Insights into the Phytochemical and Multifunctional Biological Profile of Spices from the Genus Piper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Phytochemical Characterization
2.3. Antimicrobial Assay
2.4. Antioxidant, Cholinesterase, Amylase and Glucosidase Assays
2.5. Cell Viability Assay
2.6. Melanin Assay
2.7. Tyrosinase Assay
2.7.1. Mushroom Tyrosinase Assay
2.7.2. Murine Tyrosinase Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Characterization
3.2. Antimicrobial Activity
3.3. Antioxidant Activity
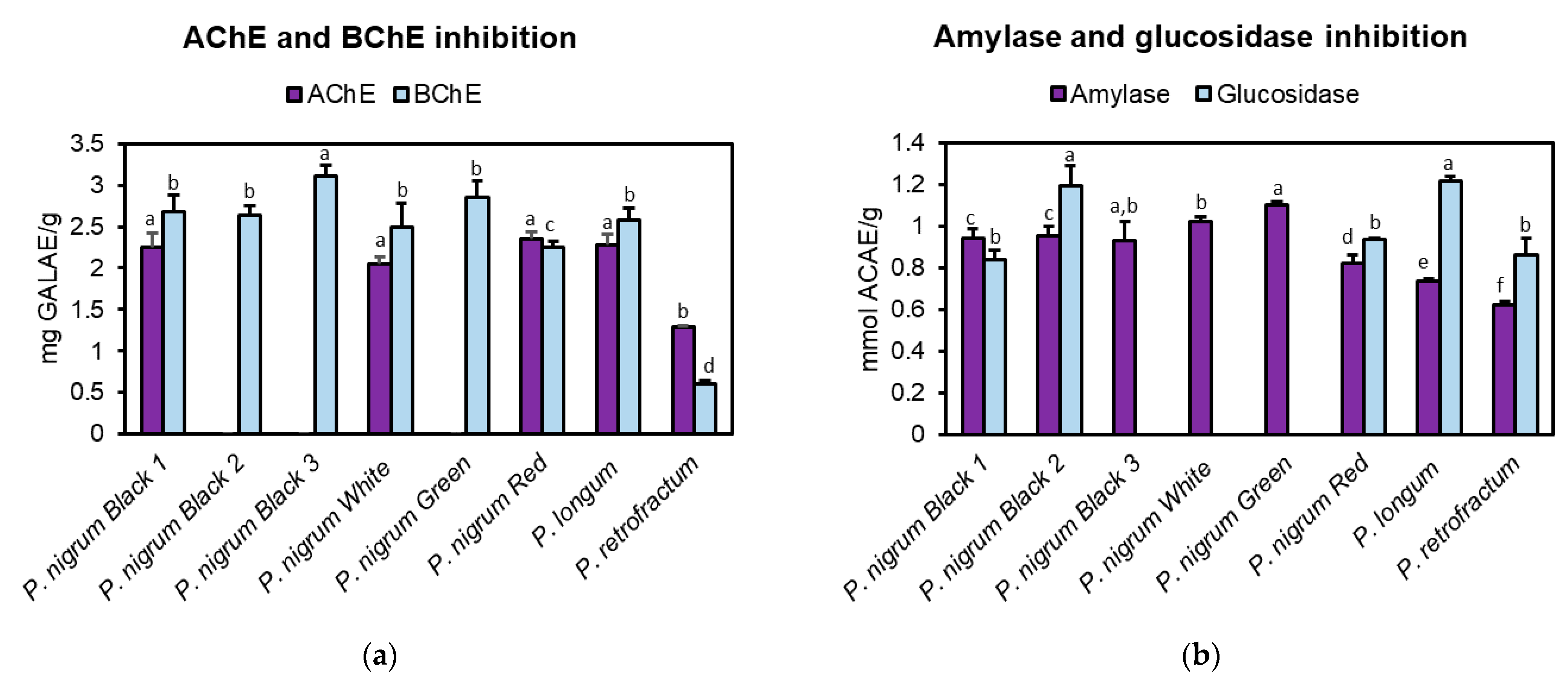
3.4. Anti-Cholinesterase Activity
3.5. Anti-Amylase and Anti-Glucosidase Activity
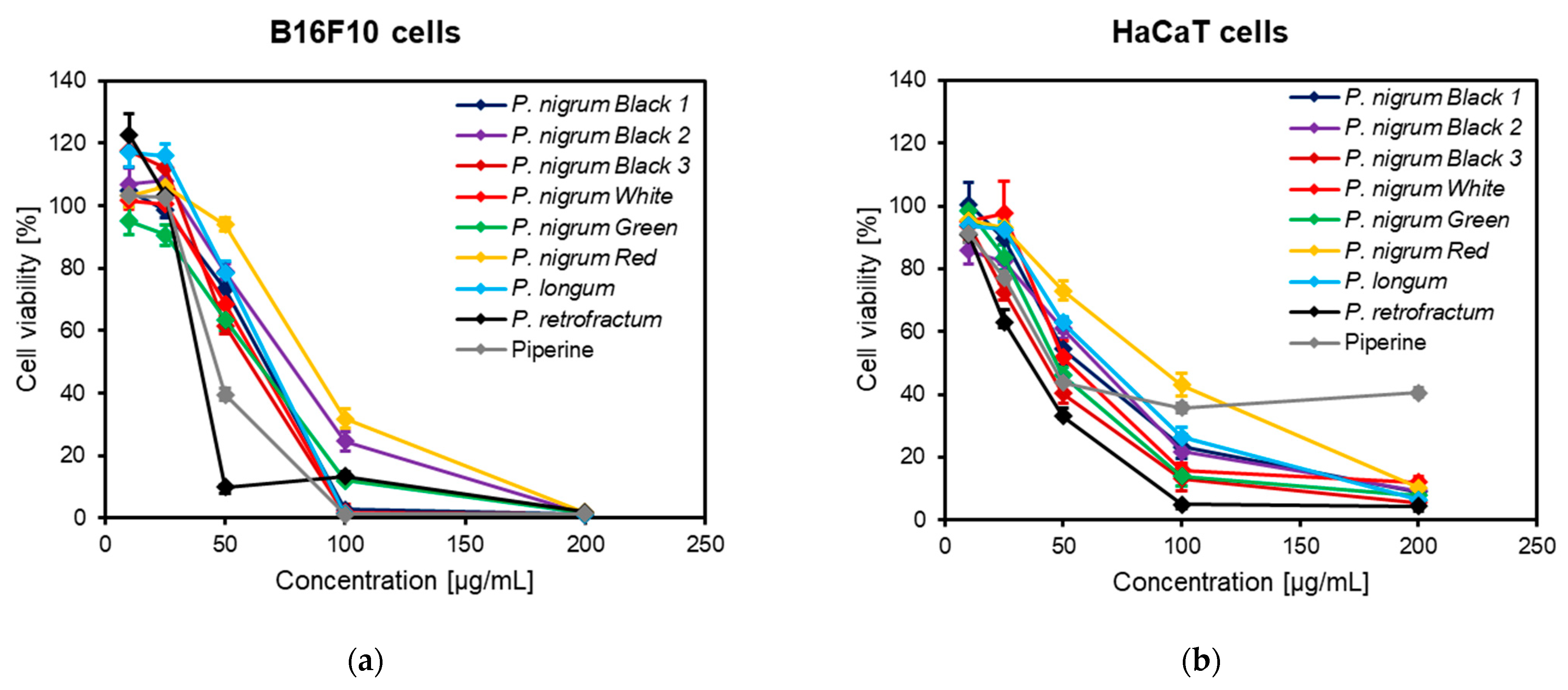
3.6. Anti-Melanogenic Activity
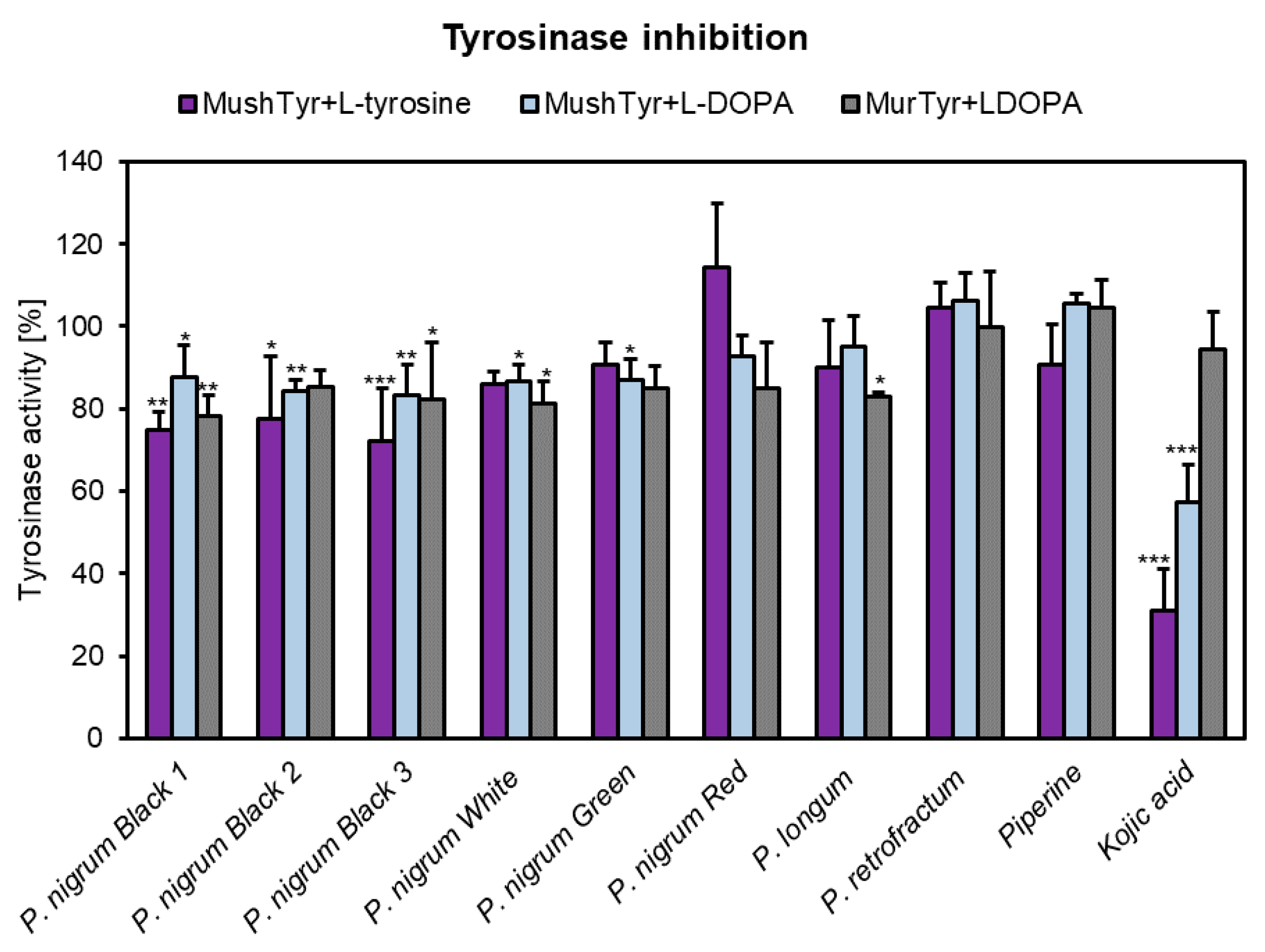
3.7. Anti-Tyrosinase Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Luca, S.V.; Minceva, M.; Gertsch, J.; Skalicka-Woźniak, K. LC-HRMS/MS-based phytochemical profiling of Piper spices: Global association of piperamides with endocannabinoid system modulation. Food Res. Int. 2021, 141, 110123. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kamboj, J.; Sharma, S. Overview for various aspects of the health benefits of Piper longum Linn. fruit. J. Acupunct. Meridian Stud. 2011, 4, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2020, 247, 112255. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Hasan, J.; Snigdha, H.S.H.; Ali, E.S.; Sharifi-Rad, J.; Martorell, M.; Mubarak, M.S. Chemical profile, traditional uses, and biological activities of Piper chaba Hunter: A review. J. Ethnopharmacol. 2020, 257, 112853. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Nam, M.; Kang, M.S.; Lee, J.O.; Lee, Y.W.; Hwang, G.-S.; Kim, H.S. Piperine regulates UCP1 through the AMPK pathway by generating intracellular lactate production in muscle cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, D.; Ohte, S.; Ohshiro, T.; Jiang, W.; Rudel, L.; Hong, B.; Si, S.; Tomoda, H. Molecular target of piperine in the inhibition of lipid droplet accumulation in macrophages. Biol. Pharm. Bull. 2008, 31, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Rho, M.-C.; Park, H.R.; Choi, J.-H.; Kang, J.Y.; Lee, J.W.; Kim, K.; Lee, H.S.; Kim, Y.K. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. J. Agric. Food Chem. 2006, 54, 9759–9763. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Moreno, I.s.; Najar-Guerrero, I.; Escarenño, N.; Flores-Soto, M.E.; Gertsch, J.r.; Viveros-Paredes, J.M. An endocannabinoid uptake inhibitor from black pepper exerts pronounced anti-inflammatory effects in mice. J. Agric. Food Chem. 2017, 65, 9435–9442. [Google Scholar] [CrossRef]
- Nicolussi, S.; Viveros-Paredes, J.M.; Gachet, M.S.; Rau, M.; Flores-Soto, M.E.; Blunder, M.; Gertsch, J. Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol. Res. 2014, 80, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 9–15. [Google Scholar]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Sabitov, A.; Gaweł-Bęben, K.; Sakipova, Z.; Strzępek-Gomółka, M.; Hoian, U.; Satbayeva, E.; Głowniak, K.; Ludwiczuk, A. Rosa platyacantha Schrenk from Kazakhstan—Natural source of bioactive compounds with cosmetic significance. Molecules 2021, 26, 2578. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Salem, N.B.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. Lwt-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Kuete, V. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar]
- Karsha, P.V.; Lakshmi, O.B. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J. Nat. Prod. Res. 2010, 1, 213–215. [Google Scholar]
- Zou, L.; Hu, Y.-Y.; Chen, W.-X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Chen, W.; Dou, Z.-M.; Chen, R.; Hu, Y.; Chen, W.; Chen, H. Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. Technol 2017, 54, 2067–2076. [Google Scholar] [CrossRef]
- Saraf, A.; Saraf, A. Phytochemical and antimicrobial studies of medicinal plant Piper longum Linn. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 213–222. [Google Scholar]
- Panphut, W.; Budsabun, T.; Sangsuriya, P. In vitro antimicrobial activity of piper retrofractum fruit extracts against microbial pathogens causing infections in human and animals. Int. J. Microbiol. 2020, 2020, 5638961. [Google Scholar] [CrossRef] [Green Version]
- Brook, I. Spectrum and treatment of anaerobic infections. J. Infect. Chemother. 2016, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, T.; Saito, M.; Harasawa, R. Salivary nitrate-nitrite conversion capacity after nitrate ingestion and incidence of Veillonella spp. in elderly individuals. J. Oral Sci. 2018, 60, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Miron, A.; Ciocârlan, N.; Brebu, M.; Roşu, C.M.; Trifan, A.; Vochiţa, G.; Gherghel, D.; Luca, S.V.; Niţă, A. Essential oils of Moldavian Thymus species: Chemical composition, antioxidant, anti-Aspergillus and antigenotoxic activities. Flav. Fragr. J. 2019, 34, 175–186. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Wolfram, E.; Skalicka-Woźniak, K.; Luca, S.V. LC-HRMS/MS phytochemical profiling of Symphytum officinale L. and Anchusa ochroleuca M. Bieb.(Boraginaceae): Unveiling their multi-biological potential via an integrated approach. J. Pharm. Biomed. Anal. 2021, 204, 114283. [Google Scholar] [CrossRef]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Oben, J.E.; Ngogang, J.Y. In vitro antioxidant activity of three Piper species. J. Herb. Pharmacother. 2008, 7, 49–64. [Google Scholar] [CrossRef]
- Chatterjee, S.; Niaz, Z.; Gautam, S.; Adhikari, S.; Variyar, P.S.; Sharma, A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans). Food Chem. 2007, 101, 515–523. [Google Scholar] [CrossRef]
- Samudram, P.; Vasuki, R.; Rajeshwari, H.; Geetha, A.; Moorthi, P.S. Antioxidant and antihepatotoxic activities of ethanolic crude extract of Melia azedarach and Piper longum. J. Med. Plants Res. 2009, 3, 1078–1083. [Google Scholar]
- Barua, C.; Singh, A.; Sen, S.; Barua, A.; Barua, I. In vitro antioxidant and antimycobacterial activity of seeds of Piper longum Linn: A comparative study. SAJ Pharm. Pharmacol. 2014, 1. [Google Scholar] [CrossRef]
- Jadid, N.; Hidayati, D.; Hartanti, S.R.; Arraniry, B.A.; Rachman, R.Y.; Wikanta, W. Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. Proc. AIP Conf. 2017, 1854, 020019. [Google Scholar]
- Mahaldar, K.; Hossain, A.; Islam, F.; Islam, S.; Islam, M.A.; Shahriar, M.; Rahman, M.M. Antioxidant and hepatoprotective activity of Piper retrofractum against paracetamol-induced hepatotoxicity in Sprague-Dawley rat. Nat. Prod. Res. 2020, 34, 3219–3225. [Google Scholar] [CrossRef]
- Dung, H.V.; Cuong, T.D.; Chinh, N.M.; Quyen, D.; Kim, J.A.; Byeon, J.S.; Woo, M.H.; Choi, J.S.; Min, B.S. Compounds from the aerial parts of Piper bavinum and their anti-cholinesterase activity. Arch. Pharm. Res. 2015, 38, 677–682. [Google Scholar] [CrossRef]
- Ferreres, F.; Oliveira, A.P.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Piper betle leaves: Profiling phenolic compounds by HPLC/DAD–ESI/MSn and anti-cholinesterase activity. Phytochem. Anal. 2014, 25, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gök, H.N.; Luca, S.V.; Ay, S.T.; Komsta, Ł.; Salmas, R.E.; Orhan, I.E.; Skalicka-Woźniak, K. Profiling the annual change of the neurobiological and antioxidant effects of five Origanum species in correlation with their phytochemical composition. Food Chem. 2021, 368, 130775. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhong, Y.; Du, H.; Luo, W.; Wen, Y.; Li, Q.; Zhu, C.; Li, Y. Anticholinesterases and antioxidant alkamides from Piper nigrum fruits. Nat. Prod. Res. 2016, 30, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Khatami, Z.; Herdlinger, S.; Sarkhail, P.; Zehl, M.; Kaehlig, H.; Schuster, D.; Adhami, H.-R. Isolation and characterization of acetylcholinesterase inhibitors from Piper longum and binding mode predictions. Planta Med. 2020, 86, 1118–1124. [Google Scholar] [CrossRef]
- Tappayuthpijarn, P.; Sattaponpan, C.; Sakpakdeecharoen, I.; Ittharat, A. Cholinesterase inhibitory and antioxidant activities of Thai traditional remedies potentially used for Alzheimer’s disease. Thai J. East Asian Stud. 2012, 17, 18–25. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Yang, S.Y.; Cuong, N.M.; Kwon, Y.I.; Jang, H.D.; Kim, Y.H. A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and α-glucosidase inhibitory activity. Bioorg. Med. Chem. Lett. 2014, 24, 4120–4124. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Barajas, E.; Buitimea-Cantúa, G.V.; Hernández-Morales, A.; Torres-Pelayo, V.d.R.; Vázquez-Martínez, J.; Buitimea-Cantúa, N.E. In vitro α-amylase and α-glucosidase enzyme inhibition and antioxidant activity by capsaicin and piperine from Capsicum chinense and Piper nigrum fruits. J. Environ. Sci. Health B 2021, 56, 282–291. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Zheleva-Dimitrova, D.; Picot-Allain, C.; Mahomoodally, M.F.; Imran, M.; Dall’Acqua, S. UHPLC-MS Characterization and biological insights of different solvent extracts of two Achillea species (A. aleppica and A. santolinoides) from Turkey. Antioxidants 2021, 10, 1180. [Google Scholar] [CrossRef]
- Pullela, S.V.; Tiwari, A.K.; Vanka, U.S.; Vummenthula, A.; Tatipaka, H.B.; Dasari, K.R.; Khan, I.A.; Janaswamy, M.R. HPLC assisted chemobiological standardization of α-glucosidase-I enzyme inhibitory constituents from Piper longum Linn-An Indian medicinal plant. J. Ethnopharmacol. 2006, 108, 445–449. [Google Scholar] [CrossRef]
- Huu, D.M.N.; Dang, P.H.; Huynh, N.V.; Dang, H.P.; Vuong, L.; Nguyen, T.L.T. Pipercyclobutanamide D, a new member of the cyclobutanamide-type alkaloid, from the roots of Piper nigrum. J. Asian Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Srisayam, M.; Weerapreeyakul, N.; Kanokmedhakul, K. Inhibition of two stages of melanin synthesis by sesamol, sesamin and sesamolin. Asian Pac. J. Trop. Biomed. 2017, 7, 886–895. [Google Scholar] [CrossRef]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.; Bzéouich, I.M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Compounds isolated from the aerial part of Crataegus azarolus inhibit growth of B16F10 melanoma cells and exert a potent inhibition of the melanin synthesis. Biomed. Pharmacother. 2015, 69, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Kim, K.; Kim, Y.-D.; Lee, S.-E. Antimelanogenic activities of piperlongumine derived from Piper longum on murine B16F10 melanoma cells in vitro and zebrafish embryos in vivo: Its molecular mode of depigmenting action. App. Biol. Chem. 2019, 62, 1–7. [Google Scholar] [CrossRef]
- Min, K.R.; Kim, K.-S.; Ro, J.S.; Lee, S.H.; Kim, J.A.; Son, J.K.; Kim, Y. Piperlonguminine from Piper longum with inhibitory effects on alpha-melanocyte-stimulating hormone-induced melanogenesis in melanoma B16 cells. Planta Med. 2004, 70, 1115–1118. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.A.; Eom, S.Y.; Lee, S.H.; Min, K.R.; Kim, Y. Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression. Pigment Cell Res. 2006, 19, 90–98. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Salleh, W.M.N.H.W.; Hashim, N.A.; Ahmad, F.; Yen, K.H. Anticholinesterase and antityrosinase activities of ten Piper species from Malaysia. Adv. Pharm. Bull. 2014, 4, 527. [Google Scholar]
- Salleh, W.; Ahmad, F.; Khong, H. Antioxidant and anti-tyrosinase activities from Piper officinarum C. DC (Piperaceae). J. Appl. Pharm. Sci. 2014, 4, 087–091. [Google Scholar]
- Hashim, N.A.; Ahmad, F.; Salleh, W.M.N.H.W.; Khamis, S. Phytochemicals and tyrosinase inhibitory activity from Piper caninum and Piper magnibaccum. Pharm. Sci. 2019, 25, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.P.; Chan, E.W.C. Antioxidant, antityrosinase and antibacterial properties of fresh and processed leaves of Anacardium occidentale and Piper betle. Food Biosci. 2014, 6, 17–23. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [Green Version]


| Sample | Voucher | Extraction Yield | TPC (mg GAE/g) | TFC (mg RE/g) | |
|---|---|---|---|---|---|
| P. nigrum L. | Black1 | PN1/102019 | 10.5% | 40.96 ± 0.42 b | 10.03 ± 0.22 b |
| Black2 | PN5/102019 | 10.9% | 40.80 ± 0.45 b | 9.12 ± 0.32 b | |
| Black3 | PN6/102019 | 11.9% | 36.71 ± 0.18 c | 1.44 ± 0.15 e | |
| White | PN2/1020219 | 7.7% | 42.11 ± 1.06 b | 4.66 ± 0.30 c | |
| Green | PN3/102019 | 12.5% | 58.90 ± 1.84 a | 18.37 ± 0.27 a | |
| Red | PN4/102019 | 14.5% | 41.41 ± 1.00 b | 5.42 ± 0.32 c | |
| P. longum L. | PL1/112019 | 18.3% | 29.53 ± 0.25 d | 3.01 ± 0.03 d | |
| P. retrofractum Vahl. | PR1/112019 | 10.1% | 32.60 ± 0.22 e | 19.70 ± 0.13 d | |
| No. | Compound | UV [nm] | HRMS (+) [m/z] | MF | HRMS/MS (+) [m/z] |
|---|---|---|---|---|---|
| 1 | Piperolactam C | 220; 340 | 310.1075 | C18H15NO4 | 193.0750; 120.0395 |
| 2 | Piperlongumine | 230; 330 | 318.1360 | C17H19NO5 | 269.0957; 221.0805; 201.0527 |
| 3 | Piperyline | 255; 310; 342 | 272.1291 | C16H17NO3 | 201.0555; 173.0572; 143.0503; 135.0405; 115.0571 |
| 4 | Piperlonguminine | 230; 340 | 274.1452 | C16H19NO3 | 161.0576; 135.0452 |
| 5 | Piperine | 240; 310; 341 | 286.1443 | C17H19NO3 | 201.0542; 173.0525; 135.0455; 112.0733 |
| 6 | Piperettines | 310; 345 | 312.1605 | C19H21NO3 | 227.0679; 199.0776; 169.0555; 141.085; 131.0405 |
| 7 | Piperolein A | 260; 340 | 316.1904 | C19H25NO3 | 231.1056; 161.0538; 135.0512 |
| 8 | Pellitorine | 210; 260 | 224.2015 | C14H25NO | 168.1414; 151.1170; 123.1196; 109.0603 |
| 9 | Pipercallosine | 210; 265; 310 | 330.2049 | C20H27NO3 | 259.1312; 241.1301; 208.1725; 135.0490 |
| 10 | Dehydropipernonaline | 210; 265 | 340.1919 | C21H25NO3 | 227.1001; 161.0554; 131.0414 |
| 11 | Pipernonaline | 215; 260; 305 | 342.2065 | C21H27NO3 | 229.1564; 199.0916; 161.0593; 135.0471 |
| 12 | Neopeollitorine B | 210; 265 | 236.2001 | C15H25NO | 151.1140; 109.0625 |
| 13 | Retrofractamide B | 210; 260 | 356.2203 | C22H29NO3 | 283.1309; 255.1371; 234.1822; 215.1868; 135.0433 |
| 14 | Piperolein B | 210; 265; 305 | 344.2205 | C21H29NO3 | 259.1299; 222.1829; 135.0395 |
| 15 | Piperundecalidine | 215; 270 | 368.2223 | C23H29NO3 | 246.1807; 215.1046; 173.0559; 135.0411 |
| 16 | Guineensine | 215; 260; 315 | 384.2586 | C24H33NO3 | 311.1985; 283.2033; 161.0754; 135.0577 |
| 17 | N-Isobutyl-2,4,12-octadecatrienamide | 220; 260 | 334.3099 | C22H39NO | 261.2253; 233.2201 |
| No. | Compound | P. nigrum | P. longum | P. retrofractum | |||||
|---|---|---|---|---|---|---|---|---|---|
| Black 1 | Black 2 | Black 3 | White | Green | Red | ||||
| mg PE/g Extract | |||||||||
| 1 | Piperolactam C | nq | nq | nq | nq | nq | nq | nq | 18.17 ± 0.25 a |
| 2 | Piperlongumine | nq | nq | nq | nq | nq | nq | nq | 14.19 ± 0.14 a |
| 3 | Piperyline | 11.66 ± 0.17 c | 12.68 ± 0.16 b | 8.62 ± 0.18 e | 10.90 ± 0.08 d | 15.40 ± 0.34 a | 5.81 ± 0.07 f | nq | nq |
| 4 | Piperlonguminine | 4.90 ± 0.12 d | 4.84 ± 0.26 d | 2.83 ± 0.13 e | 2.72 ± 0.15 f | 6.01 ± 0.14 c | 5.10 ± 0.13 d | 16.87 ± 0.13 a | 12.93 ± 0.03 b |
| 5 | Piperine | 276.70 ± 0.82 d | 299.63 ± 0.99 c | 293.07 ± 0.71 c | 308.36 ± 0.50 b | 315.19 ± 1.12 a | 154.03 ± 0.21 f | 249.79 ± 0.99 e | 93.86 ± 0.13 g |
| 6 | Piperettines | 70.60 ± 1.69 a | 64.65 ± 0.46 b | 46.12 ± 0.67 c | 66.18 ± 0.87 b | 70.69 ± 0.06 a | 21.51 ± 0.08 d | 3.64 ± 0.40 e | nq |
| 7 | Piperolein A | 28.83 ± 1.60 a | 17.96 ± 0.28 c | 9.93 ± 0.16 d | 28.97 ± 0.66 a | 23.90 ± 0.54 b | 5.84 ± 0.12 e | nq | 23.00 ± 1.21 b |
| 8 | Pellitorine | 39.68 ± 0.14 b | 15.95 ± 0.10 e | 22.41 ± 0.26 d | 26.11 ± 0.17 c | 18.78 ± 0.15 e | 9.33 ± 0.07 f | 7.90 ± 0.35 g | 206.95 ± 0.22 a |
| 9 | Pipercallosine | 3.72 ± 0.51 c | 3.28 ± 0.15 d | 3.02 ± 0.06 d | 4.72± 0.37 b | 4.57 ± 0.18 b | 2.04 ± 0.04 e | 9.25 ± 0.47 a | nq |
| 10 | Dehydropipernonaline | 22.23 ± 0.29 a | 11.18 ± 0.09 e | 18.50 ± 0.46 b | 17.12 ± 0.17 c | 12.77 ± 0.12 d | 2.68 ± 0.04 f | 12.71 ± 0.29 d | nq |
| 11 | Pipernonaline | 3.37 ± 0.12 c | nq | 2.14 ± 0.20 d | 5.60 ± 0.44 b | nq | nq | 47.62 ± 0.29 a | nq |
| 12 | Neopeollitorine B | 7.89 ± 0.65 a | 3.26 ± 0.10 c | 2.73 ± 0.20 d | 2.31 ± 0.36 d | 3.14 ± 0.01 c | nq | nq | 6.51 ± 0.25 b |
| 13 | Retrofractamide B | 22.07 ± 0.57 a | 13.28 ± 0.21 d | 6.14 ± 0.06 f | 6.75 ± 0.31 e | 19.75 ± 0.21 b | 12.65 ± 0.12 d | 14.63 ± 0.07 c | nq |
| 14 | Piperolein B | 17.26 ± 0.10 a | 12.46 ± 0.27 d | 11.02 ± 0.01 e | 13.17 ± 0.24 c | 15.35 ± 0.20 b | 3.20 ± 0.06 f | nq | 2.70 ± 0.01 g |
| 15 | Piperundecalidine | 7.66 ± 0.46 a | 4.07 ± 0.09 c | 4.86 ± 0.06 b | nq | 4.28 ± 0.16 c | 4.19 ± 0.10 c | 6.32 ± 0.19 d | 7.48 ± 0.11 a |
| 16 | Guineensine | 42.53 ± 0.08 a | 14.18 ± 0.33 e | 28.57 ± 0.12 c | 11.95 ± 0.04 f | 29.59 ± 0.16 b | 10.22 ± 0.05 g | 24.51 ± 0.25 d | 29.73 ± 0.05 b |
| 17 | N-Isobutyl-2,4,12-octadecatrienamide | 32.31 ± 0.37 d | 14.18 ± 0.21 e | 49.44 ± 0.33 b | 13.61 ± 0.19 f | 37.62 ± 0.20 c | 12.64 ± 0.05 g | 82.65 ± 0.25 a | 1.98 ± 0.05 h |
| Total piperamides | 591.42 ± 4.01 a | 525.35 ± 4.10 b | 588.42 ± 6.44 a | 247.75 ± 0.87 f | 530.67 ± 1.49 b | 453.58 ± 7.63 d | 475.89 ± 1.05 c | 423.72 ± 2.49 e | |
| Microbial Species | P. nigrum | P. longum | P. retrofractum | Reference drug | |||||
|---|---|---|---|---|---|---|---|---|---|
| Black 1 | Black 2 | Black 3 | White | Green | Red | ||||
| MIC (mg/mL) | MIC (mg/L) | ||||||||
| S. aureus ATCC 25923 | 4 | 0.25 | 0.5 | 0.125 | 0.5 | 1 | 4 | 0.25 | 0.98 1 |
| S. epidermidis ATCC 12228 | >4 | 2 | 4 | 4 | 0.125 | 0.5 | 4 | 4 | 0.98 1 |
| M. luteus ATCC 10240 | 0.125 | 0.125 | 0.25 | 0.125 | 0.25 | 0.5 | 1 | 0.5 | 0.12 1 |
| B. cereus ATCC 10876 | 0.5 | 1 | 0.5 | 0.5 | 2 | 2 | 4 | 0.5 | 0.98 1 |
| E. faecalis ATCC 29212 | >4 | >4 | >4 | 4 | 4 | >4 | 4 | >4 | 1.95 1 |
| S. mutans ATCC 25175 | 4 | 4 | 4 | 4 | 4 | 4 | >4 | 4 | 0.98 1 |
| A. israelii ATCC 10049 | 0.125 | 0.125 | 0.0625 | 0.0625 | 0.125 | 0.5 | 0.5 | 0.25 | 0.5 1 |
| C. perfringens ATCC 13124 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | 2 | 1.95 3 |
| C. jejunii ATCC 33291 | 4 | >4 | 2 | 4 | 4 | >4 | >4 | >4 | 0.125 2 |
| S. Typhimurium ATCC14028 | >4 | >4 | >4 | >4 | 4 | >4 | >4 | >4 | 0.061 2 |
| E. coli ATCC 25922 | >4 | >4 | >4 | >4 | 4 | >4 | >4 | >4 | 0.015 2 |
| P. mirabilis ATCC 12453 | >4 | >4 | >4 | >4 | 2 | 2 | >4 | >4 | 0.03 2 |
| K. pneumoniae ATCC 13883 | >4 | >4 | >4 | >4 | 4 | >4 | >4 | >4 | 0.122 2 |
| P. aeruginosa ATCC 9027l | >4 | >4 | >4 | >4 | 4 | >4 | >4 | >4 | 0.488 2 |
| B. fragilis ATCC 10240 | 0.5 | 0.25 | 0.25 | 0.25 | 0.5 | 1 | 1 | 1 | 0.98 3 |
| P. intermedia ATCC 25611 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | >4 | 0.5 | 0.25 | 0.488 3 |
| F. nucleatum ATCC 25586 | 0.125 | 0.125 | 0.0625 | 0.125 | 0.0625 | 0.125 | 0.25 | 0.0625 | 1.95 3 |
| V. parvula ATCC 10790 | 0.25 | 0.25 | 0.125 | 0.25 | 0.25 | 1 | 1 | 2 | 1.95 3 |
| C glabrata ATCC 90030 | 2 | 2 | 2 | 4 | 2 | 4 | 4 | 2 | 0.24 4 |
| C. albicans ATCC 102231 | 1 | 1 | 0.5 | 1 | 1 | 1 | 2 | 1 | 0.48 4 |
| C. parapsilosis ATCC 22019 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | 2 | 0.5 | 0.24 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, S.V.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czech, K.; Trifan, A.; Zengin, G.; Korona-Glowniak, I.; Minceva, M.; Gertsch, J.; Skalicka-Woźniak, K. Insights into the Phytochemical and Multifunctional Biological Profile of Spices from the Genus Piper. Antioxidants 2021, 10, 1642. https://doi.org/10.3390/antiox10101642
Luca SV, Gaweł-Bęben K, Strzępek-Gomółka M, Czech K, Trifan A, Zengin G, Korona-Glowniak I, Minceva M, Gertsch J, Skalicka-Woźniak K. Insights into the Phytochemical and Multifunctional Biological Profile of Spices from the Genus Piper. Antioxidants. 2021; 10(10):1642. https://doi.org/10.3390/antiox10101642
Chicago/Turabian StyleLuca, Simon Vlad, Katarzyna Gaweł-Bęben, Marcelina Strzępek-Gomółka, Karolina Czech, Adriana Trifan, Gokhan Zengin, Izabela Korona-Glowniak, Mirjana Minceva, Jürg Gertsch, and Krystyna Skalicka-Woźniak. 2021. "Insights into the Phytochemical and Multifunctional Biological Profile of Spices from the Genus Piper" Antioxidants 10, no. 10: 1642. https://doi.org/10.3390/antiox10101642








