Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Concentration of Total Plasma Protein
2.3. Concentration of Fibronectin
2.4. FN Molecular Forms Revealed by FN Immunoblotting
2.5. Frequency of Occurrence and Relative Amounts of Plasma FN-Fibrin Complexes
2.6. Receiver Operating Characteristics (ROC) Curve Analysis
3. Discussion
4. Materials and Methods
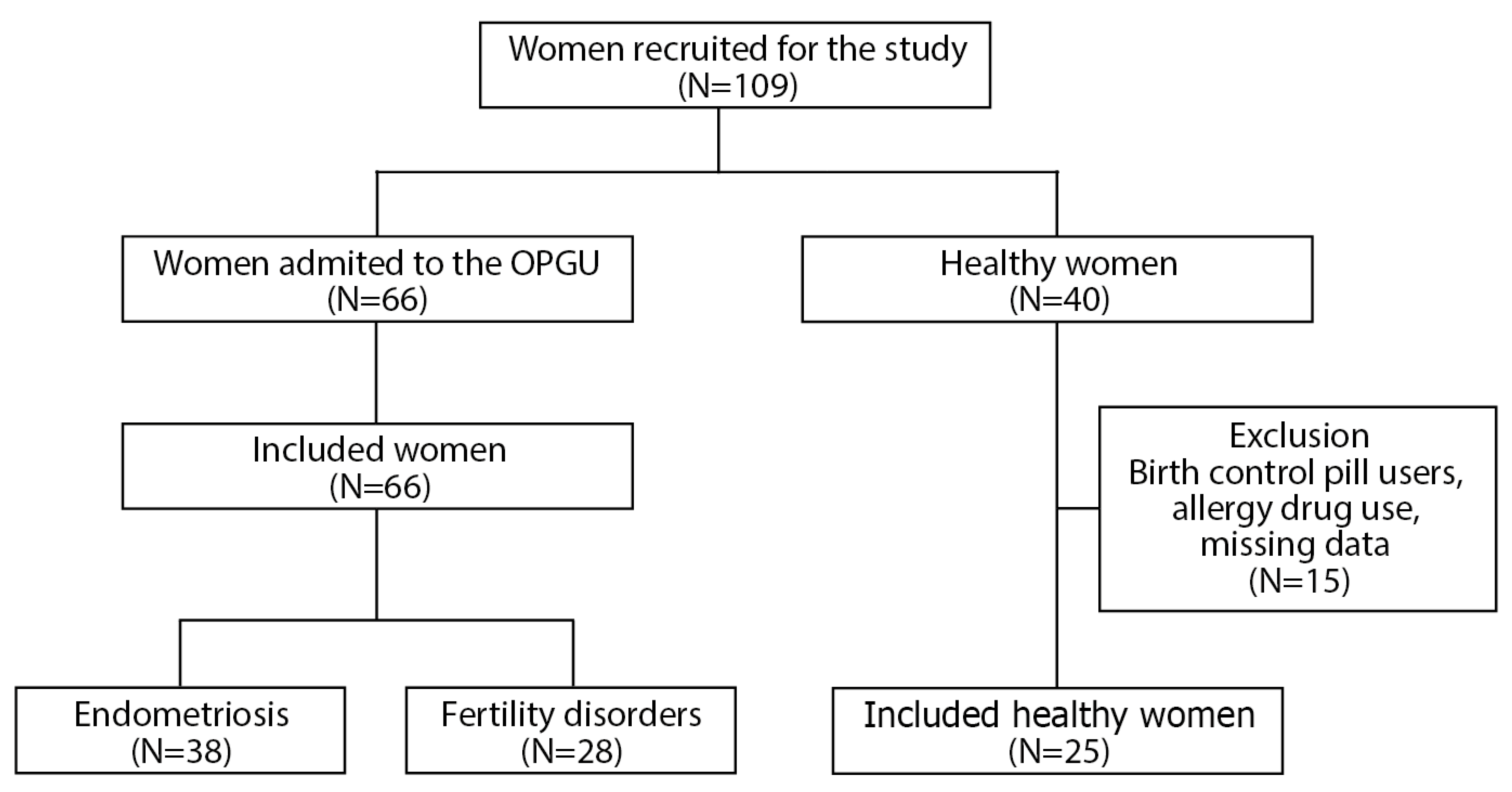
4.1. The Recruitment of Women
4.2. Blood Collection
4.3. Sample Pre-Treatment for Analysis
4.4. Determination of FN Concentration
4.5. Revealing of FN-Fibrin Complexes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fassbender, A.; Burney, R.O.; D’Hooghe, T.; Giudice, L. Update on Biomarkers for the Detection of Endometriosis. BioMed Res. Int. 2015, 2015, 130854. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Dittfeld, L.; Lazzeri, P.; Tomaiuolo, R.; Tasciotti, E. Unraveling the Balance between Genes, Microbes, Lifestyle and the Environment to Improve Healthy Reproduction. Genes 2021, 12, 605. [Google Scholar] [CrossRef]
- Tarín, J.J.; García-Pérez, M.A.; Hamatani, T.; Cano, A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod. Biol. Endocrinol. 2015, 13, 31. [Google Scholar] [CrossRef]
- Owiredu, W.K.B.A.; Ofori, P.N.; Turpin, C.A.; Obirikorang, C.; Acheampong, E.; Anto, E.O.; Owiredu, E.-W.; Adu, E.A. Weight management merits attention in women with infertility: A cross-sectional study on the association of anthropometric indices with hormonal imbalance in a Ghanaian population. BMC Res. Notes 2019, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Pylyp, L.Y.; Spynenko, L.O.; Verhoglyad, N.V.; Mishenko, A.O.; Mykytenko, D.O.; Zukin, V.D. Chromosomal abnormalities in products of conception of first-trimester miscarriages detected by conventional cytogenetic analysis: A review of 1000 cases. J. Assist. Reprod. Genet. 2017, 35, 265–271. [Google Scholar] [CrossRef]
- Tao, X.; Ge, S.-Q.; Chen, L.; Cai, L.-S.; Hwang, M.-F.; Wang, C.-L. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics 2018, 73. [Google Scholar] [CrossRef]
- Diamond, M.P.; Legro, R.; Coutifaris, C.; Alvero, R.; Robinson, R.D.; Casson, P.A.; Christman, G.M.; Huang, H.; Hansen, K.R.; Baker, V.; et al. Sexual function in infertile women with polycystic ovary syndrome and unexplained infertility. Am. J. Obstet. Gynecol. 2017, 217. 191.e1–191.e19. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, 529. [Google Scholar] [CrossRef]
- Keckstein, J.; Becker, C.M.; Canis, M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Nisolle, M.; Roman, H.; Saridogan, E.; Tanos, V.; et al. Recommendations for the Surgical Treatment of Endometriosis. Part 2: Deep Endometriosis. Hum. Reprod. Open 2020, 2020, hoaa002. [Google Scholar]
- Nirgianakis, K.; Ma, L.; McKinnon, B.; Mueller, M.D. Recurrence Patterns after Surgery in Patients with Different Endometriosis Subtypes: A Long-Term Hospital-Based Cohort Study. J. Clin. Med. 2020, 9, 496. [Google Scholar] [CrossRef]
- Lousse, J.-C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal Endometriosis Is an Inflammatory Disease. Front. Biosci. 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Moradi, M.; Parker, M.A.; Sneddon, A.; Lopez, V.; Ellwood, D. Impact of endometriosis on women’s lives: A qualitative study. BMC Womens Health 2014, 14, 123. [Google Scholar] [CrossRef]
- Asghari, S.; Valizadeh, A.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Endometriosis: Perspective, lights, and shadows of etiology. Biomed. Pharmacother. 2018, 106, 163–174. [Google Scholar] [CrossRef]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Barbosa, C.P.; De Souza, A.M.B.; Bianco, B.; Christofolini, D.M. The effect of hormones on endometriosis development. Minerva Ginecol. 2011, 63, 375–386. [Google Scholar]
- Johnston, J.L.; Reid, H.; Hunter, D. Diagnosing endometriosis in primary care: Clinical update. Br. J. Gen. Pract. 2015, 65, 101–102. [Google Scholar] [CrossRef]
- van der Zanden, M.; de Kok, L.; Nelen, W.L.D.M.; Braat, D.D.M.; Nap, A.W. Strengths and weaknesses in the diagnostic process of endometriosis from the patients’ perspective: A focus group study. Diagnosis 2020, 8, 333–339. [Google Scholar] [CrossRef]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 21, 2840. [Google Scholar] [CrossRef]
- Harrington, D.J.; Lessey, B.A.; Rai, V.; Bergqvist, A.; Kennedy, S.; Manek, S.; Barlow, D.H.; Mardon, H.J. Tenascin is differentially expressed in endometrium and endometriosis. J. Pathol. 1999, 187, 242–248. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Aredo, J.V.; Heyrana, K.J.; Karp, B.I.; Shah, J.P.; Stratton, P. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin. Reprod. Med. 2017, 35, 088–097. [Google Scholar] [CrossRef]
- Bulun, S.; Yilmaz, B.; Sison, C.A.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Yang, W.-C.V.; Au, H.-K.; Chang, C.-W.; Chen, H.-W.; Chen, P.-H.; Chen, C.-C.; Tang, Y.-L.; Wang, I.-T.; Tzeng, C.-R. Matrix remodeling and endometriosis. Reprod. Med. Biol. 2005, 4, 93–99. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and Endometriosis. Front. Biosci. 2016, 21, 941–948. [Google Scholar]
- Machairiotis, N.; Vasilakaki, S.; Thomakos, N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021, 9, 54. [Google Scholar] [CrossRef]
- Coutinho, L.M.; Ferreira, M.C.; Rocha, A.L.L.; Carneiro, M.M.; Reis, F.M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. [Google Scholar] [CrossRef]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Filip, L.; Duică, F.; Prădatu, A.; Crețoiu, D.; Suciu, N.; Crețoiu, S.; Predescu, D.-V.; Varlas, V.; Voinea, S.-C. Endometriosis Associated Infertility: A Critical Review and Analysis on Etiopathogenesis and Therapeutic Approaches. Medicina 2020, 56, 460. [Google Scholar] [CrossRef]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, H. Fibronectin maintains the balance between hemostasis and thrombosis. Cell. Mol. Life Sci. 2016, 73, 3265–3277. [Google Scholar] [CrossRef]
- Wang, Y.; Reheman, A.; Spring, C.M.; Kalantari, J.; Marshall, A.H.; Wolberg, A.S.; Gross, P.L.; Weitz, J.I.; Rand, M.L.; Mosher, D.F.; et al. Plasma Fibronectin Supports Hemostasis and Regulates Thrombosis. J. Clin. Invest. 2014, 124, 4281–4293. [Google Scholar] [CrossRef] [PubMed]
- Makogonenko, E.; Tsurupa, G.; Ingham, K.; Medved, L. Interaction of Fibrin(ogen) with Fibronectin: Further Characterization and Localization of the Fibronectin-Binding Site. Biochemistry 2002, 41, 7907–7913. [Google Scholar] [CrossRef]
- Bae, E.; Sakai, T.; Mosher, D.F. Assembly of Exogenous Fibronectin by Fibronectin-null Cells Is Dependent on the Adhesive Substrate. J. Biol. Chem. 2004, 279, 35749–35759. [Google Scholar] [CrossRef]
- White, E.; Muro, A.F. Fibronectin splice variants: Understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life 2011, 63, 538–546. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2013, 12, 313–316. [Google Scholar] [CrossRef]
- Kauma, S.; Clark, M.R.; White, C.; Halme, J. Production of fibronectin by peritoneal macrophages and concentration of fibronectin in peritoneal fluid from patients with or without endometriosis. Obstet. Gynecol. 1988, 72, 13–18. [Google Scholar]
- Chen, Y.; Li, H.; Cheng, H.-Y.; Rui-Qiong, M.; Ye, X.; Cui, H.; Hong-Lan, Z.; Chang, X.-H. Fibrinogen alpha chain is up-regulated and affects the pathogenesis of endometriosis. Reprod. Biomed. Online 2019, 39, 893–904. [Google Scholar] [CrossRef] [PubMed]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef]
- Aziz-Seible, R.S.; Casey, C.A. Fibronectin: Functional character and role in alcoholic liver disease. World J. Gastroenterol. 2011, 17, 2482–2499. [Google Scholar] [CrossRef]
- Barker, T.H.; Engler, A.J. The provisional matrix: Setting the stage for tissue repair outcomes. Matrix Biol. 2017, 60–61, 1–4. [Google Scholar] [CrossRef]
- Holzer, I.; Machado Weber, A.; Marshall, A.; Freis, A.; Jauckus, J.; Strowitzki, T.; Germeyer, A. GRN, NOTCH3, FN1, and PINK1 Expression in Eutopic Endometrium—Potential Biomarkers in the Detection of Endometriosis—A Pilot Study. J. Assist. Reprod. Genet. 2020, 37, 2723–2732. [Google Scholar] [CrossRef]
- Nassif, J.; Abbasi, S.A.; Nassar, A.; Abu-Musa, A.; Eid, A.A. The role of NADPH-derived reactive oxygen species production in the pathogenesis of endometriosis: A novel mechanistic approach. J. Boil. Regul. Homeost. Agents 2016, 30, 31–40. [Google Scholar]
- Ekarattanawong, S.; Tanprasertkul, C.; Somprasit, C.; Chamod, P.; Tiengtip, R.; Bhamarapravatana, K.; Suwannarurk, K. Possibility of using superoxide dismutase and glutathione peroxidase as endometriosis biomarkers. Int. J. Womens Health 2017, 9, 711–716. [Google Scholar] [CrossRef]
- Lemańska-Perek, A.; Polańska, B.; Krzyżanowska-Gołąb, D.; Kątnik-Prastowska, I. Occurrence of soluble supra-molecular FN–fibrin complexes in the plasma of children with recurrent respiratory infection. Ann. Clin. Biochem. Int. J. Lab. Med. 2014, 52, 441–447. [Google Scholar] [CrossRef]
- Lemańska-Perek, A.; Krzyżanowska-Gołąb, D.; Pupek, M.; Klimeczek, P.; Witkiewicz, W.; Kątnik-Prastowska, I. Analysis of Soluble Molecular Fibronectin-Fibrin Complexes and EDA-Fibronectin Concentration in Plasma of Patients with Atherosclerosis. Inflammation 2016, 39, 1059–1068. [Google Scholar] [CrossRef]
- Pupek, M.; Pawłowicz, R.; Lindner, K.; Krzyżanowska-Gołąb, D.; Lemańska-Perek, A.; Panaszek, B.; Kątnik-Prastowska, I. Occurrence of fibronectin–fibrin complexes in plasma of patients with multimorbidity due to the inflammaging phenomenon. Exp. Gerontol. 2016, 77, 19–28. [Google Scholar] [CrossRef]
- Pupek, M.; Krzyżanowska-Gołąb, D.; Kotschy, D.; Witkiewicz, W.; Kwiatkowska, W.; Kotschy, M.; Kątnik-Prastowska, I. Time-dependent changes in extra-domain A-fibronectin concentration and relative amounts of fibronectin-fibrin complexes in plasma of patients with peripheral arterial disease after endovascular revascularisation. Int. Wound J. 2018, 15, 649–659. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Berghausen-Mazur, M.; Kątnik-Prastowska, I.; Orczyk-Pawiłowicz, M. Delivery-associated presence of supramolecular fibronectin-fibrin complexes in puerperal and cord plasma. J. Matern. Neonatal Med. 2018, 32, 3581–3588. [Google Scholar] [CrossRef]
- Jawor, P.; Krzyżanowska-Gołąb, D.; Bajzert, J.; Stefaniak, T.; Kątnik-Prastowska, I. Changes of plasma fibronectin and fibronectin-fibrin complexes in dams of stillborn dairy calves. Ir. Vet. J. 2020, 73, 17. [Google Scholar] [CrossRef]
- Schaal-Jensen, R.; Kiehr, B.; Boesen, H.T.; Krabbe, J.S.; Sommer, C.; Jacobsen, H.; Oleksiewicz, M.B. Characterization of High Molecular Weight Plasma Protein Complexes Induced by Clotting Factor RFXIII-Treatment in the Cynomolgus Monkey. J. Thromb. Haemost. 2007, 5, 2070–2078. [Google Scholar] [CrossRef]
- Cho, J.; Mosher, D.F. Role of fibronectin assembly in platelet thrombus formation. J. Thromb. Haemost. 2006, 4, 1461–1469. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, H. Fibronectin: Extra Domain Brings Extra Risk? Blood 2015, 125, 3043–3044. [Google Scholar] [CrossRef][Green Version]
- Krzyżanowska-Gołąb, D.; Lemańska-Perek, A.; Pupek, M.; Lindner, K.; Polańska, B.; Porębska, I.; Kątnik-Prastowska, I. Identification of Soluble Supramolecular FN-fibrin Complexes in Human Plasma. J. Immunoass. Immunochem. 2014, 35, 412–427. [Google Scholar] [CrossRef]
- Matalliotaki, C.; Matalliotakis, M.; Rahmioglu, N.; Mavromatidis, G.; Matalliotakis, I.; Koumantakis, G.; Zondervan, K.; Spandidos, D.; Goulielmos, G.N.; Zervou, M.I. Role of FN1 and GREB1 gene polymorphisms in endometriosis. Mol. Med. Rep. 2019, 20, 111–116. [Google Scholar] [CrossRef]
- Chen, I.; Veth, V.B.; Choudhry, A.J.; Murji, A.; Zakhari, A.; Black, A.Y.; Agarpao, C.; Maas, J.W. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database Syst. Rev. 2020, 2020, CD003678. [Google Scholar] [CrossRef]
- Kokot, I.; Piwowar, A.; Jędryka, M.; Sołkiewicz, K.; Kratz, E. Diagnostic Significance of Selected Serum Inflammatory Markers in Women with Advanced Endometriosis. Int. J. Mol. Sci. 2021, 22, 2295. [Google Scholar] [CrossRef]
- Clarke, M.; Burzynski, L.; Humphry, M.; Pyrillou, K.; Wiggins, K.; Chan, J.; Figg, N.; Kitt, L.; Summers, C.; Tatham, K.; et al. The Coagulation and Immune Systems are Directly Linked through the Activation of Interleukin-1α by Thrombin. Immunity 2019, 50, 1033–1042. [Google Scholar] [CrossRef]
- Rocha, A.; Reis, F.M.; Taylor, R.N. Angiogenesis and Endometriosis. Obstet. Gynecol. Int. 2013, 2013, 859619. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, e12622. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal Endometriosis, Ovarian Endometriosis, and Adenomyotic Nodules of the Rectovaginal Septum Are Three Different Entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Yates, C.C.; Bodnar, R.; Wells, A. Matrix Control of Scarring. Cell. Mol. Life Sci. 2011, 68, 1871–1881. [Google Scholar] [CrossRef]
- van der Poll, T.; de Boer, J.D.; Levi, M. The Effect of Inflammation on Coagulation and Vice Versa. Curr. Opin. Infect. Dis. 2011, 24, 273–278. [Google Scholar] [CrossRef]
- Ding, D.; Liu, X.; Duan, J.; Guo, S.-W. Platelets are an unindicted culprit in the development of endometriosis: Clinical and experimental evidence. Hum. Reprod. 2015, 30, 812–832. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Hu, C.; Pang, B.; Ma, Z.; Yi, H. Immunophenotypic Profiles in Polycystic Ovary Syndrome. Mediat. Inflamm. 2020, 2020, 5894768. [Google Scholar] [CrossRef]
- Keski-Nisula, L.T.; Aalto, M.-L.; Kirkinen, P.P.; Kosma, V.-M.; Heinonen, S.T. Myometrial Inflammation in Human Delivery and Its Association with Labor and Infection. Am. J. Clin. Pathol. 2003, 120, 217–224. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A.; Ascaso, F.J.; Huerva, V. Age-Related Macular Degeneration in the Aspect of Chronic Low-Grade Inflammation (Pathophysiological ParaInflammation). Mediat. Inflamm. 2014, 2014, 930671. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goldsmith, L.T.; Taylor, R.N.; Bellet, D.; Taylor, H.S. Inflammation in Reproductive Disorders. Reprod. Sci. 2009, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.L.; Reis, F.M.; Petraglia, F. New trends for the medical treatment of endometriosis. Expert Opin. Investig. Drugs 2012, 21, 905–919. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum. Reprod. Update 2000, 6, 45–55. [Google Scholar] [CrossRef]
- Machado, D.E.; Abrao, M.S.; Berardo, P.T.; Takiya, C.M.; Nasciutti, L.E. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil. Steril. 2008, 90, 148–155. [Google Scholar] [CrossRef]
- Adamson, G.D. Endometriosis classification. Curr. Opin. Obstet. Gynecol. 2011, 23, 213–220. [Google Scholar] [CrossRef]
- Orczyk-Pawiłowicz, M.; Hirnle, L.; Berghausen-Mazur, M.; Kątnik-Prastowska, I. Terminal glycotope expression on milk fibronectin differs from plasma fibronectin and changes over lactation. Clin. Biochem. 2015, 48, 167–173. [Google Scholar] [CrossRef]
- Perutelli, P.; Boeri, E.; Mori, P.G. A rapid and sensitive method for the analysis of von Willebrand factor multimeric structure. Haematologica 1997, 82, 510. [Google Scholar]



| Endometriosis N = 38 (% (n/N)) | Fertility Disorders N = 28 (% (n/N)) | Normal Group N = 25 (% (n/N)) | Chi-Square Test χ2 | p-Value | |
|---|---|---|---|---|---|
Race/ethnicity
| 100% (38/38) | 100% (28/28) | 100% (25/25) | NA | NA |
| Women′s age | 22.8 | 0.0008 | |||
| (mean ± SD) | 32.7 ± 5.3 | 35.2 ± 3.7 | 27.0 ± 5.2 | ||
| 26.3% (10/38) | 3.6% (1/28) | 80.0% (20/25) | ||
| 34.2% (13/38) | 50.0% (14/28) | 4.0% (1/25) | ||
| 31.6% (12/38) | 32.1% (9/28) | 16.0% (4/25) | ||
| 7.9% (3/38) | 14.3% (4/28) | 0.0% (0/25) | ||
| Women′s BMI, kg/m2 | 16.7 | 0.01 | |||
| (mean ± SD) | 21.7 ± 2.7 | 24.3 ± 4.1 | 22.4 ± 2.7 | ||
| 13.5% (5/37) | 0.0% (0/28) | 8.0% (2/25) | ||
| 78.4% (29/37) | 71.4% (20/28) | 72.0% (18/25) | ||
| 8.1% (3/37) | 10.7% (3/28) | 20.0% (5/25) | ||
| 0.0% (0/37) | 17.9% (5/28) | 0.0% (0/25) | ||
| Parity | 5.7 | 0.2 | |||
| 89.5% (34/38) | 92.8% (26/28) | 100.0% (19/19) | ||
| 2.6% (1/38) | 7.1% (2/28) | 0.0% (0/19) | ||
| 7.9% (3/38) | 0.0% (0/28) | 0.0% (0/19) | ||
| Miscarriages | 5.7 | 0.2 | |||
| 81.6% (31/38) | 92.8% (26/28) | 100.0% (19/19) | ||
| 15.8% (6/38) | 3.6% (1/28) | 0.0% (0/19) | ||
| 2.6% (1/38) | 3.6% (1/28) | 0.0% (0/19) | ||
| Stages of endometriosis according to rASRM classification | NA | NA | |||
| 23.7% (9/38) | 0.0 | 0.0 | ||
| 76.3% (29/38) | 0.0 | 0.0 | ||
| Hypothyroidism | 13.2% (5/38) | 50.0% (14/28) | 0.0 | NA | NA |
| Insulin resistance | 7.9% (3/38) | 32.1% (9/28) | 0.0 | NA | NA |
| Polycystic ovary syndrome | 2.6% (1/38) | 10.7% (3/28) | 0.0 | NA | NA |
| Frequency of Occurrence and Relative Amount of FN Forms Mean Value of Relative Amount ± SD Median (25th–75th) Range | p-Value E vs. N Group | p-Value FD vs. N Group | ||||
|---|---|---|---|---|---|---|
| Plasma FN Forms | No Band MW (kDa) | Endometriosis (E) N = 38 | Fertility Disorders (FD) N = 28 | Normal (N) N = 25 | ||
| Protein concentration [g/L] | 62.16 ± 12.93 61.58 (55.03–67.26) 45.38–115.39 | 59.72 ± 9.92 60.52 (53.52–64.01) 36.93–84.04 | 60.97 ± 7.21 59.89 (57.73–65.81) 45.96–73.84 | p > 0.82 | p > 0.47 | |
| FN concentration [mg/L] | 292.61 ± 96.17 283.91 (227.11–364.76) 125.40–505.46 | 287.53 ± 122.68 262.85 (209.98–352.33) 110.95–687.68 | 226.55 ± 91.98 216.00 (183.79–248.00) 124.00–546.58 | p < 0.002 | p < 0.02 | |
| FN monomer ± degradations fragments | ∼220–280 | Not detected | Not detected | 0.12 (3/25) 1.96 ± 5.45 0.00 (0.00–0.00) 0.00–18.03 | NA | NA |
| FN dimer | ∼500 | 1 (38/38) 49.45 ± 22.19 43.15 (30.97–66.67) 17.96–87.86 | 1 (28/28) 43.43 ± 22.32 40.27 (26.11–68.00) 9.68–83.10 | 1 (25/25) 84.80 ± 18.88 100.00 (69.30–100.00) 53.67–100.00 | p < 0.000001 | p < 0.000001 |
| FN-fibrin complexes | I ∼750 | 1 (38/38) 29.75 ± 6.88 32.08 (26.20–33.74) 12.14–39.25 | 1 (28/28) 30.20 ± 5.58 30.92 (26.87–33.86) 16.90–41.94 | 0.48 (12/25) 12.95 ± 15.75 0.00 (0.00–28.48) 0.00–45.24 | p < 0.00002 | p < 0.00006 |
| II ∼1000 | 0.61 (23/38) 10.70 ± 9.60 13.87 (0.00–19.41) 0.00–25.89 | 0.75 (21/28) 14.69 ± 11.61 16.21 (2.38–22.25) 0.00–48.39 | 0.04 (1/25) 0.29 ± 1.44 0.00 (0.00–0.00) 0.00–7.20 | p < 0.00005 | p < 0.000001 | |
| III ∼1300 | 0.55 (21/38) 6.91 ± 6.86 7.29 (0.00–12.96) 0.00–18.72 | 0.64 (18/28) 7.66 ± 7.02 7.47 (0.00–13.21) 0.00–21.74 | Not detected | NA | NA | |
| IV ∼1600 | 0.45 (17/38) 2.94 ± 4.09 0.00 (0.00–5.12) 0.00–15.92 | 0.42(12/28) 3.38 ± 4.94 0.00 (0.00–6.00) 0.00–17.39 | Not detected | NA | NA | |
| V ∼1900 | 0.05 (2/38) 0.24 ± 1.09 0.00 (0.00–0.00) 0.00–6.12 | 0.14 (4/28) 0.65 ± 1.83 0.00 (0.00–0.00) 0.00–7.45 | Not detected | NA | NA | |
| Parameter | AUC | AUC with 95% Confidence Interval (Lower–Upper) | Cut-Off Point | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|---|---|
| Endometriosis | ||||||
| FN concentration [mg/L] | 0.722 | 0.590–0.854 | 228.36 | 0.7368 | 0.28 | <0.001 |
| FN dimer 500 kDa | 0.868 | 0.781–0.956 | 87.86 | 1 | 0.4 | <0.0001 |
| FN-fibrin complex 750 kDa | 0.805 | 0.681–0.929 | 12.14 | 1 | 0.4 | <0.0001 |
| Fertility Disorders | ||||||
| FN concentration [mg/L] | 0.676 | 0.528–0.824 | 284.39 | 0.5 | 0.12 | <0.02 |
| FN dimer 500 kDa | 0.904 | 0.828–0.981 | 53.45 | 0.6786 | 0 | <0.0001 |
| FN-fibrin complex 750 kDa | 0.809 | 0.681–0.936 | 16.90 | 1 | 0.4 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Kuberka, J.; Kubik, P.; Chrobak, A.; Pająk, J.; Chełmońska-Soyta, A.; Orczyk-Pawiłowicz, M. Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders. Int. J. Mol. Sci. 2021, 22, 11410. https://doi.org/10.3390/ijms222111410
Lis-Kuberka J, Kubik P, Chrobak A, Pająk J, Chełmońska-Soyta A, Orczyk-Pawiłowicz M. Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders. International Journal of Molecular Sciences. 2021; 22(21):11410. https://doi.org/10.3390/ijms222111410
Chicago/Turabian StyleLis-Kuberka, Jolanta, Paulina Kubik, Agnieszka Chrobak, Jarosław Pająk, Anna Chełmońska-Soyta, and Magdalena Orczyk-Pawiłowicz. 2021. "Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders" International Journal of Molecular Sciences 22, no. 21: 11410. https://doi.org/10.3390/ijms222111410
APA StyleLis-Kuberka, J., Kubik, P., Chrobak, A., Pająk, J., Chełmońska-Soyta, A., & Orczyk-Pawiłowicz, M. (2021). Fibronectin Molecular Status in Plasma of Women with Endometriosis and Fertility Disorders. International Journal of Molecular Sciences, 22(21), 11410. https://doi.org/10.3390/ijms222111410






