and ↑ ATG12 |
| Ref. | Type | Cell/Animal | Intervention | Major Findings |
|---|---|---|---|---|
| Skin Cancer | ||||
| [67] | In vitro | HaCaT | Pretreatment Del (1, 5, 10, 15, and 20 μM), 24 h, and UVB (15–30 mJ/cm2), 24 h | ↑ Cell viability, ↓ apoptosis, ↓ cleaved PARP, ↓ lipid peroxidation, ↓ PCNA degradation, ↓ BAX, ↑ Bcl-2, and inhibited ↓ procaspase-3, -8, and -9 |
| In vivo | SKH-1 hairless mouse skin | Del 1 mg/0.1 mL DMSO and UVB (180 mJ/cm2) | ↓ CPDs, ↓ 8-OHdG, and ↓ apoptotic cells | |
| [68] | In vitro | HaCaT | Del (5 and 10 μM) and UVB (100 mJ/cm2) | ↑ Elastic modulus |
| [27] | In vitro | JB6 P+ | Del (5, 10, 20, and 40 μM), 30 min+ TPA 4 h | Del (10 μM) inhibited 43% neoplastic transformation, ↓ COX-2, ↓PGE2, ↓ AP-1, ↓ NF-κB, ↓ p-ERK, ↓ p-90RSK, ↓ p-MSK, suppressed Raf1 and MEK1 activities |
| [26] | In vitro | JB6 P+ | Del (10, 20, 40, 60, 80, and 100 μM), 5 days + TPA10 ng/mL | ↑ Nrf2, ↑ Hmox1, ↑ Nqo1, ↑ SOD1, ↓ CpG methylation, ↓ DNMTs, and ↓ HDACs |
| Breast Cancer | ||||
| [77] | In vitro | MCF-10A | Del (5, 10, 20, and 40 μM), 3 h + HGF (40 ng/mL), 30 min | ↓ Cell viability, suppressed p-Met, ↓ p-FAK, ↓ p-src, ↓ p-Crk, ↓ p-JNK, ↓ p-SHP-2, ↓ p-Gab1, and ↓ GRB2, inhibited Ras-ERK MAPK and PI3K/AKT NF-κB/p65 pathways |
| [5] | In vitro | HCC1806, MDA231, MDA468, SKBR3, MDA453, BT474, and MCF7 | Del (12.5, 25, 50, and 100 µg/mL), 6 days; effective at 50 µg/mL, 48 h | ↑ Apoptosis, blocked anchorage independent growth and migration, ↓ p-HER2, ↓ p-Akt, and ↓ p-ERK1/2 |
| [62] | In vitro | MDA-MB-453 and BT474 | Del (20, 40, 80 µM), 48 h | ↓ Proliferation, ↑ cleaved caspase-9 and -3, ↑ LC3-II/LC3-I, ↑ Atg5-Atg12, inhibited mTOR signaling, and activated AMPK signaling |
| [82] | In vitro | MCF-7 | Delphinidin-3-glucoside (12.5, 25, 50, and 100 µM), 48 h | ↑ Antiproliferative effect |
| [83] | In vitro | MDA-MB-231 | Del (12.5, 25, and 50 µM), 24 h | ↓ Proliferation concentration-dependent manner, ↑ p53, ↓ Bcl-2, and ↓ p-GSK3β |
| In vivo | Female nude mice | 10 mg/kg | ↑ Apoptosis | |
| [85] | In vitro | MCF-7 | Del (15, 30, 60, and 90 µM) + PMA (100 nM), 24 h | ↓ Invasion and migration, ↓ MMP-9, ↓ p-p38, ↓ p-JNK, inhibited translocation of p65, and ↑ IκBα |
| Breast Cancer | ||||
| [87] | In vitro | MCF10A | Del (10, 20, and 40 µM), 24 h | ↓ HOTAIR expression, ↓ Akt, and ↑ IRF1 |
| In vivo | Xenografted female BALB/c athymic mice | Del (40 mg/kg/day) | Inhibited breast tumor growth and ↓ HOTAIR expression | |
| [88] | In vitro | MDA-MB-231, MCF-7, and MDA-MB-453 | 40 μM, 24 h | ↓ HOTAIR expression, ↑ miR-34a, ↓ β-catenin, ↓ Gsk3β, ↓ c-Myc, ↓ cyclin-D1, and ↓ MMP-7 |
| In vivo | MNU-induced female SD rats | 100 mg/kg/rat/day | Lower (43.7%) cancer incidence, ↓ proliferation, and no adverse effect | |
| [91] | In vitro | MCF-7 | HA (0, 1, 2, and 3 mg/mL), 24 h | ↑ LC3-II, ↑ p-AMPK, and ↓ mitochondrial membrane potential |
| Hepatic Cancer | ||||
| [92] | In vitro | HepG2 | Del (50, 100, 150, and 200 μM), 24 h | ↑ BAX, ↓ Bcl-2, ↑ DNA fragmentation, ↑ LDH leakage, ↑ c-Jun, ↑ p-JNK, and ↑ intracellular ROS |
| [93] | In vitro | SMMC7721 | Del (100 and 150 μM), 24 h | ↑ LC3 lipidation and ↑ cellular vacuolization |
| [95] | In vitro | Huh7 and PLC/PRF/5 | Del (30, 40, 80, and 100 μM), 24 h or 48 h | EGF-induced ↓ EMT, ↓ EGFR, ↓ MMP2, ↓ ERK, ↓AKT, ↑ E-cadherin, ↓ vimentin, ↓ Snail, ↓ MMP-2, and ↓ EGFR/AKT/ERK |
| Leukemia | ||||
| [96] | In vitro | HL-60 | Del and anthocyanidins (100 μM), 6 h | ↑ c-Jun, ↑ p-JNK, ↑ cleaved caspase-3, and ↑ apoptosis |
| [97] | In vitro | HL-60 | Del (3 mg/mL), 24 h | ↑ Apoptosis, ↑ p-p38, ↑ p-c-jun, ↑ cleaved caspase-8, ↑ cleaved caspase-3, ↑ cytochrome C (in cytosol), ↑ Fas, and ↑ FasL |
| [98] | In vitro | HL-60 | Delphinidin-3-sambubioside (25, 50, 75, 100, and 125 μM), 6 h | ↑ DNA fragmentation, ↑ apoptosis, ↑ activation of caspase-9, -8, and -3, ↑ cytochrome C (in cytosol), ↓ BID, and loss of mitochondrial membrane potential |
| [101] | In vitro | HL-60 | HA extract (69% delphinidin) (0, 0.1, 0.3, 0.5, and 0.7 mg/mL), 24 h | Cell cycle arrest at the G2/M phase and activation of ATM/cellular checkpoint kinase pathway |
| [102] | In vitro | HL-60 | Del (10, 30, 100 μM), 24 h | Inhibited GLO I (IC50 = 1.9 μM) and ↑ apoptosis |
| [103] | In vitro | Jurkat | Delphinidin-3-O-glucoside and delphinidin-3-O-rutinoside (100 μM), 24 h | ↑ ROS generation, ↓ p-Akt, ↓ p-Bad, ↓ Bcl-2, and ↓ UHRF |
| Bladder and Mesenchymal Tumors | ||||
| [20] | In vitro | T24 | Del (10, 20, 30, 40, 50 and 60 μg/mL), 24 h | ↓ Proliferation, ↑ apoptosis, and ↑ ROS generation |
| [106] | In vitro | HT1080 | Del-3-glu (10 and 100 μM), 36 h | Inhibits MMP-2 (IC50 = 16.0 μM) and MMP-9 (IC50 = 13.6 μM) |
| Glioma | ||||
| [110] | In vitro | U87MG and LN18 | DPN (10, 25, and 50 μM), 24 h | ↓ Viability, ↓ p-Akt, ↓ NF-κB, ↓ VEGF, ↓ b-FGF, ↓ EGFR, ↓ MMP-9, ↓ MMP-2, ↑ caspase-8, ↑ truncated BID, ↑ BAX, ↑ caspase-3, ↑ caspase-6, and ↓ Bcl-2 |
| [114] | In vitro | U-87 MG | Del (35 and 50 μM), 24 h | ↓ Migration, ↓ TGFβ/Smad2, ↓ TGFβ/ERK, ↓ fibronectin, and ↓ Snail |
| Osteosarcoma | ||||
| [21] | In vitro | HOS and U2OS human osteosarcoma | Del (10, 25, 50, 75, and 100 µM), 24 h | ↑ apoptosis, ↑ E-cadherin, ↓ N-cadherin, ↓ Snail, ↓ Slug, ↓ EMT, ↓ ERK, and P38 phosphorylation |
| [115] | In vitro | U2OS | Del (10, 50, 100, and 200 μM), 48 h | ↓ Cell viability, ↑ ROS, ↑ LC3-II, ↑ autophagosome formation, and ↓ p62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Choi, H.-K.; Kim, Y.-K.; Lee, H.-J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. https://doi.org/10.3390/ijms222111500
Sharma A, Choi H-K, Kim Y-K, Lee H-J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. International Journal of Molecular Sciences. 2021; 22(21):11500. https://doi.org/10.3390/ijms222111500
Chicago/Turabian StyleSharma, Anshul, Hyo-Kyoung Choi, Yeon-Kye Kim, and Hae-Jeung Lee. 2021. "Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives" International Journal of Molecular Sciences 22, no. 21: 11500. https://doi.org/10.3390/ijms222111500
APA StyleSharma, A., Choi, H.-K., Kim, Y.-K., & Lee, H.-J. (2021). Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. International Journal of Molecular Sciences, 22(21), 11500. https://doi.org/10.3390/ijms222111500







