Nickel Laterites—Mineralogical Monitoring for Grade Definition and Process Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Sample Preparation
2.2. X-ray Diffraction (XRD)
2.3. Rietveld Quantification
2.4. Cluster Analysis
- Comparison of all scans in a document with each other, resulting in a correlation matrix representing the dissimilarities of all data points of any given pair of scans.
- Agglomerative hierarchical cluster analysis puts the scans in different classes defined by their similarity. The output of this step is displayed as a dendrogram, where each scan starts at the left side as an individual cluster. The clusters amalgamate in a stepwise fashion until they are all united in one single group.
- The best possible grouping (=number of separate clusters) is estimated by the KGS test [19] or by the largest relative step on the dissimilarity scale. This number can be adapted manually too. Additionally, the most representative scan and the two most outlying scans within each cluster is determined and marked.
- Next to hierarchical clustering you can use three independent tools, namely Principal Components Analysis (PCA), Metric Multi-Dimensional Scaling (MMDS), or t-Stochastic Neighbor Embedding (t-SNE) to define clusters; they are all shown in pseudo-three-dimensional plots.
2.5. Fuzzy Clustering
- Probabilities < 0.2 indicate members, which surely do not belong to this cluster.
- Probabilities > 0.7 indicate members, which certainly do belong to a specific cluster.
- Probabilities between 0.2 and 0.7 indicate members, which could belong to more than one cluster. These should be inspected in more detail.
2.6. X-ray Fluorescence (XRF)
3. Results and Discussion
3.1. Cluster Analysis of Nickel Laterite
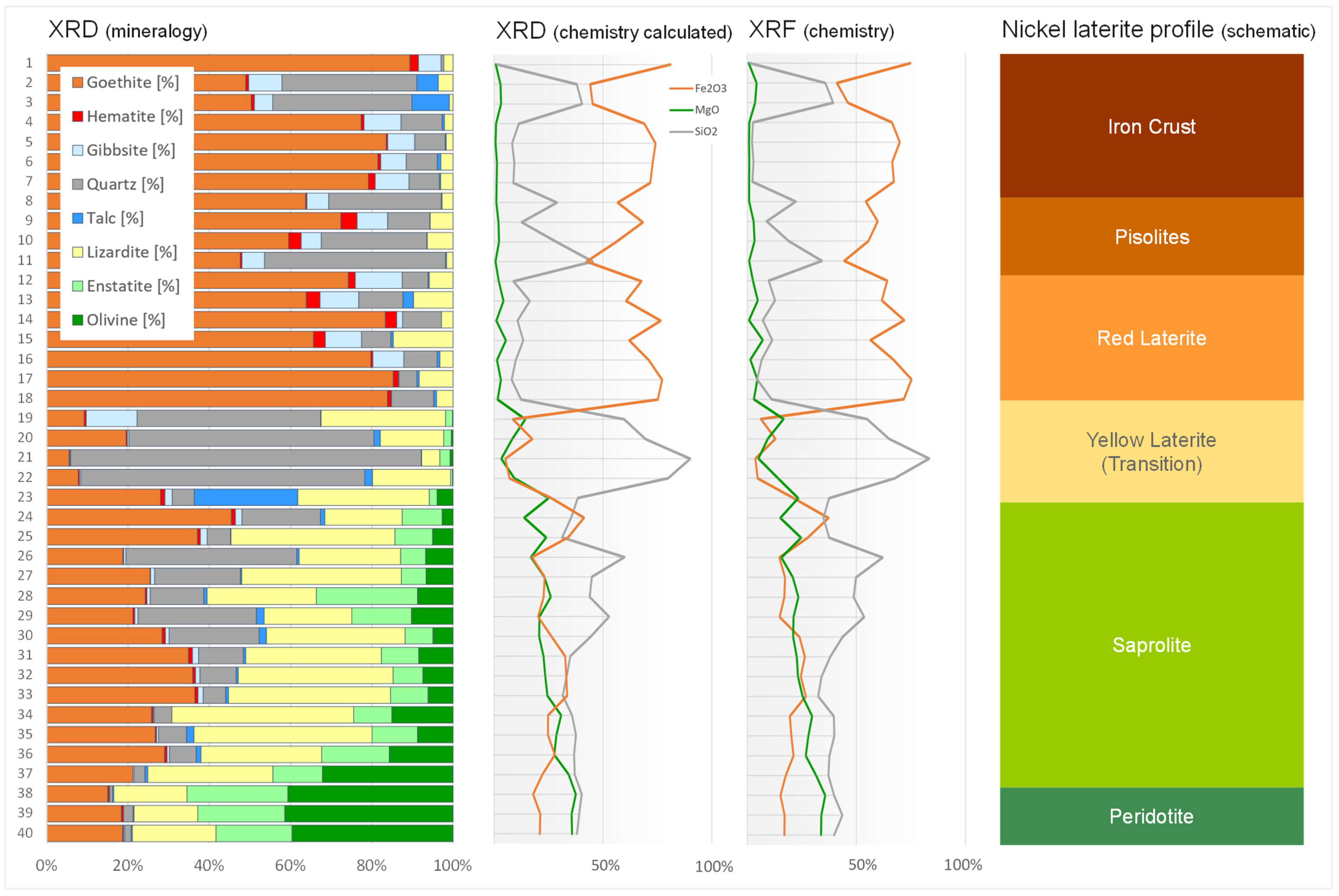
3.2. Mineral Identification and Quantification
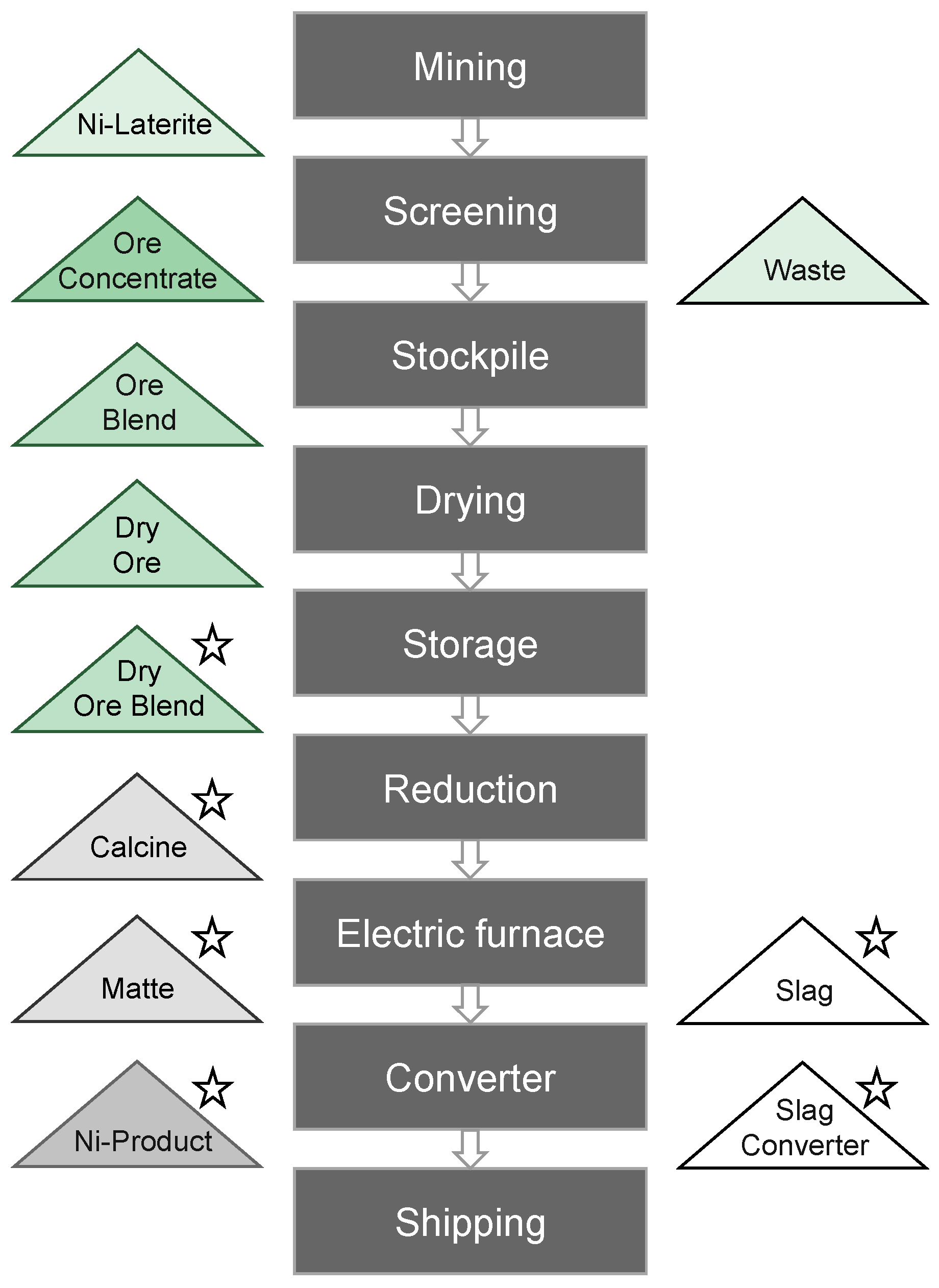
3.3. Mineralogical Monitoring during Pyrometallurgical Processing of Nickel Laterites
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Butt, C.R.M.; Cluzel, D. Nickel Laterite Ore Deposits: Weathered Serpentinites. Elements 2013, 9, 123–128. [Google Scholar] [CrossRef]
- Garvin, M.M.; Simon, M.J. A detailed assessment of global nickel resource trends and endowments. Soc. Econ. Geol. Inc. Econ. Geol. 2014, 109, 1813–1814. [Google Scholar]
- DERA. Batterierohstoffe für Die Elektromobilität; DERA Themenheft: Berlin, Germany, 2021; p. 26. [Google Scholar]
- Meshram, P.; Pandey, B.D. Advanced review on extraction of nickel from primary and secondary sources. Miner. Process. Extr. Metall. Rev. 2019, 40, 157–193. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickelliferrous laterite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Tian, H.; Pan, J.; Zhu, D.; Yang, C.; Guo, Z.; Xue, Y. Imporved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J. Mater. Res. Technol. 2020, 9, 2578–2589. [Google Scholar] [CrossRef]
- Lv, X.W.; Bai, C.G.; He, S.P.; Huang, Q.Y. Mineral change of Philippine and Indonesian nickel laterite ore during sintering and mineralogy of the sinter. ISIJ Int. 2010, 50, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Brand, N.W.; Butt, C.R.M.; Elias, M. Nickel laterites: Classification and features. AGSO J. Aust. Geol. Geophys. 1998, 17, 81–88. [Google Scholar]
- Maurizot, P.; Sevin, B.; Iseppi, M.; Giband, T. Nickel-Bearing Laterite Deposits in Accretionary Context and the Case of New Caledonia: From the Large-Scale Structure of Earth to Our Everyday Appliances. GSA Today 2019, 29, 4–10. [Google Scholar] [CrossRef]
- Brindley, G.W.; Hang, P.T. The nature of garnierites—I. Structures, Chemical Composition and Color Characteristic. Clays Clay Miner. 1973, 21, 27–40. [Google Scholar] [CrossRef]
- Brindley, G.W.; Maksimovic, Z. The nature and nomenclature of hydrous nickel-containing silicates. Clay Miner. 1974, 10, 271–277. [Google Scholar] [CrossRef]
- Wells, M.A.; Ramanaidou, E.R.; Verrall, M.; Tessarolo, C. Mineralogy and crystal chemistry of “garnierites” in the Goro lateritic nickel deposit, New Caledonia. Eur. J. Miner. 2009, 21, 467–483. [Google Scholar] [CrossRef]
- Horn, R.A.; Bacon, W.G. Goro Nickel-Cobalt Project Located in French Overseas Territorial Community (Collectivite Territoriale) of New Caledonia; Goro Nickel Technical Report; Goro Nickel: Noumea, New Caledonia, 2002; p. 116. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- COD Crystallography Open Database. Available online: http://www.crystallography.net/cod/ (accessed on 24 October 2021).
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method. International Union of Crystallography; Oxford University Press: Oxford, UK, 1993; p. 298. [Google Scholar]
- Kelley, L.A.; Gardner, S.P.; Sutcliffe, M.J. An automated approach for clustering an ensemble of NMR-derived protein structures into conformationally related subfamilies. Protein Eng. Des. Sel. 1996, 9, 1063–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, B.; Chen, J. The application of cluster analysis in X-ray diffraction phase analysis. J. Appl. Crystallogr. 1992, 25, 336–339. [Google Scholar] [CrossRef] [Green Version]
- Van der Maaten, L.J.P.; Hinton, G.E. Visualizing High-Dimensional Data Using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Sato, M.; Sato, Y.; Jain, L.C. Fuzzy Clustering Models and Applications, Studies in Fuzziness and Soft Computing; Springer: NewYork, NY, USA, 1997; Volume 9, p. 122. [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M. Cluster Analysis, 5th ed.; Wiley: London, UK, 2011; 346p. [Google Scholar]
- Garnier, J. Essai sur la geologie et les ressources minerales de la Nouvelle-Caledonie. Ann. Des Mines 1867, 6, 1–92. [Google Scholar]
- Faust, G.T. The hydrous nickel-magnesium silicgroup—The garnierite group. Am. Min. 1966, 51, 279–298. [Google Scholar]
- Glasser, M.E. Note sur une espèce minérale nouvelle, la népouite, silicate hydraté de nickel et de magnésie. Bull. De La Société Française De Minéralogie 1907, 30, 17–28. [Google Scholar] [CrossRef]
- Brindley, G.W.; Wan, H.M. Composition, structures, and thermal behavior of nickel-containing minerals in the lizardite-nepouite series. Am. Mineral. 1975, 60, 863–871. [Google Scholar]
- Gamaletsos, P.N.; Kalatha, S.; Godelitsas, A.; Economou-Eliopoulos, M.; Göttlicher, J.; Steininger, R. Arsenic distribution and speciation in the bauxitic Fe-Ni-laterite ore deposit of the Patitira mine, Lokris area (Greece). J. Geochem. Explor. 2018, 194, 189–197. [Google Scholar] [CrossRef]
- Samouhos, M.; Godelitsas, A.; Nomikou, C.; Taxiarchou, M.; Tsakiridis, P.; Zavasnik, J.; Gamaletsos, P.N.; Apostolikas, A. New insights into nanomineralogy and geochemistry of Ni-laterite ores from central Greece (Larymna and Evia depositis). Geochemistry 2018, 12, 5. [Google Scholar]
- Alvarez, M.; Sileo, E.E.; Rueda, E.H. Structure and reactivity of synthetic Co-substituted goethites. Am. Miner. 2008, 93, 584–590. [Google Scholar] [CrossRef]
- Gasser, U.G.; Jeanroy, E.; Mustin, C.; Barres, O.; Nüesch, R.; Berthelin, J.; Herbillon, A.J. Properties of synthetic goethites with Co for Fe substitution. Clay Miner. 1996, 31, 465–476. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; VHC Verlagsgesellschaft: Weinheim, Germany, 1996; pp. 35–47. [Google Scholar]
- Cornell, R.M.; Giovanoli, R. Effect of cobalt on the formation of crystalline iron oxides from ferrihydrite in alkaline media. Clays Clay Min. 1989, 37, 65–70. [Google Scholar] [CrossRef]
- Cornell, R.M. Simultaneous incooperation od Mn, Ni, Co in the goethite (α-FeOOH) structure. Clay Miner. 1991, 2, 427–430. [Google Scholar] [CrossRef]
- Pozas, R.; Rojas, T.C.; Ocaña, M.; Serna, C.J. The Nature of Co in Synthetic Co-substituted Goethites. Clays Clay Miner. 2004, 52, 760–766. [Google Scholar] [CrossRef]
- Blake, R.L.; Hessevick, R.E.; Zoltai, T.; Finger, L.W. Refinement of the hematite structure. Am. Min. 1966, 51, 123–129. [Google Scholar]
- Saalfeld, H.; Wedde, M. Refinement of the crystal structure of gibbsite, Al(OH)3. Z. Für Krist. 1974, 139, 129–135. [Google Scholar] [CrossRef]
- Gualtieri, A. Accuracy of XRPD QPA using the combined Rietveld–RIR method. J. Appl. Crystallogr. 2000, 33, 267–278. [Google Scholar] [CrossRef]
- Guggenheim, S.; Zhan, W. Effect of temperature on the structures of lizardite-1T and lizardite-2H1. Can. Min. 1998, 36, 1587–1594. [Google Scholar]
- Perdikatsis, B.; Burzlaff, H. Strukturverfeinerung am Talk Mg3[(OH)2Si4O10]. Z. Für Krist. Cryst. Mater. 1981, 156, 177–186. [Google Scholar] [CrossRef]
- Nestola, F.; Gatta, G.D.; Ballaran, T.B. The effect of Ca substitution on the elastic and structural behavior of orthoenstatite. Am. Miner. 2006, 91, 809–815. [Google Scholar] [CrossRef]
- Ottonello, G.; Princivalle, F.; Della Giusta, A. Temperature, composition, and fO2 effects on intersite distribution of Mg and Fe2+ in olivines. Phys. Chem. Miner. 1990, 17, 301–312. [Google Scholar] [CrossRef]
- Bessemer, H. Sir Henry Bessemer, F.R.S. An Autobiography; Offices of “Engineering”: London, UK, 1905; p. 176. [Google Scholar]
- Nakagiri, N.; Manghnani, M.H.; Ming, L.C.; Kimura, S. Crystal structure of magnetite under pressure. Phys. Chem. Miner. 1986, 13, 238–244. [Google Scholar] [CrossRef]
- Mancini, F.; Sillanpaa, R.; Marshall, B.; Papunen, H. Magnesian hornblende from a metamorphosed ultramafic body in southwestern Finland: Crystal chemistry and petrological implications. Can. Mineral. 1996, 34, 835–844. [Google Scholar]
- Elliot, A.D. Structure of pyrrhotite 5C (Fe9S10). Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 271–279. [Google Scholar] [CrossRef]
- Lottermoser, W.; Steiner, K.; Grodzicki, M.; Jiang, K.; Scharfetter, G.; Bats, J.W.; Redhammer, G.; Treutmann, W.; Hosoya, S.; Amthauer, G. The electric field gradient in synthetic fayalite α-Fe2SiO4 at moderate temperatures. Phys. Chem. Miner. 2002, 29, 112–121. [Google Scholar] [CrossRef]
- Leineweber, A.; Jacobs, H.; Hull, S. Ordering of Nitrogen in Nickel Nitride Ni3N Determined by Neutron Diffraction. Inorg. Chem. 2001, 40, 5818–5822. [Google Scholar] [CrossRef]
- Fleet, M.E. The crystal structure of heazlewoodite, and metallic bonds in sulfide minerals. Am. Min. 1977, 62, 341–345. [Google Scholar]
- Woodward, P.M.; Suard, E.; Karen, P. Structural Tuning of Charge, Orbital, and Spin Ordering in Double-Cell Perovskite Series between NdBaFe2O5 and HoBaFe2O5. J. Am. Chem. Soc. 2003, 125, 8889–8899. [Google Scholar] [CrossRef] [PubMed]
- Bertaut, F. La structure de sulfure de fer. J. De Phys. Et Du Radium 1954, 15, 775. [Google Scholar]
- Setiawan, I.; Febrina, E.; Subagja, R.; Harjanto, S.; Firdiyono, F. Investigations on mineralogical characteristics of Indonesian nickel laterite ores during the roasting process. IOP Conf. Ser. Mater. Sci. Eng. 2019, 541, 012038. [Google Scholar] [CrossRef]










| Cluster No. | Sample No. | Laterite | Transition | Saprolite (High Liz) | Saprolite (High Oli/Ens) | Mixture * |
|---|---|---|---|---|---|---|
| 1 | 1 | 0.8 | 0.2 | 0.1 | 0.1 | |
| 2 | 2 | 0.4 | 0.8 | 0.2 | 0.0 | X |
| 2 | 3 | 0.5 | 0.7 | 0.1 | 0.1 | X |
| 1 | 4 | 0.7 | 0.4 | 0.2 | 0.0 | |
| 1 | 5 | 0.8 | 0.3 | 0.1 | 0.1 | |
| 1 | 6 | 0.8 | 0.4 | 0.1 | 0.0 | |
| 1 | 7 | 0.9 | 0.4 | 0.1 | 0.1 | |
| 2 | 8 | 0.6 | 0.7 | 0.1 | 0.0 | X |
| 1 | 9 | 0.7 | 0.1 | 0.3 | 0.1 | |
| 1 | 10 | 0.8 | 0.2 | 0.2 | 0.1 | |
| 2 | 11 | 0.5 | 0.7 | 0.1 | 0.1 | X |
| 1 | 12 | 0.8 | 0.4 | 0.2 | 0.1 | |
| 1 | 13 | 0.7 | 0.2 | 0.1 | 0.1 | |
| 1 | 14 | 0.9 | 0.3 | 0.1 | 0.1 | |
| 1 | 15 | 0.7 | 0.3 | 0.3 | 0.1 | |
| 1 | 16 | 0.8 | 0.4 | 0.1 | 0.0 | |
| 2 | 17 | 0.5 | 0.4 | 0.5 | 0.1 | X |
| 2 | 18 | 0.3 | 0.8 | 0.3 | 0.1 | X |
| 2 | 20 | 0.2 | 0.7 | 0.3 | 0.0 | X |
| 3 | 23 | 0.2 | 0.3 | 0.7 | 0.2 | |
| 2 | 24 | 0.5 | 0.5 | 0.4 | 0.0 | X |
| 3 | 25 | 0.2 | 0.1 | 0.9 | 0.3 | |
| 3 | 26 | 0.1 | 0.3 | 0.8 | 0.2 | |
| 3 | 27 | 0.1 | 0.1 | 0.7 | 0.4 | |
| 3 | 28 | 0.2 | 0.2 | 0.8 | 0.2 | |
| 3 | 29 | 0.1 | 0.4 | 0.7 | 0.1 | |
| 3 | 30 | 0.1 | 0.3 | 0.8 | 0.2 | |
| 3 | 31 | 0.2 | 0.2 | 0.9 | 0.2 | |
| 3 | 32 | 0.2 | 0.1 | 0.9 | 0.3 | |
| 3 | 33 | 0.2 | 0.1 | 0.9 | 0.2 | |
| 4 | 34 | 0.1 | 0.0 | 0.6 | 0.6 | X |
| 3 | 35 | 0.2 | 0.1 | 0.8 | 0.3 | |
| 3 | 36 | 0.2 | 0.2 | 0.8 | 0.4 | |
| 4 | 37 | 0.1 | 0.1 | 0.6 | 0.7 | |
| 4 | 38 | 0.1 | 0.1 | 0.4 | 0.7 | |
| 4 | 39 | 0.1 | 0.1 | 0.4 | 0.8 | |
| 4 | 40 | 0.1 | 0.1 | 0.4 | 0.8 |
| Mineral | Formula | References |
|---|---|---|
| Goethite | FeOOH | [27] |
| Hematite | Fe2O3 | [36] |
| Gibbsite | Al(OH)3 | [37] |
| Quartz | SiO2 | [38] |
| Lizardite | (Mg,Ni)3(Si2O5)(OH)4 | [39] |
| Talc | Mg3[(OH)2Si4O10] | [40] |
| Enstatite (Pyroxene) | Mg15.44Ca0.56Si16O48 | [41] |
| Forsterite (Olivine) | Mg7.17Fe0.8Ni0.02Mn0.01Si4O16 | [42] |
| # | Goe * [%] | Hem * [%] | Gib * [%] | Qua * [%] | Tal * [%] | Liz * [%] | Ens * [%] | Oli * [%] | Rwp | Ni [%] | Co [%] | Fe2O3 [%] | MgO [%] | SiO2 [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 89.4 | 2.1 | 5.6 | 0.7 | 0.0 | 2.2 | 0.0 | 0.0 | 4.6 | 0.6 | 0.0 | 74.7 | 0.7 | 1.2 |
| 2 | 49.0 | 0.7 | 8.2 | 33.2 | 5.3 | 3.6 | 0.0 | 0.0 | 3.5 | 1.6 | 0.1 | 41.1 | 4.2 | 35.8 |
| 3 | 50.4 | 0.8 | 4.5 | 34.3 | 9.2 | 0.9 | 0.0 | 0.0 | 4.6 | 0.7 | 0.1 | 46.1 | 3.6 | 39.3 |
| 4 | 77.4 | 0.7 | 9.1 | 10.2 | 0.5 | 2.1 | 0.0 | 0.0 | 1.9 | 1.6 | 0.6 | 66.3 | 0.9 | 2.6 |
| 5 | 83.6 | 0.3 | 6.7 | 7.5 | 0.2 | 1.7 | 0.0 | 0.0 | 3.1 | 1.2 | 0.2 | 69.9 | 0.7 | 2.1 |
| 6 | 81.6 | 0.7 | 6.3 | 7.7 | 0.8 | 3.0 | 0.0 | 0.0 | 2.4 | 1.9 | 0.3 | 66.5 | 0.9 | 2.8 |
| 7 | 79.2 | 1.7 | 8.3 | 7.5 | 0.3 | 3.0 | 0.0 | 0.0 | 2.7 | 1.7 | 0.4 | 67.2 | 0.8 | 2.3 |
| 8 | 63.8 | 0.3 | 5.4 | 27.7 | 0.2 | 2.7 | 0.0 | 0.0 | 2.5 | 1.2 | 0.2 | 54.4 | 0.8 | 22.2 |
| 9 | 72.5 | 4.0 | 7.5 | 10.4 | 0.1 | 5.6 | 0.0 | 0.0 | 6.6 | 0.7 | 0.0 | 59.8 | 2.9 | 8.9 |
| 10 | 59.5 | 3.1 | 4.9 | 26.0 | 0.1 | 6.3 | 0.0 | 0.0 | 6.9 | 0.9 | 0.0 | 55.5 | 3.3 | 19.0 |
| 11 | 47.6 | 0.4 | 5.6 | 44.5 | 0.2 | 1.6 | 0.0 | 0.0 | 3.2 | 0.9 | 0.1 | 44.4 | 0.8 | 34.2 |
| 12 | 74.2 | 1.7 | 11.5 | 6.4 | 0.3 | 5.8 | 0.0 | 0.0 | 2.9 | 1.4 | 0.1 | 64.2 | 2.6 | 9.8 |
| 13 | 63.9 | 3.4 | 9.5 | 10.9 | 2.6 | 9.7 | 0.0 | 0.0 | 9.6 | 0.8 | 0.0 | 61.7 | 4.8 | 12.7 |
| 14 | 83.3 | 2.8 | 1.4 | 9.6 | 0.0 | 2.8 | 0.0 | 0.0 | 3.3 | 0.3 | 0.0 | 72.0 | 0.7 | 7.1 |
| 15 | 65.7 | 2.9 | 8.9 | 7.2 | 0.6 | 14.7 | 0.0 | 0.0 | 4.7 | 1.1 | 0.0 | 56.5 | 7.1 | 11.4 |
| 16 | 79.8 | 0.5 | 7.6 | 8.2 | 0.7 | 3.2 | 0.0 | 0.0 | 2.3 | 1.5 | 0.2 | 67.1 | 1.4 | 6.6 |
| 17 | 85.3 | 1.3 | 0.1 | 4.4 | 0.6 | 8.3 | 0.0 | 0.0 | 5.5 | 0.8 | 0.1 | 75.3 | 4.6 | 4.5 |
| 18 | 83.8 | 0.9 | 0.1 | 10.4 | 0.7 | 4.0 | 0.0 | 0.0 | 8.9 | 0.9 | 0.0 | 71.8 | 3 | 11.1 |
| 19 | 9.2 | 0.5 | 12.5 | 45.1 | 0.1 | 30.6 | 1.7 | 0.1 | 9.9 | 2.4 | 0.1 | 6.2 | 16.6 | 54.9 |
| 20 | 19.6 | 0.3 | 0.4 | 60.4 | 1.6 | 15.6 | 1.9 | 0.4 | 6.2 | 1.2 | 0.0 | 12.9 | 9.4 | 65 |
| 21 | 5.5 | 0.2 | 0.2 | 86.2 | 0.1 | 4.5 | 2.5 | 0.7 | 6.1 | 0.5 | 0.0 | 3.7 | 5.1 | 83.4 |
| 22 | 7.8 | 0.3 | 0.3 | 69.9 | 1.9 | 19.2 | 0.5 | 0.1 | 8.3 | 0.9 | 0.0 | 4.7 | 14.2 | 67.5 |
| 23 | 28.0 | 1.1 | 1.8 | 5.4 | 25.5 | 32.4 | 1.9 | 3.9 | 9.4 | 2.2 | 0.1 | 21.2 | 23.4 | 37.5 |
| 24 | 45.4 | 1.0 | 1.6 | 19.3 | 1.1 | 19.0 | 9.9 | 2.6 | 4.6 | 1.4 | 0.0 | 37.2 | 15.1 | 34.9 |
| 25 | 37.2 | 0.7 | 1.6 | 5.8 | 0.1 | 40.4 | 9.3 | 5.0 | 7.2 | 2.6 | 0.1 | 27.7 | 24.7 | 37.6 |
| 26 | 18.8 | 0.2 | 0.5 | 42.1 | 0.6 | 25.0 | 6.2 | 6.7 | 8.5 | 1.4 | 0.1 | 14.7 | 15.6 | 62.1 |
| 27 | 25.5 | 0.1 | 1.0 | 21.1 | 0.3 | 39.3 | 6.1 | 6.6 | 8.7 | 2.4 | 0.0 | 17.3 | 20.9 | 49.9 |
| 28 | 24.3 | 0.4 | 0.8 | 13.2 | 0.8 | 26.9 | 24.9 | 8.7 | 8.3 | 1.9 | 0.1 | 17 | 23.4 | 48.8 |
| 29 | 21.2 | 0.5 | 0.8 | 29.2 | 1.9 | 21.6 | 14.7 | 10.2 | 8.5 | 1.6 | 0.0 | 14.8 | 21.2 | 53.6 |
| 30 | 28.4 | 0.8 | 0.9 | 22.2 | 1.8 | 34.1 | 6.9 | 4.9 | 7.1 | 2.6 | 0.1 | 23.9 | 21.0 | 43.7 |
| 31 | 34.9 | 1.0 | 1.5 | 11.0 | 0.6 | 33.4 | 9.2 | 8.4 | 6.9 | 2.5 | 0.1 | 26.4 | 22.7 | 38.1 |
| 32 | 35.9 | 0.7 | 1.1 | 8.9 | 0.5 | 38.1 | 7.3 | 7.4 | 7.8 | 2.4 | 0.1 | 24.5 | 23.2 | 34.1 |
| 33 | 36.5 | 0.8 | 1.3 | 5.5 | 0.7 | 39.9 | 9.3 | 6.1 | 7.1 | 2.3 | 0.1 | 26.8 | 25.3 | 32.5 |
| 34 | 25.8 | 0.4 | 0.2 | 4.4 | 0.0 | 44.7 | 9.4 | 15.0 | 9.7 | 3.3 | 0.0 | 19.5 | 29.7 | 39.7 |
| 35 | 26.7 | 0.3 | 0.5 | 6.9 | 1.8 | 43.8 | 11.2 | 8.7 | 9.8 | 3.2 | 0.0 | 20.2 | 28 | 39.9 |
| 36 | 29.0 | 0.6 | 0.6 | 6.6 | 1.2 | 29.7 | 16.6 | 15.7 | 6.9 | 2.5 | 0.0 | 21.2 | 26.8 | 37.6 |
| 37 | 21.2 | 0.0 | 0.3 | 2.7 | 0.7 | 30.8 | 12.2 | 32.1 | 8.4 | 2.6 | 0.0 | 17.6 | 31.6 | 37.2 |
| 38 | 15.0 | 0.3 | 0.2 | 0.7 | 0.3 | 18.0 | 24.8 | 40.6 | 7.0 | 1.5 | 0.0 | 15.2 | 35.7 | 39.8 |
| 39 | 18.4 | 0.5 | 0.0 | 2.4 | 0.2 | 15.7 | 21.4 | 41.4 | 6.3 | 1.4 | 0.0 | 17.1 | 33.9 | 43.6 |
| 40 | 18.7 | 0.2 | 0.3 | 1.6 | 0.3 | 20.6 | 18.7 | 39.6 | 7.1 | 1.9 | 0.0 | 17.0 | 33.8 | 39.9 |
| Mineral | Formula | References |
|---|---|---|
| Magnetite | Fe3O4 | [44] |
| Hornblende | (Ca,Na)2–3(Mg,Fe,Al)5(Al,Si)8O22(OH,F)2 | [45] |
| Pyrrhotite | Fe(1–x)S | [46] |
| Fayalite (Olivine) | Fe2SiO4 | [47] |
| Nickel | Ni | [48] |
| Heazlewoodite | Ni3S2 | [49] |
| Iron | Fe | [50] |
| Troilite | FeS | [51] |
| Value | Tool |
|---|---|
| Optimization of ore blends from various nickel laterite deposits | Cluster analysis |
| Adjustment of superheat in the feed and optimization of energy costs | Mineralogy of ore blend |
| Control of mineralization acidity | Silicate composition |
| Prevention of highly corrosive slag causing erosion of the refractories | Silicate composition |
| Better reducibility in the furnace | Olivine content |
| Boost nickel recovery rates and reduction of metal loss in slag | Slag composition |
| Increase of cobalt recoveries. | Co-bearing minerals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, U. Nickel Laterites—Mineralogical Monitoring for Grade Definition and Process Optimization. Minerals 2021, 11, 1178. https://doi.org/10.3390/min11111178
König U. Nickel Laterites—Mineralogical Monitoring for Grade Definition and Process Optimization. Minerals. 2021; 11(11):1178. https://doi.org/10.3390/min11111178
Chicago/Turabian StyleKönig, Uwe. 2021. "Nickel Laterites—Mineralogical Monitoring for Grade Definition and Process Optimization" Minerals 11, no. 11: 1178. https://doi.org/10.3390/min11111178
APA StyleKönig, U. (2021). Nickel Laterites—Mineralogical Monitoring for Grade Definition and Process Optimization. Minerals, 11(11), 1178. https://doi.org/10.3390/min11111178






