Effect of Post-Weld Heat Treatment on the Solid-State Diffusion Bonding of 6061 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
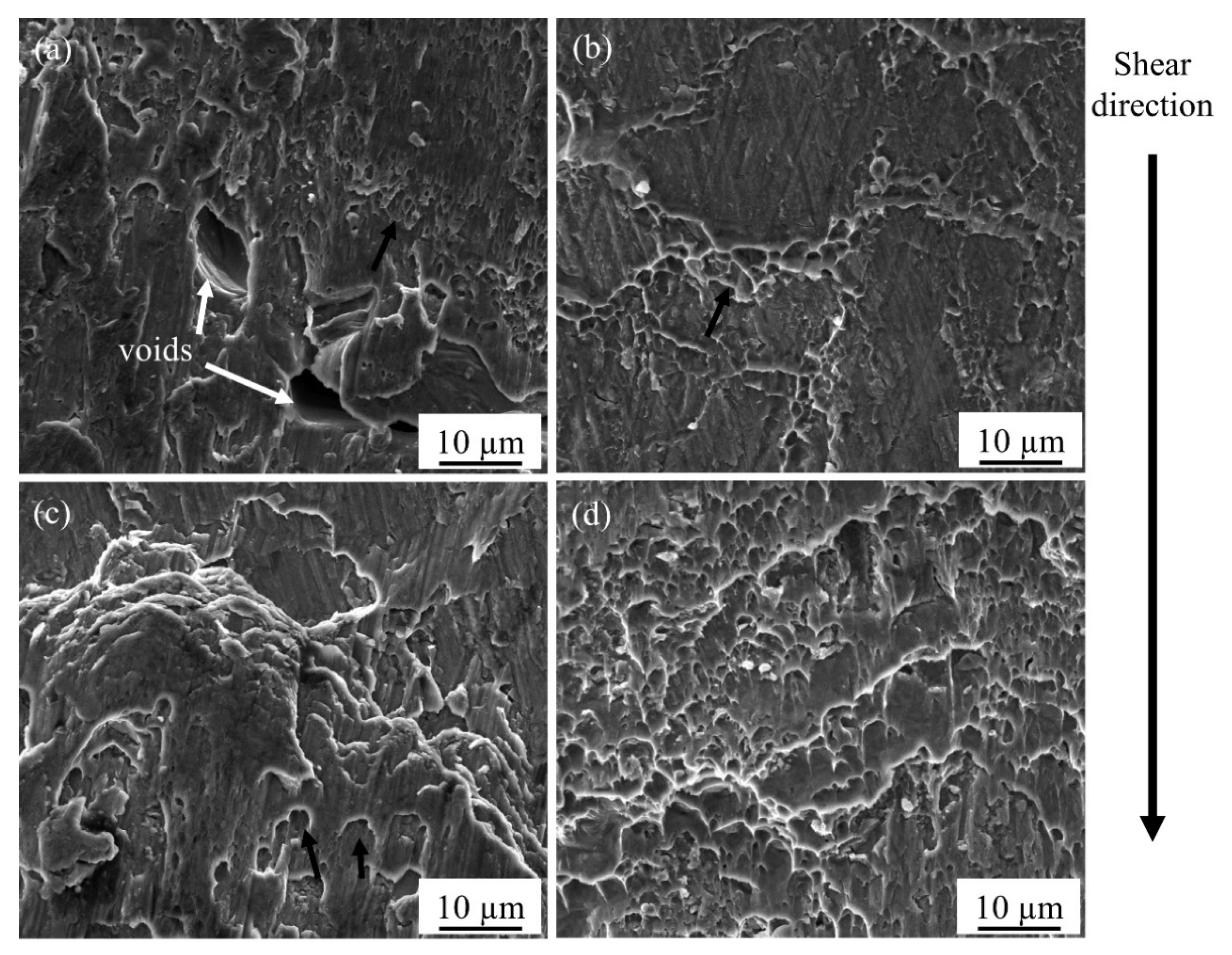
3.1. Interfacial Microstructure and Mechanical Properties of the Fabricated AA 6061 Joints

3.2. Effect of PWHT on Diffusion Bonded AA6061 Joints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Batahgy, A.; Kutsuna, M. Laser Beam Welding of AA5052, AA5083, and AA6061 Aluminum Alloys. Adv. Mater. Sci. Eng. 2009, 2009, 974182. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, X.; Liu, L. Research on laser-TIG hybrid welding of 6061-T6 aluminum alloys joint and post heat treatment. Metals (Basel) 2020, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.S.; Balasubramanian, V.; Sanavullah, M. Influences of pulsed current tungsten inert gas welding parameters on the tensile properties of AA 6061 aluminium alloy. Mater. Des. 2006, 28, 2080–2092. [Google Scholar] [CrossRef]
- Haryadi, G.D.; Dewa, R.T.; Ekaputra, I.M.W.; Suprihanto, A. Investigation of post-weld heat treatment (T6) and welding orientation on the strength of TIG-welded AL6061. Open Eng. 2020, 10, 753–761. [Google Scholar] [CrossRef]
- Almohaisen, A.M.N.; Hassan, K.S. Improving the Mechanical Properties of Aluminum Alloys 6061-T6 Friction Stir Welding Joints Using Ultrasonic Peening. IOP Conf. Ser. Mater. Sci. Eng. 2020, 881, 012093. [Google Scholar] [CrossRef]
- Helal, Y.; Boumerzoug, Z.; Fellah, L. Microstructural evolution and mechanical properties of dissimilar friction stir lap welding aluminum alloy 6061-T6 to ultra low carbon steel. Energy Procedia 2019, 157, 208–215. [Google Scholar] [CrossRef]
- Dai, W.; Xue, S.-B.; Ji, F.; Lou, J.; Sun, B.; Wang, S.-Q. Brazing 6061 aluminum alloy with Al-Si-Zn filler metals containing Sr. Int. J. Miner. Metall. Mater. 2013, 20, 365–370. [Google Scholar] [CrossRef]
- Dai, W.; Xue, S.; Lou, J.; Wang, S. Microstructure and properties of 6061 aluminum alloy brazing joint with AlSiZn filler metal. Mater. Trans. 2012, 53, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Tsao, L.C.; Tsai, T.C.; Wu, C.S.; Chuang, T.H. Brazeability of the 6061-T6 aluminum alloy with Al-Si-20Cu-based filler metals. J. Mater. Eng. Perform. 2001, 10, 705–709. [Google Scholar] [CrossRef]
- Eivani, A.R.; Ahmadian, M.; Vafaeenezhad, H. Effect of process conditions on the evolution of microstructure and mechanical properties of AA3003 vacuum furnace brazing joints. Mater. Res. Express 2020, 7, 016561. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Tsao, L.; Li, T.; Chuang, T. Joining 6061 aluminum alloy with Al–Si–Cu filler metals. J. Alloys Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Schällibaum, J.; Burbach, T.; Münch, C.; Weiler, W.; Wahlen, A. Transient liquid phase bonding of AA 6082 aluminium alloy: Transientes Flüssigphasenfügen der Aluminiumlegierung AA 6082. Materwiss. Werksttech. 2015, 46, 704–712. [Google Scholar] [CrossRef]
- Wang, X.G.; Li, X.G.; Wang, C.G. Transient liquid phase bonding of aluminium alloy using two-step heating process. Sci. Technol. Weld. Join. 2012, 17, 414–418. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, C.H.; Seo, K.; Shin, S.Y.; Lee, C.H. Diffusion Bonding of Al 6061 Alloys Using an Eutectic Reaction of Al-Ag-Cu. Key Eng. Mater. 2005, 297–300, 2772–2777. [Google Scholar] [CrossRef]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, C.; Lim, C.H.; Cho, D.C.; Shin, S.Y. Fluxless Brazing of Al6061 Alloys Using Ag-28Cu Insert. Mater. Sci. Forum 2005, 486–487, 173–176. [Google Scholar] [CrossRef]
- Shin, S.Y.; Ko, M.W.; Cho, D.C.; Lee, C.H.; Shin, K.S.; Park, K. Microstructure and mechanical properties of Al 6061 joints diffusion brazed using Cu interlayer. J. Mater. Sci. Lett. 2002, 21, 903–906. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, W.; Han, Y.; Fu, X.; Xu, Y.; Hou, H. Effect of alloying elements gradient on solid-state diffusion bonding between aerospace aluminum alloys. Materials 2018, 11, 1446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, G.; Wang, Y.; Shen, Q.; Zhang, L. An investigation on diffusion bonding of aluminum and magnesium using a Ni interlayer. Mater. Lett. 2012, 83, 189–191. [Google Scholar] [CrossRef]
- Cooke, K.O.; Atieh, A.M. Current trends in dissimilar diffusion bonding of titanium alloys to stainless steels, aluminium and magnesium. J. Manuf. Mater. Process. 2020, 4, 39. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Theoretical model for diffusion bonding. Met. Sci. 1982, 16, 49–56. [Google Scholar] [CrossRef]
- Kawakatsu, I.; Kitayama, S. Study on Diffusion Bonding of Metals. Trans. Jpn. Inst. Met. 1977, 18, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Zuruzi, A.; Li, H.; Dong, G. Effects of surface roughness on the diffusion bonding of Al alloy 6061 in air. Mater. Sci. Eng. A 1999, 270, 244–248. [Google Scholar] [CrossRef]
- Saleh, A.A. Microstructure and strength of diffusion bonded 2014 AA alloys using copper interlayer. Open J. Appl. Sci. 2019, 9, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Saleema, N.; Sarkar, D.; Paynter, R.; Gallant, D.; Eskandarian, M. A simple surface treatment and characterization of AA 6061 aluminum alloy surface for adhesive bonding applications. Appl. Surf. Sci. 2012, 261, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cao, J.; Tian, X.; Li, R.; Feng, J. Low-temperature diffusion bonding of pure aluminum. Appl. Phys. A 2013, 113, 101–104. [Google Scholar] [CrossRef]
- Zinong, T.; Bing, Z.; Jun, J.; Zhiqiang, L.; Jianguo, L. A study on the hot roll bonding of aluminum alloys. Procedia Manuf. 2020, 50, 56–62. [Google Scholar] [CrossRef]
- ASM International. Heat Treating of Aluminum Alloys. In ASM Handbook; Totten, G.E., Ed.; ASM International: Materials Park, OH, USA, 1991; pp. 841–879. [Google Scholar]
- Davis, J.R. Light Metals and Alloys: Aluminum and Aluminum Alloys. In Alloying: Understanding the Basics; Davis, J.R., Ed.; ASM International: Materials Park, OH, USA, 2001; pp. 351–416. [Google Scholar]
- Vargel, C. Influence of alloy composition. Corros. Alum. 2020, 127–155. [Google Scholar] [CrossRef]
- Summers, P.T.; Chen, Y.; Rippe, C.M.; Allen, B.; Mouritz, A.P.; Case, S.W.; Lattimer, B.Y. Overview of aluminum alloy mechanical properties during and after fires. Fire Sci. Rev. 2015, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Hatch, J.E. Microstructure of Alloys. In Aluminum: Properties and Physical Metallurgy; Hatch, J.E., Ed.; ASM International: Materials Park, OH, USA, 1984; pp. 58–104. [Google Scholar] [CrossRef]
- Abid, J.; Raza, H.; Akhtar, A.; Gohar, G.A.; Ullah, S.; Akram, M.; Raza, Y.; Bukhari, M.D. Effect of surface roughness on shear strength of bonded joints of aluminum Al 6061 T6 substrate. VW Appl. Sci. 2020, 2, 87–91. [Google Scholar] [CrossRef]
- Ahmad, R.; Bakar, M.A. Effect of a post-weld heat treatment on the mechanical and microstructure properties of AA6061 joints welded by the gas metal arc welding cold metal transfer method. Mater. Des. 2011, 32, 5120–5126. [Google Scholar] [CrossRef]
- Gussev, M.; Sridharan, N.; Norfolk, M.; Terrani, K.; Babu, S. Effect of post weld heat treatment on the 6061 aluminum alloy produced by ultrasonic additive manufacturing. Mater. Sci. Eng. A 2017, 684, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Elangovan, K.; Balasubramanian, V. Influences of post-weld heat treatment on tensile properties of friction stir-welded AA6061 aluminum alloy joints. Mater. Charact. 2008, 59, 1168–1177. [Google Scholar] [CrossRef]
- Hosoda, N.; Nakai, M.; Eto, T. The effect of microstructure on mechanical properties of forged 6061 aluminum alloy. In Proceedings of the 9th International Conference on Aluminium Alloys, Brisbane, Australia, 2–5 August 2004; Nie, J.F., Morton, A.J., Muddle, B.C., Eds.; Institute of Materials Engineering Australasia Ltd.: Melbourne, Australia, 2004; pp. 1382–1387. [Google Scholar]








| Al | Mg | Si | Cu | Cr | Mn |
|---|---|---|---|---|---|
| Bal. | 1.10 | 0.61 | 0.25 | 0.12 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Sun, Y.-K.; Lai, Y.-C.; Chang, S.-Y.; Chuang, T.-H. Effect of Post-Weld Heat Treatment on the Solid-State Diffusion Bonding of 6061 Aluminum Alloy. Appl. Sci. 2021, 11, 9660. https://doi.org/10.3390/app11209660
Chen C-H, Sun Y-K, Lai Y-C, Chang S-Y, Chuang T-H. Effect of Post-Weld Heat Treatment on the Solid-State Diffusion Bonding of 6061 Aluminum Alloy. Applied Sciences. 2021; 11(20):9660. https://doi.org/10.3390/app11209660
Chicago/Turabian StyleChen, Chun-Hao, Yu-Kai Sun, Yu-Chang Lai, Shih-Ying Chang, and Tung-Han Chuang. 2021. "Effect of Post-Weld Heat Treatment on the Solid-State Diffusion Bonding of 6061 Aluminum Alloy" Applied Sciences 11, no. 20: 9660. https://doi.org/10.3390/app11209660
APA StyleChen, C.-H., Sun, Y.-K., Lai, Y.-C., Chang, S.-Y., & Chuang, T.-H. (2021). Effect of Post-Weld Heat Treatment on the Solid-State Diffusion Bonding of 6061 Aluminum Alloy. Applied Sciences, 11(20), 9660. https://doi.org/10.3390/app11209660





