The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of Seed Priming on Germination Parameters
2.1.1. Final Germination Rate (FG %)
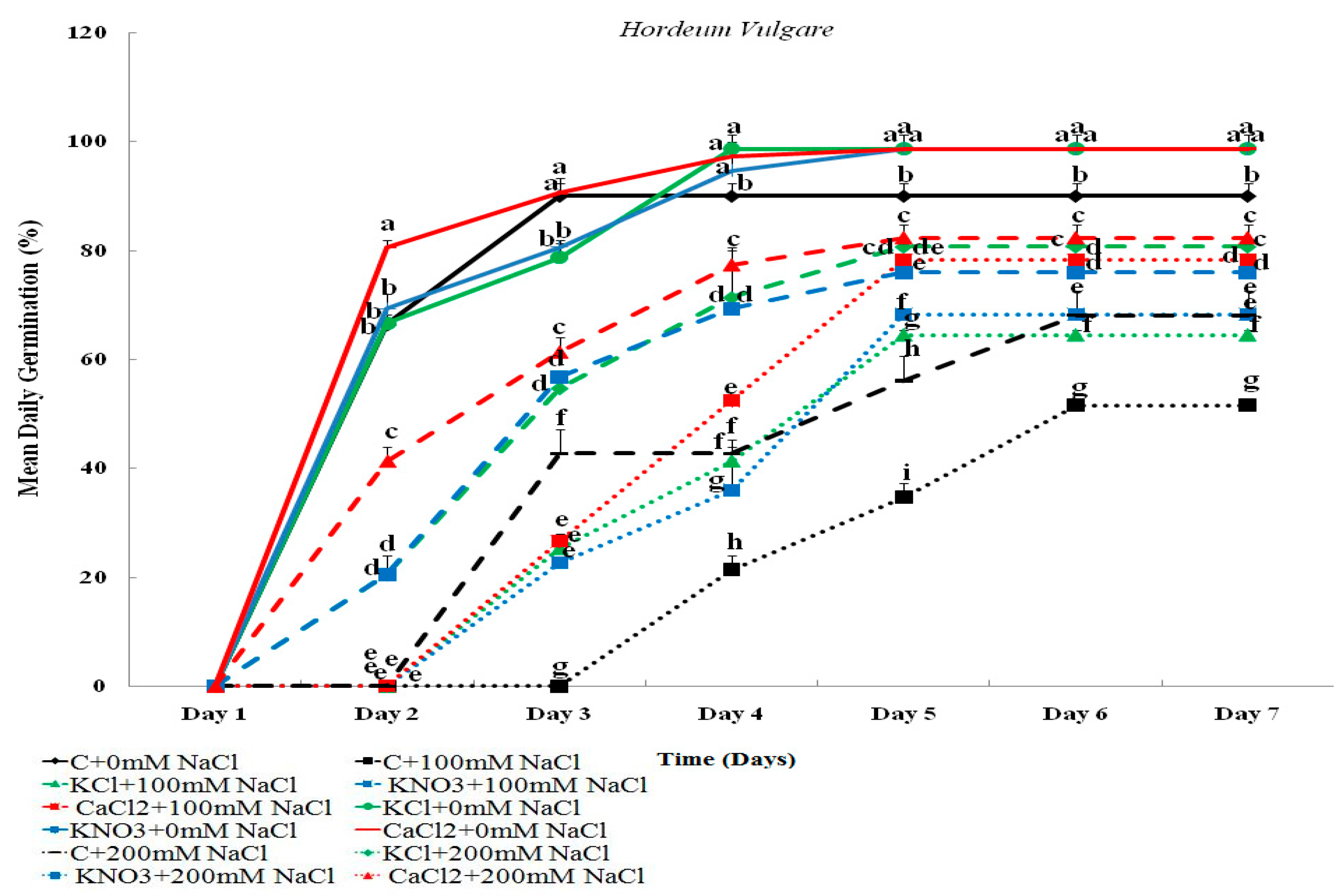
2.1.2. Mean Daily Germination (MDG)
2.2. Effect of Seed Priming on Seedling Growth
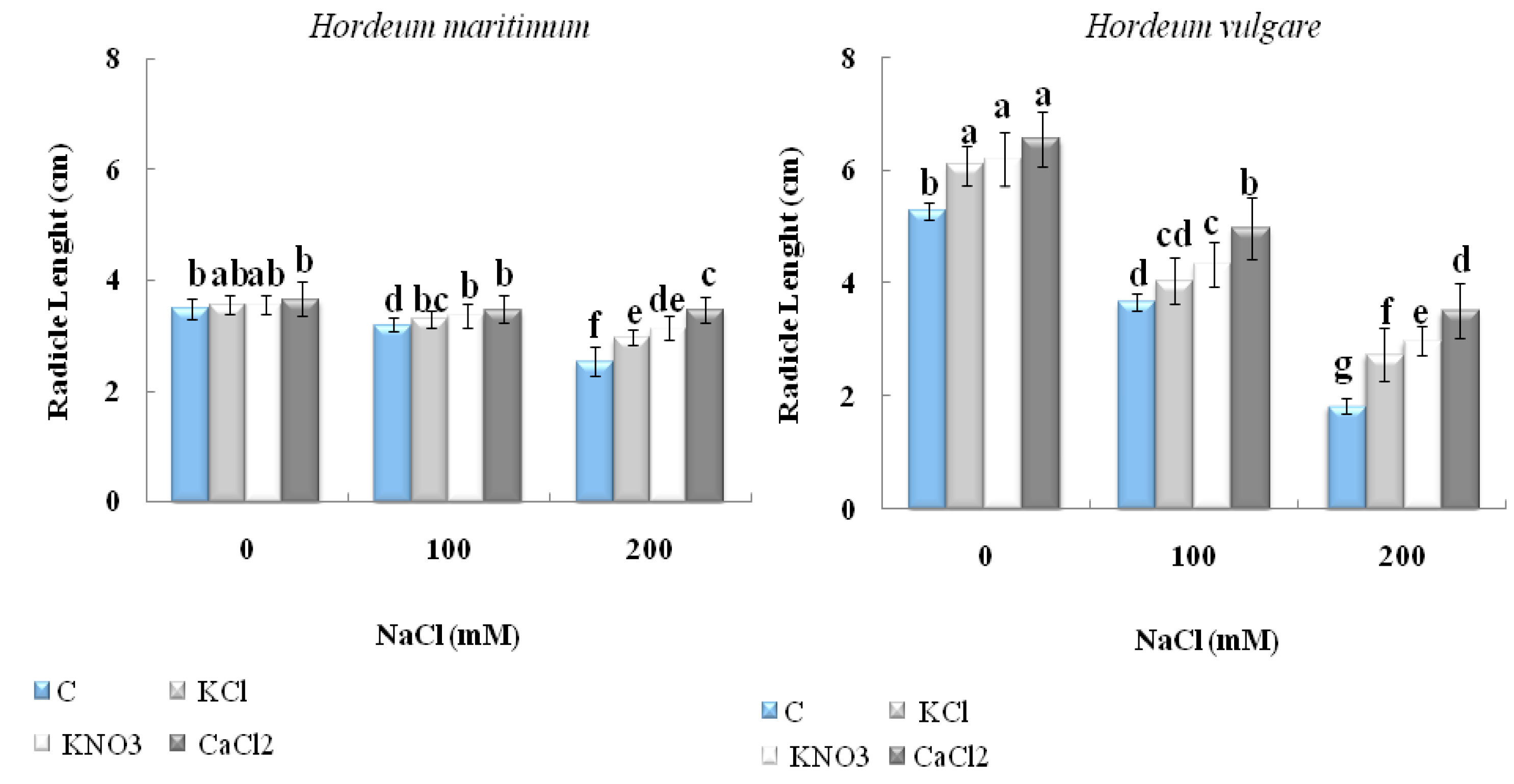
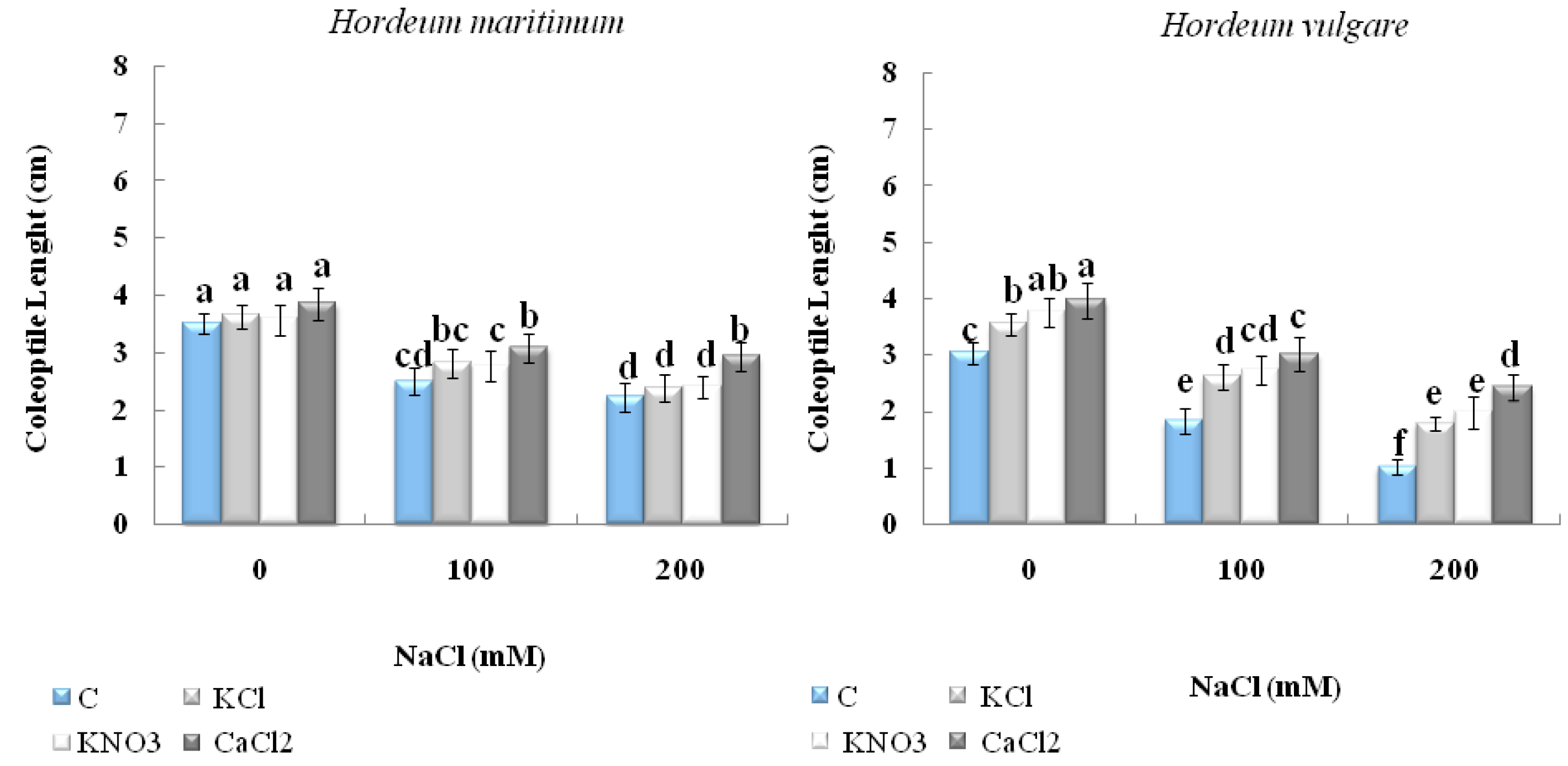
2.2.1. Seedling Length
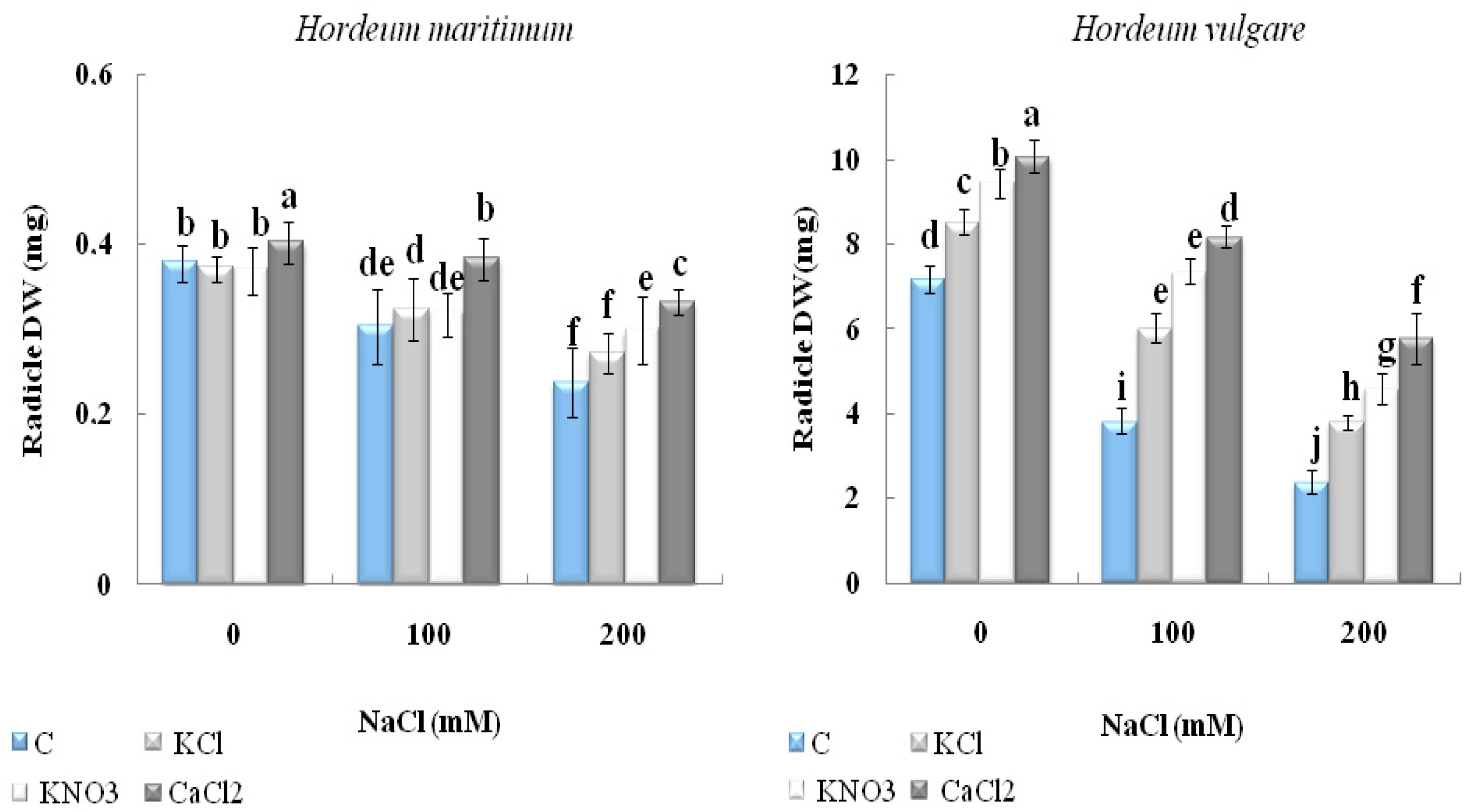
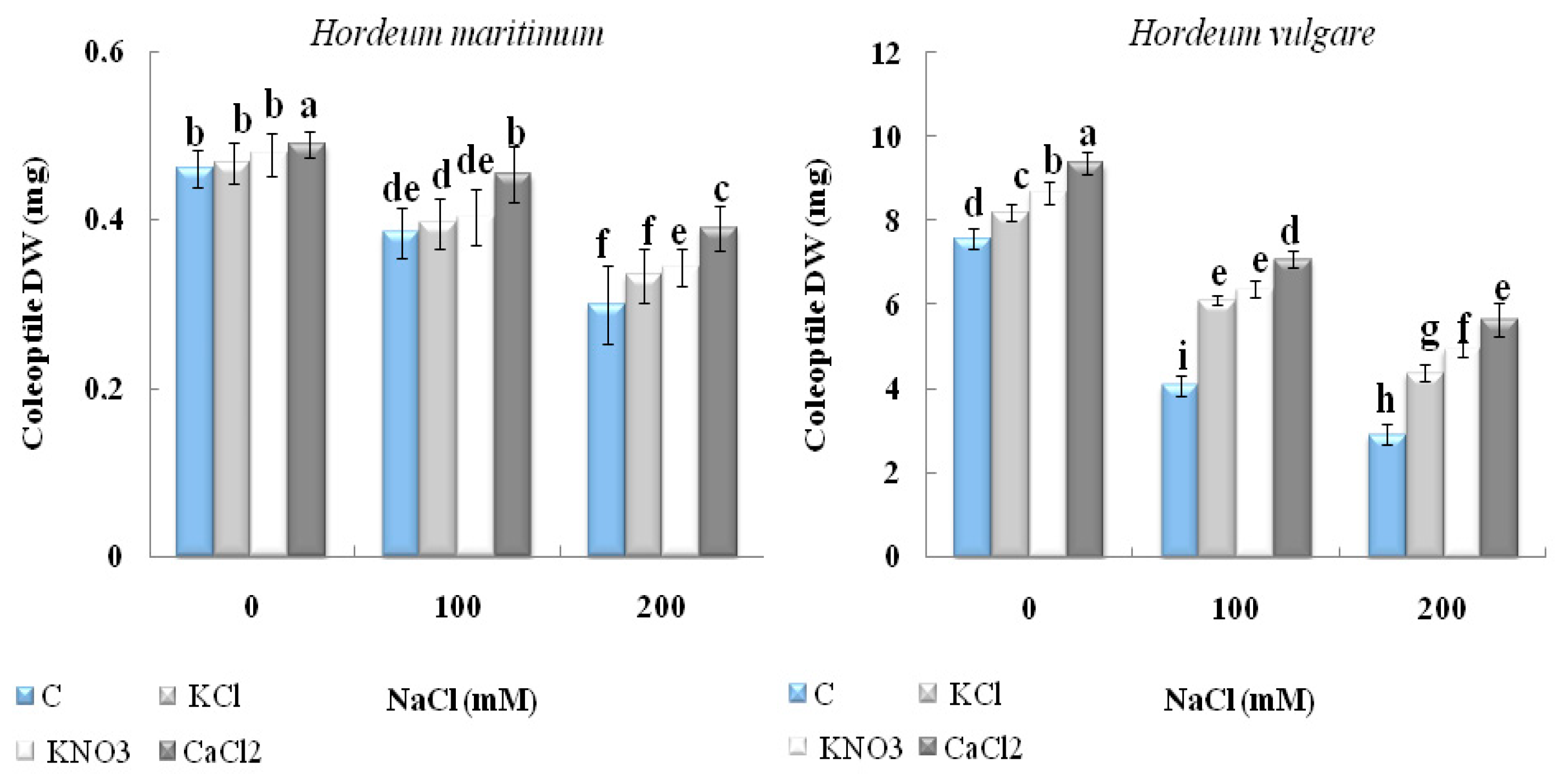
2.2.2. Dry Biomass
2.2.3. Mineral Nutrition
Iron
Calcium
Magnesium
Potassium
Sodium
Sodium/Potassium (Na/K) Ratio
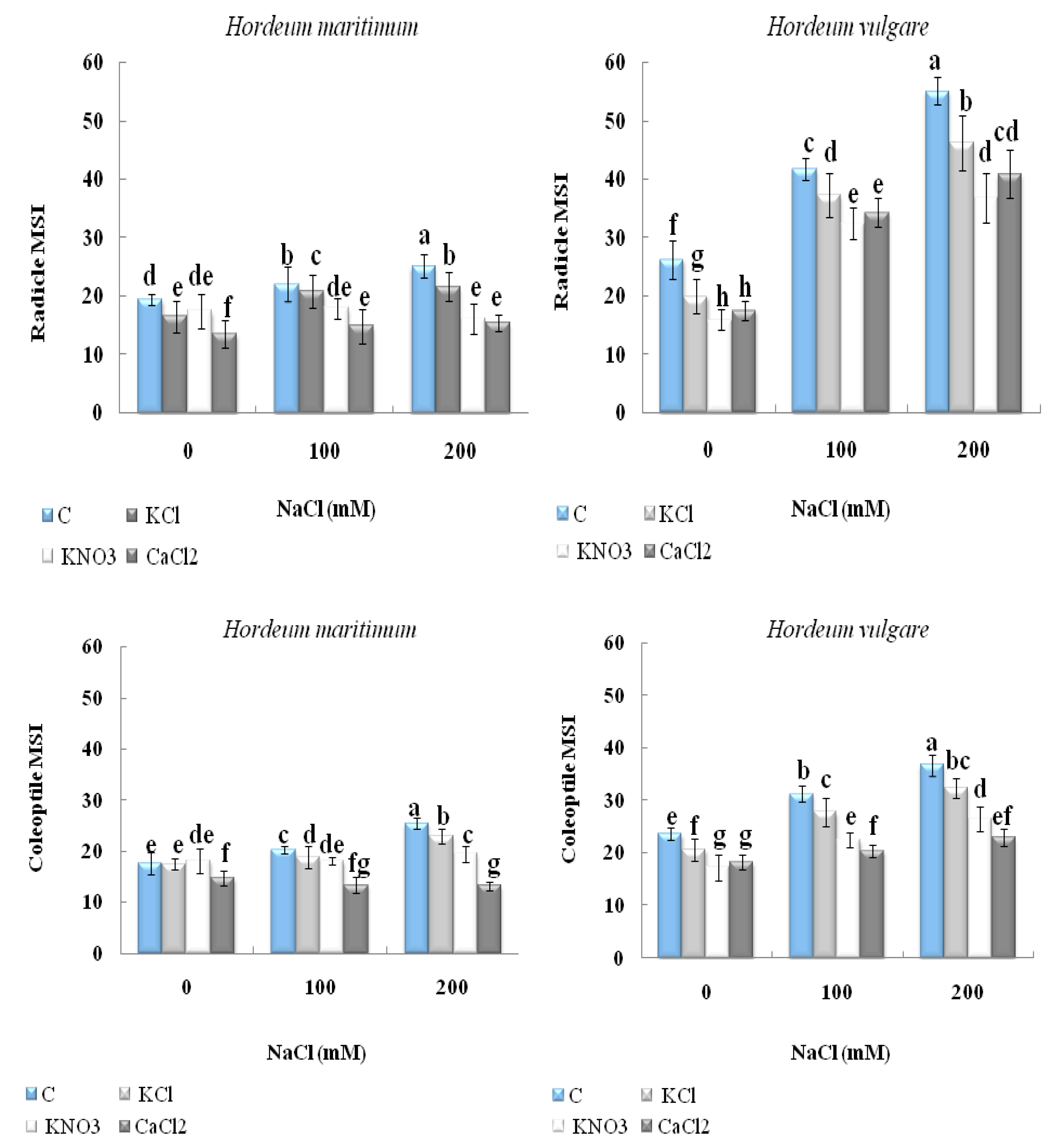
2.3. Effect of Seed Priming on Seedling Electrolyte Leakage
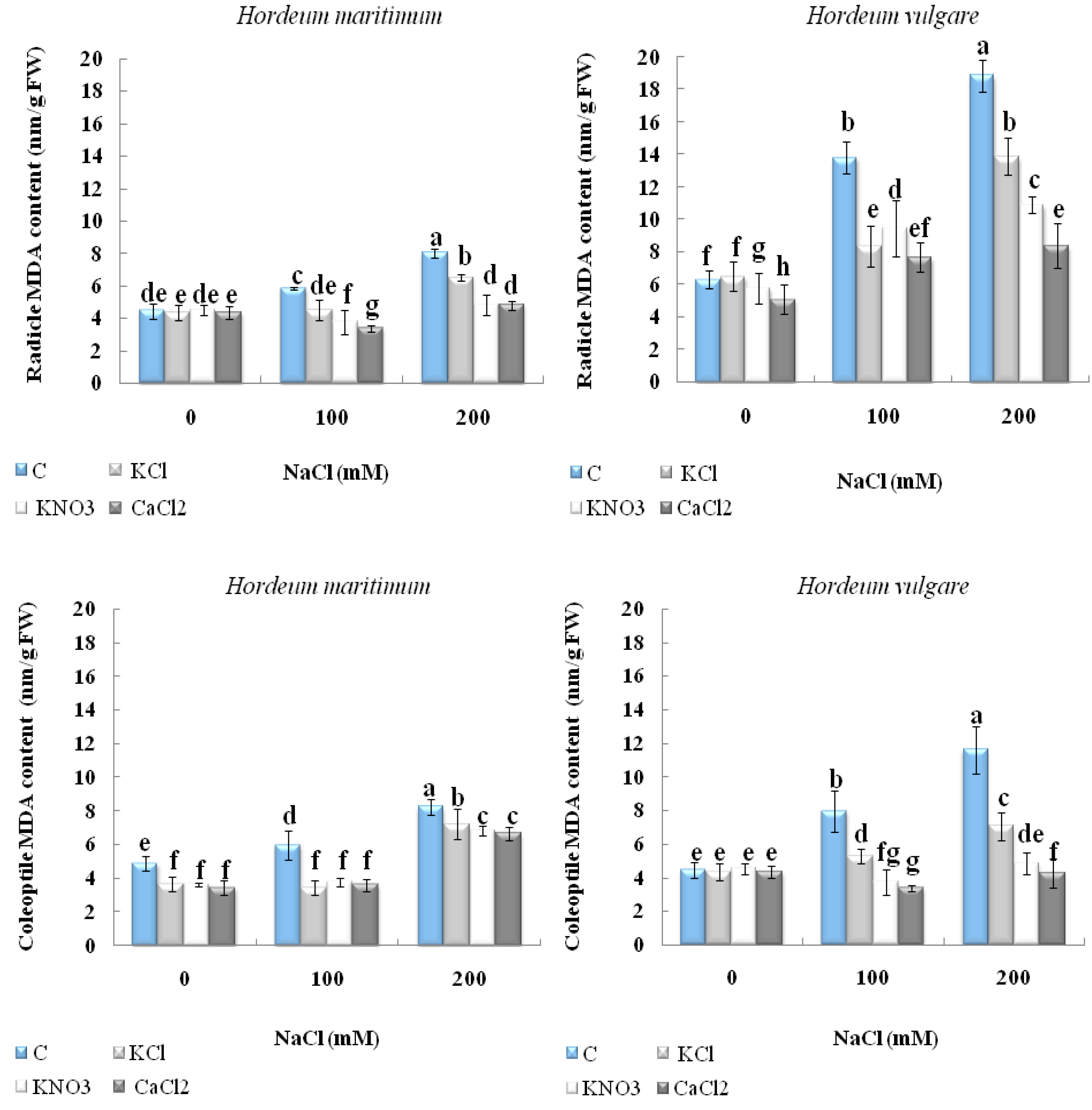
2.4. Effect of Seed Priming onLipid Peroxidation
2.5. Pearson’s Correlation Matrix Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Germination Parameters
4.2.1. Final Germination Percentage (FG %)
4.2.2. Mean Daily Germination (MDG)
4.3. Seedlings Growth
4.4. Nutrient Extraction and Analysis
4.5. Electrolyte Leakage
4.6. Malondialdehyde Content (MDA)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubbard, M.; Germida, J.; Vujanovic, V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2018, 77, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Wen, D.; Vanden Langenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Fan, R.; Zheng, R.; Li, C.-M.; Yu, D.-Y. Proteomic analysis of seed germination under salt stress in soybeans. J. Zhejiang Univ. Sci. B 2011, 12, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puyang, X.; An, M.; Han, L.; Zhang, X. Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol. Environ. Saf. 2015, 117, 96–106. [Google Scholar] [CrossRef]
- Anaya, F.; Fghire, R.; Issa Ali, O.; Wahbi, S.; Loutfi, K. Effet du stress salin sur la germination de fève (Vicia faba L.). In Proceedings of the 5ème Rencontre Nationale Gestion et Protection de l’Environnement G-ENVIRO5, Casablanca, Morocco, 28 May 2013. [Google Scholar]
- Yadav, P.; Maya, K.; Zakwan, A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Res. J. Seed Sci. 2011, 4, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Omid, A.; Farzad, S.-Z. Osmo and hydro priming improvement germination characteristics and enzyme activity of Mountain Rye (Secale montanum) seeds under drought stress. J. Stress Physiol. Biochem. 2012, 8, 253–261. [Google Scholar]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [Green Version]
- Boukari, N.; Jelali, N.; Renaud, J.B.; Youssef, R.B.; Abdelly, C.; Hannoufa, A. Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of Alfalfa. Environ. Exp. Bot. 2019, 167, 103820. [Google Scholar] [CrossRef]
- Kazemi, K.; Eskandari, H. Does priming improve seed performance under salt and drought stress. J. Basic Appl. Sci. Res. 2012, 2, 3503–3507. [Google Scholar]
- Gholami, M.; Mokhtarian, F.; Baninasab, B. Seed halopriming improves the germination performance of black seed (Nigella sativa) under salinity stress conditions. J. Crop. Sci. Biotechnol. 2015, 18, 21–26. [Google Scholar] [CrossRef]
- Nasri, N.; Kaddour, R.; Mahmoudi, H.; Baatour, O.; Bouraoui, N.; Lachaâl, M. The effect of osmopriming on germination, seedling growth and phosphatase activities of lettuce under saline condition. Afr. J. Biotechnol. 2011, 10, 14366–14372. [Google Scholar]
- Navin, P.; Pravin, P.; Kumar, T.S. Osmopriming of tomato genotypes with polyethylene glycol 6000 induces tolerance to salinity stress. Biosciences 2014, 7, 4412–4417. [Google Scholar]
- Abraha, B.; Yohannes, G. The role of seed priming in improving seedling growth of maize (Zea mays L.) under salt stress at field conditions. Agric. Sci. 2013, 4, 666–672. [Google Scholar]
- Tabatabaei, S. The effect of priming on germination indexes and seed reserve utilization of maize seeds under salinity stress. Seed Res. (J. Seed Sci. Technol.) 2014, 3, 44. [Google Scholar]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Farhoudi, R.; Saeedipour, S.; Mohammadreza, D. The effect of NaCl seed priming on salt tolerance, antioxidant enzyme activity, proline and carbohydrate accumulation of muskmelon (Cucumis melo L.) under saline condition. Afr. J. Agric. Res. 2011, 6, 1363–1370. [Google Scholar]
- Song, J.-Y.; Roe, J.-H. The role and regulation of Trxl, a cytosolic thioredoxin in Schizosaccharomyces pombe. J. Microbiol. 2008, 46, 408–414. [Google Scholar] [CrossRef]
- Elphick, C.; Sanders, D.; Maathuis, F. Critical role of divalent cations and Na+ efflux in Arabidopsis thaliana salt tolerance. Plant Cell Environ. 2001, 24, 733–740. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, N.; Jain, A.; Arya, K. Alleviation of salt stress in Cucumis sativus L. through seed priming with calcium chloride. Indian J. Appl. Res. 2011, 3, 22–25. [Google Scholar]
- Verma, J.; Srivastava, A. Physiological basis of salt stress resistance in pigeon pea (Cajanus cajan L.)-II. Pre-sowing seed soaking treatment in regulating early seedling metabolism during seed germination. Plant Physiol. Bio-Chem.-New Delhi 1998, 25, 89–94. [Google Scholar]
- Kaya, C.; Ak, B.E.; Higgs, D. Response of salt-stressed strawberry plants to supplementary calcium nitrate and/or potassium nitrate. J. Plant Nutr. 2003, 26, 543–560. [Google Scholar] [CrossRef]
- Elouaer, M.A.; Hannachi, C. Seed priming to improve germination and seedling growth of safflower (Carthamus tinctorius) under salt stress. Eur. Asian J. Biosci. 2012, 6, 76–84. [Google Scholar] [CrossRef]
- Ruan, S.; Xue, Q.; Tylkowska, K. The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci. Technol. 2002, 30, 61–67. [Google Scholar]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. Plant Physiol. 2002, 9, 172–174. [Google Scholar]
- Rabhi, M.; Ferchichi, S.; Jouini, J.; Hamrouni, M.H.; Koyro, H.-W.; Ranieri, A.; Abdelly, C.; Smaoui, A. Phyto desalination of a salt-affected soil with the halophyte Sesuvium portulacastrum L. to arrange in advance the requirements for the successful growth of a glycophytic crop. Bioresour. Technol. 2010, 101, 6822–6828. [Google Scholar]
- Abdelly, C.; Lachaâl, M.; Grignon, C.; Soltani, A.; Hajji, M. Association épisodiqued’halophytes stricts et de glycophytes dans un écosystème hydromorphesaléen zone semi-aride. Agronomie 1995, 15, 557–568. [Google Scholar] [CrossRef]
- Hafsi, C.; Lakhdhar, A.; Rabhi, M.; Debez, A.; Abdelly, C.; Ouerghi, Z. Interactive effects of salinity and potassium availability on growth, water status, and ionic composition of Hordeum maritimum. J. Plant Nutr. Soil Sci. 2007, 170, 469–473. [Google Scholar] [CrossRef]
- Zavariyan, A.M.; Rad, M.Y.; Asghari, M. Effect of seed priming by potassium nitrate on germination and biochemical indices in Silybummarianum L. under salinity stress. Int. J. Life Sci. 2015, 9, 23–29. [Google Scholar]
- Hessini, K.; Ferchichi, S.; Ben Youssef, S.; Werner, K.H.; Cruz, C.; Gandour, M. How does salinity duration affect growth and productivity of cultivated barley? Agron. J. 2015, 107, 174–180. [Google Scholar] [CrossRef]
- Shelden, M.C.; Dias, D.A.; Jayasinghe, N.S.; Bacic, A.; Roessner, U. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J. Exp. Bot. 2016, 67, 3731–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017, 7, 2035. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. Plant Stress Physiol. 2012, 1, 59–93. [Google Scholar]
- Chalbi, N.; Hessini, K.; Gandour, M.; Mohamed, S.N.; Smaoui, A.; Abdelly, C.; Youssef, N.B. Are changes in membrane lipids and fatty acid composition related to salt-stress resistance in wild and cultivated barley? J. Plant Nutr. Soil Sci. 2013, 176, 138–147. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants—A review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Peleg, Z.; Apse, M.P.; Blumwald, E. Engineering salinity and water-stress tolerance in crop plants: Getting closer to the field. Adv. Bot. Res. 2011, 57, 405–443. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of salinity on the in vitro development of glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS J. Integr. Biol. 2011, 15, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Proseus, T.E.; Boyer, J.S. Calcium deprivation disrupts enlargement of Chara corallina cells: Further evidence for the calcium pectate cycle. J. Exp. Bot. 2012, 63, 3953–3958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mahi, H.; Pérez-Hormaeche, J.; De Luca, A.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.; Norlyn, J.D.; Rush, D.W.; Kingsbury, R.W.; Kelley, D.B.; Cunningham, G.A.; Wrona, A.F. Saline culture of crops: A genetic approach. Science 1980, 210, 399–404. [Google Scholar] [CrossRef]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1109. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomic and transcriptomic analyses reveal the reasons why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Environ. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Seckin, B.; Turkan, I.; Sekmen, A.H.; Ozfidan, C. The role of antioxidant defense systems at differential salt tolerance of hordeum marinum huds. (Sea barley grass) and Hordeum vulgare L. (cultivated barley). Environ. Exp. Bot. 2010, 69, 76–85. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Maiti, R.; Pramanik, K. Vegetable seed priming: A low cost, simple and powerful techniques for farmers’ livelihood. Int. J. Bio-Resour. Stress Manag. 2013, 4, 475–481. [Google Scholar]
- Batista, T.B.; Binotti, F.F.D.S.; Cardoso, E.D.; Bardiviesso, E.M.; Costa, E. Aspectos fisiológicos e qualidade de mudas da pimenteiraem respostaao vigor e condicionamento das sementes. Bragantia 2015, 74, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Ahmadizadeh, M.; Rahbarian, P. Enhancing stand establishment of tomato cultivars under salt stress condition. South West. J. Hortic. Biol. Environ. 2014, 5, 19–42. [Google Scholar] [CrossRef]
- Saeidi, M.R.; Abdolghaium, A.; Hassanzadeh, M.; Rouhi, A.; Nikzad, P. Investigation of seed priming on some germination aspects of different canola cultivars. J. Food Agric. Environ. 2008, 6, 188. [Google Scholar]
- Liu, W.; Yuan, X.; Zhang, Y.; Xuan, Y.; Yan, Y. Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiol. Plant. 2014, 36, 2183–2193. [Google Scholar] [CrossRef]
- Anil, V.S.; Rajkumar, P.; Kumar, P.; Mathew, M. A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast: Modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. J. Biol. Chem. 2008, 283, 3497–3506. [Google Scholar] [CrossRef] [Green Version]
- Köster, P.; Wallrad, L.; Edel, K.H.; Faisal, M.; Alatar, A.A.; Kudla, J. The battle of two ions: Ca2+ signalling against Na+ stress. Plant Biol. 2019, 21, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jafar, M.Z.; Farooq, M.; Cheema, M.; Afzal, I.; Basra, S.M.; Wahid, M.A.; Aziz, T.; Shahid, M. Improving the performance of wheat by seed priming under saline conditions. J. Agron. Crop. Sci. 2011, 198, 38–45. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, J.; Hussain, M.; Jabbar, A.; Nadeem, G.A.; Sajid, M.; Subtain, M.U.; Shabbir, I. Seed priming a technique. Int. J. Agric. Crop. Sci. 2013, 6, 1373. [Google Scholar]
- Shehzad, M.; Ayub, M.; Ahmad, A.; Yaseen, M. Influence of priming techniques on emergence and seedling growth of forage sorghum (Sorghum bicolor L.). J. Anim. Plant. Sci. 2012, 22, 154–158. [Google Scholar]
- Ruttanaruangboworn, A.; Chanprasert, W.; Tobunluepop, P.; Onwimol, D. Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). J. Integr. Agric. 2017, 16, 605–613. [Google Scholar] [CrossRef]
- Osborne, J.; Fox, J.; Mercer, S. Germination response under elevated salinities of six semi-arid bluebush species (Western Australia). In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Berlin, Germany, 1993; pp. 323–338. [Google Scholar]
- Farhat, N.; Sassi, H.; Zorrig, W.; Abdelly, C.; Barhoumi, Z.; Smaoui, A.; Rabhi, M. Is excessive Ca the main factor responsible for Mg deficiency in Sulla carnosa on calcareous soils? J. Soils Sediments 2015, 15, 1483–1490. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. J. Plant Physiol. 2000, 157, 54–58. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; pp. 302–310. [Google Scholar]


| Treatment (mM NaCl) | C (Unprimed Seeds) + 0 | C (Unprimed Seeds) + 100 | C (Unprimed Seeds) + 200 | KCl + 100 | KCl + 200 | KNO3 + 100 | KNO3 + 200 | CaCl2 + 100 | CaCl2 + 200 |
|---|---|---|---|---|---|---|---|---|---|
| H.vulgare (L. Manel) | |||||||||
| Fe Co (mg/gDW) | 4994 ± 29 a | 2639 ± 141 c | 1414 ± 213 e | 3009 ± 331 bc | 2235 ± 126 d | 2974 ± 331 bc | 2540 ± 309 cd | 3359 ± 249 b | 2679 ± 357 c |
| Fe R (mg/gDW) | 2920 ± 45 a | 1919 ± 161 d | 1276 ± 90 f | 2688 ± 199 b | 1784 ± 102 e | 2524 ± 202 b | 2116 ± 79 cd | 2912 ± 128 a | 2292 ± 178 c |
| Ca Co (mg/gDW) | 18.25 ± 1.2 a | 11.68 ± 0.59 d | 7.60 ± 0.59 e | 14.91 ± 0.7 c | 11.04 ± 0.55 d | 13.63 ± 0.79 c | 11.10 ± 1.24 d | 19.75 ± 1.03 a | 16.66 ± 1.73 b |
| Ca R (mg/gDW) | 14.93 ± 0.9 c | 8.84 ± 1.14 f | 5.66 ± 1.14 g | 11.3 ± 0.74 de | 10.21 ± 0.76 e | 12.8 ± 1.36 cd | 1146 ± 0.9 de | 17.94 ± 0.78 a | 13.32 ± 1.26 b |
| Mg Co (mg/gDW) | 22.73 ± 1.5 a | 16.19 ± 1.11 d | 10.65 ± 1.11 e | 20.51 ± 1.14 b | 18.73 ± 1.37 bc | 20.16 ± 1.6 bc | 18.15 ± 0.68 c | 24.09 ± 1.68 a | 24.26 ± 1.7 a |
| Mg R (mg/gDW) | 25.15 ± 1.33 a | 17.58 ± 0.74 c | 11.62 ± 0.74 d | 23.5 ± 1.98 ab | 18.96 ± 0.99 c | 22.17 ± 2.36 b | 17.33 ± 0.4 c | 25.43 ± 2.26 a | 23.5 ± 1.99 ab |
| H. maritimum | |||||||||
| Fe Co (mg/gDW) | 1178 ± 34 a | 851 ± 26 e | 725 ± 19.04 f | 1051 ± 43 b | 896 ± 50 de | 1041 ± 41 b | 953 ± 18 c | 1022 ± 37 b | 918 ± 35 cd |
| Fe R (mg/gDW) | 992 ± 24 b | 921 ± 43 c | 646 ± 33.92 f | 916 ± 60 c | 737 ± 38 e | 982 ± 45 bc | 838 ± 31 d | 1055 ± 42 a | 936 ± 44 bc |
| Ca Co (mg/gDW) | 0.887 ± 0.049 a | 0.579 ± 0.06 cd | 0.373 ± 0.057 e | 0.639 ± 0.055 bc | 0.526 ± 0.060 d | 0.605 ± 0.055 c | 0.525 ± 0.031 d | 0.855 ± 0.042 a | 0.711 ± 0.035 b |
| Ca R (mg/gDW) | 0.391 ± 0.023 ab | 0.314 ± 0.030 c | 0.181 ± 0.03 e | 0.323 ± 0.031 c | 0.199 ± 0.026 e | 0.359 ± 0.015 b | 0.194 ± 0.013 e | 0.417 ± 0.025 a | 0.238 ± 0.023 d |
| Mg Co (mg/gDW) | 0.228 ± 0.026 a | 0.187 ± 0.017 c | 0.115 ± 0.017 e | 0.186 ± 0.024 c | 0.141 ± 0.009 de | 0.158 ± 0.017 d | 0.166 ± 0.016 d | 0.227 ± 0.016 ab | 0.2 ± 0.018 b |
| Mg R (mg/gDW) | 0.217 ± 0.024 a | 0.166 ± 0.009 c | 0.113 ± 0.009 f | 0.186 ± 0.013 b | 0.149 ± 0.013 d | 0.218 ± 0.017 a | 0.134 ± 0.008 e | 0.202 ± 0.017 ab | 0.146 ± 0.017 d |
| Treatment (mM NaCl) | C (Unprimed Seeds) + 0 | C (Unprimed Seeds) + 100 | C (Unprimed Seeds) + 200 | KCl + 100 | KCl + 200 | KNO3 + 100 | KNO3 + 200 | CaCl2 + 100 | CaCl2 + 200 |
|---|---|---|---|---|---|---|---|---|---|
| H.vulgare (L. Manel) | |||||||||
| Na Co (mg/gDW) | 0.066 ± 0.005 g | 0.879 ± 0.049 d | 1.63 ± 0.042 a | 0.67 ± 0.06 e | 1.262 ± 0.043 b | 0.617 ± 0.091 ef | 0.912 ± 0.052 d | 0.566 ± 0.051 f | 1.03 ± 0.07 c |
| Na R (mg/gDW) | 0.08 ± 0.011 h | 0.539 ± 0.023 d | 1.01 ± 0.023 a | 0.458 ± 0.037 b | 0.744 ± 0.031 b | 0.371 ± 0.044 f | 0.769 ± 0.05 b | 0.318 ± 0.033 g | 0.67 ± 0.027 c |
| K Co (mg/gDW) | 0.9 ± 0.036 a | 0.65 ± 0.02 c | 0.45 ± 0.013 e | 0.67 ± 0.03 bc | 0.599 ± 0.038 d | 0.719 ± 0.041 b | 0.658 ± 0.03 c | 0.695 ± 0.021 bc | 0.6 ± 0.016 d |
| K R (mg/gDW) | 0.778 ± 0.048 a | 0.365 ± 0.021 c | 0.15 ± 0.014 f | 0.391 ± 0.018 c | 0.2 ± 0.019 e | 0.453 ± 0.03 b | 0.228 ± 0.021 de | 0.403 ± 0.035 c | 0.26 ± 0.01 d |
| Na/K Co(mg/gDW) | 0.073 ± 0.007 g | 1.34 ± 0.116 d | 3.55 ± 0.058 a | 0.98 ± 0.089 e | 1.53 ± 0.153 c | 0.86 ± 0.142 ef | 1.924 ± 0.153 b | 0.814 ± 0.072 f | 1.29 ± 0.1 d |
| Na/K R (mg/gDW) | 0.054 ± 0.044 f | 1.46 ± 0.157 d | 6.739 ± 0.587 a | 1.16 ± 0.06 de | 3.75 ± 0.321 b | 0.824 ± 0.114 e | 3.414 ± 0.443 b | 0.807 ± 0.117 e | 2.573 ± 0.2 c |
| H. maritimum | |||||||||
| Na Co (mg/gDW) | 0.114 ± 0.015 g | 1.397 ± 0.1 c | 1.936 ± 0.059 a | 1.091 ± 0.083 e | 1.568 ± 0.77 b | 0.935 ± 0.086 f | 1.418 ± 0.084 c | 0.959 ± 0.076 f | 1.24 ± 0.056 d |
| Na R (mg/gDW) | 0.134 ± 0.011 e | 0.942 ± 0.058 bc | 1.134 ± 0.058 a | 0.91 ± 0.044 c | 1.005 ± 0.029 b | 0.876 ± 0.066 d | 0.957 ± 0.029 bc | 0.871 ± 0.048 d | 0.87 ± 0.05 d |
| K Co (mg/gDW) | 1.296 ± 0.056 a | 1.191 ± 0.03 b | 0.871 ± 0.067 e | 1.206 ± 0.02 b | 1.009 ± 0.06 d | 1.24 ± 0.06 ab | 0.936 ± 0.069 de | 1.213 ± 0.034 b | 1.095 ± 0.02 c |
| K R (mg/gDW) | 0.963 ± 0.33 ab | 0.89 ± 0.034 bc | 0.720 ± 0.04 e | 0.966 ± 0.03 ab | 0.84 ± 0.059 de | 0.87 ± 0.049 c | 0.761 ± 0.058 e | 0.989 ± 0.045 a | 0.92 ± 0.03 b |
| Na/K Co(mg/gDW) | 0.088 ± 0.013 f | 1.173 ± 0.08 c | 2.265 ± 0.169 a | 0.90 ± 0.066 d | 1.561 ± 0.145 b | 0.751 ± 0.067 e | 1.520 ± 0.099 b | 0.792 ± 0.08 de | 1.13 ± 0.067 c |
| Na/K R (mg/gDW) | 0.217 ± 0.024 e | 0.166 ± 0.009 c | 0.113 ± 0.009 a | 0.186 ± 0.013 cd | 0.149 ± 0.013 b | 0.218 ± 0.017 cd | 0.134 ± 0.008 b | 0.202 ± 0.017 d | 0.146 ± 0.017 cd |
| Treatments | 100 mM NaCl | 200 mM NaCl | 100 mM NaCl + KCl | 200 mM NaCl + KCl | 100 mM NaCl + KNO3 | 200 mM NaCl + KNO3 | 100 mM NaCl + CaCl2 | 200 mM NaCl + CaCl2 | |
|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||
| C L | −0.172 | −0.615 | 0.099 | −0.222 | 0.351 | −0.102 | 0.472 | 0.190 | |
| R L | 0.058 | −0.537 | 0.253 | −0.405 | 0.146 | −0.204 | 0.554 | 0.134 | |
| C DW | −0.298 | −0.565 | 0.140 | −0.302 | 0.407 | −0.143 | 0.570 | 0.191 | |
| R DW | −0.318 | −0.670 | 0.237 | −0.236 | 0.308 | −0.081 | 0.507 | 0.253 | |
| C MSI | 0.270 | 0.642 | 0.038 | 0.274 | −0.313 | −0.049 | −0.446 | −0.416 | |
| R MSI | 0.098 | 0.708 | −0.130 | 0.333 | −0.383 | −0.155 | −0.282 | −0.189 | |
| C MDA | 0.328 | 0.766 | −0.108 | 0.154 | −0.313 | −0.152 | −0.396 | −0.278 | |
| R MDA | 0.226 | 0.751 | −0.348 | 0.254 | −0.133 | −0.022 | −0.409 | −0.319 | |
| C Ca | −0.164 | −0.580 | 0.164 | −0.230 | 0.034 | −0.224 | 0.657 | 0.342 | |
| R Ca | −0.293 | −0.626 | −0.031 | −0.150 | 0.120 | −0.019 | 0.657 | 0.342 | |
| C Mg | −0.252 | −0.735 | 0.123 | −0.031 | 0.092 | −0.081 | 0.435 | 0.449 | |
| R Mg | −0.200 | −0.691 | 0.287 | −0.086 | 0.177 | −0.221 | 0.446 | 0.287 | |
| C Fe | 0.020 | −0.730 | 0.246 | −0.227 | 0.225 | −0.040 | 0.461 | 0.044 | |
| R Fe | −0.196 | −0.664 | 0.363 | −0.294 | 0.243 | −0.052 | 0.526 | 0.074 | |
| C K | 0.096 | −0.809 | 0.202 | −0.161 | 0.392 | 0.109 | 0.281 | −0.112 | |
| R K | 0.210 | −0.556 | 0.301 | −0.381 | 0.522 | −0.281 | 0.344 | −0.160 | |
| C Na | −0.074 | 0.754 | −0.304 | 0.347 | −0.363 | −0.037 | −0.419 | 0.098 | |
| R Na | −0.121 | 0.684 | −0.260 | 0.229 | −0.408 | 0.272 | −0.501 | 0.105 | |
| C Na/K | −0.086 | 0.901 | −0.246 | −0.004 | −0.303 | 0.172 | −0.324 | −0.109 | |
| R Na/K | −0.221 | 0.814 | −0.281 | 0.227 | −0.347 | 0.161 | −0.350 | −0.003 | |
| TG% | −0.065 | −0.733 | 0.283 | −0.239 | 0.196 | −0.152 | 0.457 | 0.254 | |
| Treatments | 100 mM NaCl | 200 mM NaCl | 100 mM NaCl + KCl | 200 mM NaCl + KCl | 100 mM NaCl + KNO3 | 200 mM NaCl + KNO3 | 100 mM NaCl + CaCl2 | 200 mM NaCl + CaCl2 | |
|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||
| C L | −0.099 | −0.291 | 0.107 | −0.243 | 0.107 | −0.291 | 0.363 | 0.347 | |
| R L | −0.006 | −0.779 | 0.121 | −0.190 | 0.195 | 0.140 | 0.287 | 0.232 | |
| C DW | 0.064 | −0.552 | 0.137 | −0.234 | 0.192 | −0.262 | 0.534 | 0.120 | |
| R DW | −0.059 | −0.410 | 0.099 | −0.422 | 0.057 | −0.016 | 0.591 | 0.160 | |
| C MSI | 0.090 | 0.626 | −0.031 | 0.334 | −0.097 | 0.097 | −0.497 | −0.521 | |
| R MSI | 0.206 | 0.511 | 0.100 | 0.325 | −0.147 | −0.228 | −0.410 | −0.357 | |
| C MDA | 0.060 | 0.536 | −0.463 | 0.349 | −0.426 | 0.219 | −0.458 | 0.181 | |
| R MDA | 0.178 | 0.731 | −0.150 | 0.330 | −0.374 | −0.109 | −0.487 | −0.118 | |
| C Ca | −0.058 | −0.606 | 0.099 | −0.201 | 0.009 | −0.204 | 0.671 | 0.290 | |
| R Ca | 0.158 | −0.430 | 0.198 | −0.349 | 0.357 | −0.372 | 0.614 | −0.177 | |
| C Mg | 0.150 | −0.585 | 0.133 | −0.318 | −0.150 | −0.066 | 0.552 | 0.284 | |
| R Mg | 0.015 | −0.537 | 0.222 | −0.157 | 0.567 | −0.312 | 0.394 | −0.192 | |
| C Fe | −0.279 | −0.714 | 0.410 | −0.123 | 0.375 | 0.071 | 0.309 | −0.049 | |
| R Fe | 0.120 | −0.665 | 0.107 | −0.405 | 0.294 | −0.118 | 0.503 | 0.163 | |
| C K | 0.250 | −0.594 | 0.291 | −0.228 | 0.396 | −0.423 | 0.310 | −0.001 | |
| R K | 0.097 | −0.572 | 0.361 | −0.119 | 0.004 | −0.417 | 0.446 | 0.198 | |
| C Na | 0.085 | 0.731 | −0.262 | 0.280 | −0.440 | 0.110 | −0.412 | −0.091 | |
| R Na | −0.023 | 0.732 | −0.118 | 0.227 | −0.280 | 0.037 | −0.299 | −0.275 | |
| C Na/K | −0.069 | 0.780 | −0.278 | 0.232 | −0.398 | 0.200 | −0.366 | −0.100 | |
| R Na/K | −0.099 | 0.760 | −0.2622 | 0.149 | −0.166 | 0.250 | −0.374 | −0.257 | |
| TG% | 0.027 | −0.568 | 0.2434 | −0.243 | 0.135 | −0.270 | 0.459 | 0.216 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Youssef, R.; Jelali, N.; Boukari, N.; Albacete, A.; Martinez, C.; Alfocea, F.P.; Abdelly, C. The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress. Plants 2021, 10, 2264. https://doi.org/10.3390/plants10112264
Ben Youssef R, Jelali N, Boukari N, Albacete A, Martinez C, Alfocea FP, Abdelly C. The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress. Plants. 2021; 10(11):2264. https://doi.org/10.3390/plants10112264
Chicago/Turabian StyleBen Youssef, Rim, Nahida Jelali, Nadia Boukari, Alfonso Albacete, Cristina Martinez, Francisco Perez Alfocea, and Chedly Abdelly. 2021. "The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress" Plants 10, no. 11: 2264. https://doi.org/10.3390/plants10112264
APA StyleBen Youssef, R., Jelali, N., Boukari, N., Albacete, A., Martinez, C., Alfocea, F. P., & Abdelly, C. (2021). The Efficiency of Different Priming Agents for Improving Germination and Early Seedling Growth of Local Tunisian Barley under Salinity Stress. Plants, 10(11), 2264. https://doi.org/10.3390/plants10112264








