Volatile Organic Compounds Emitted by Aspergillus flavus Strains Producing or Not Aflatoxin B1
Abstract
:1. Introduction
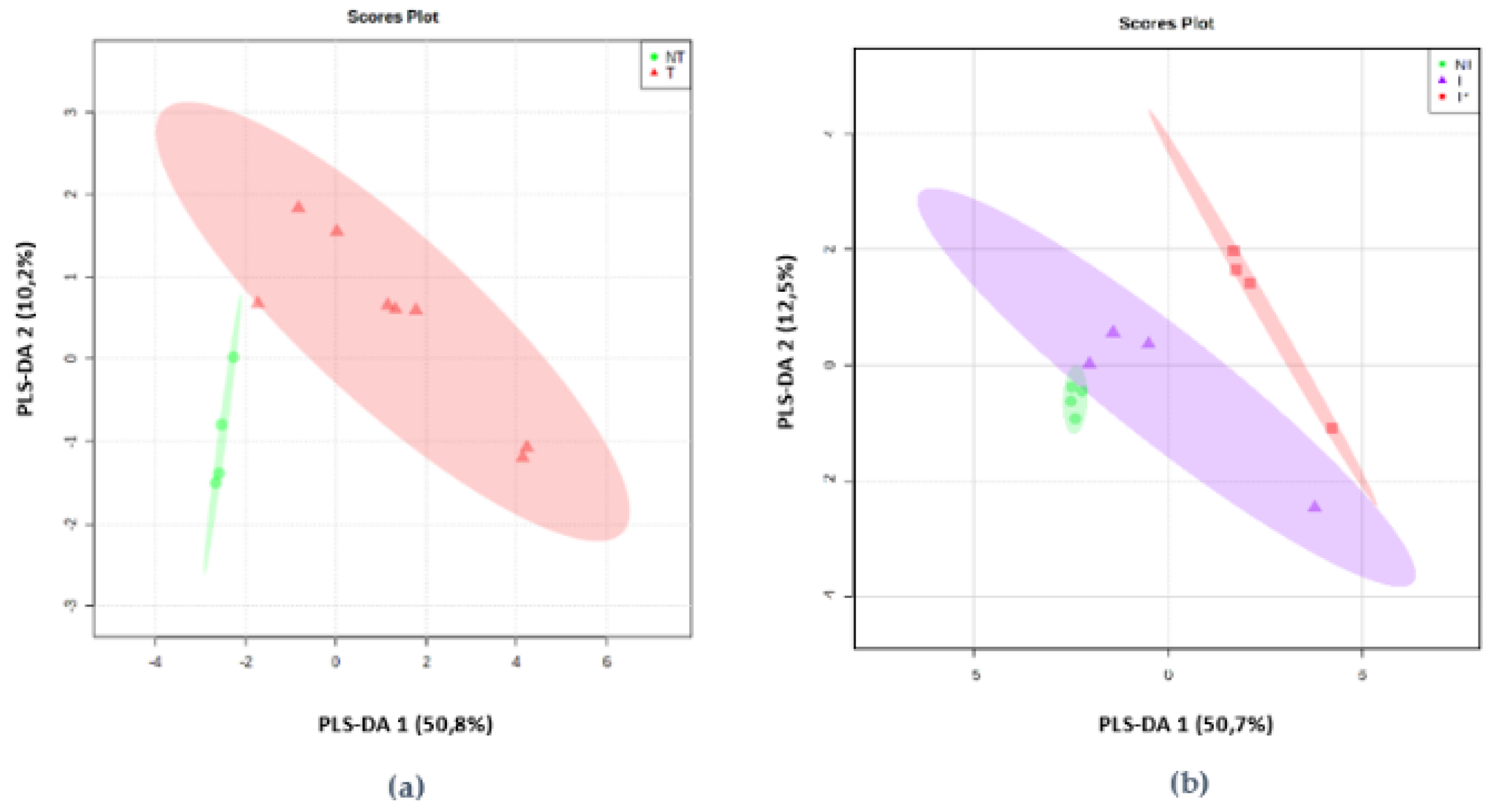
2. Results
2.1. Aflatoxin Concentrations
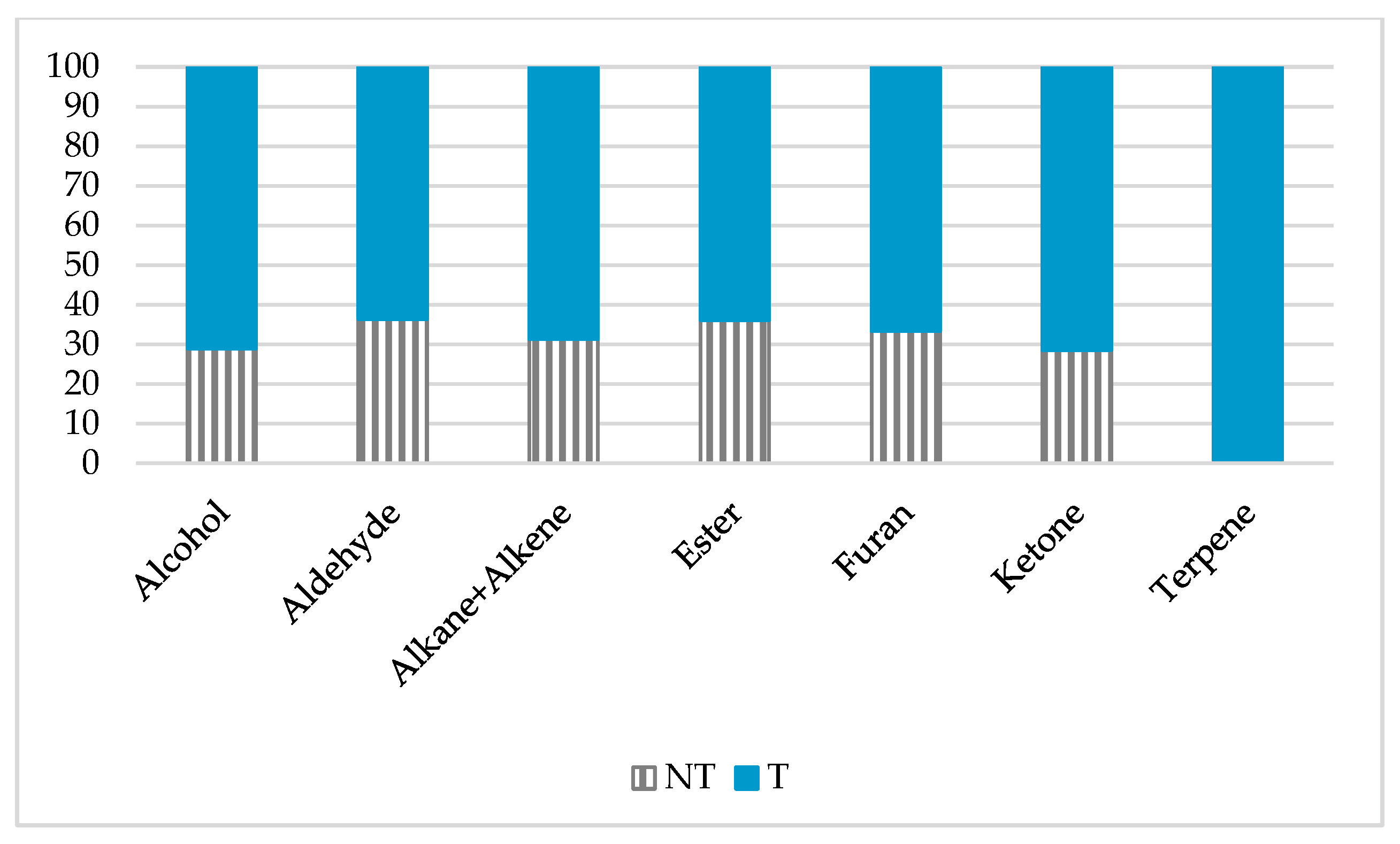
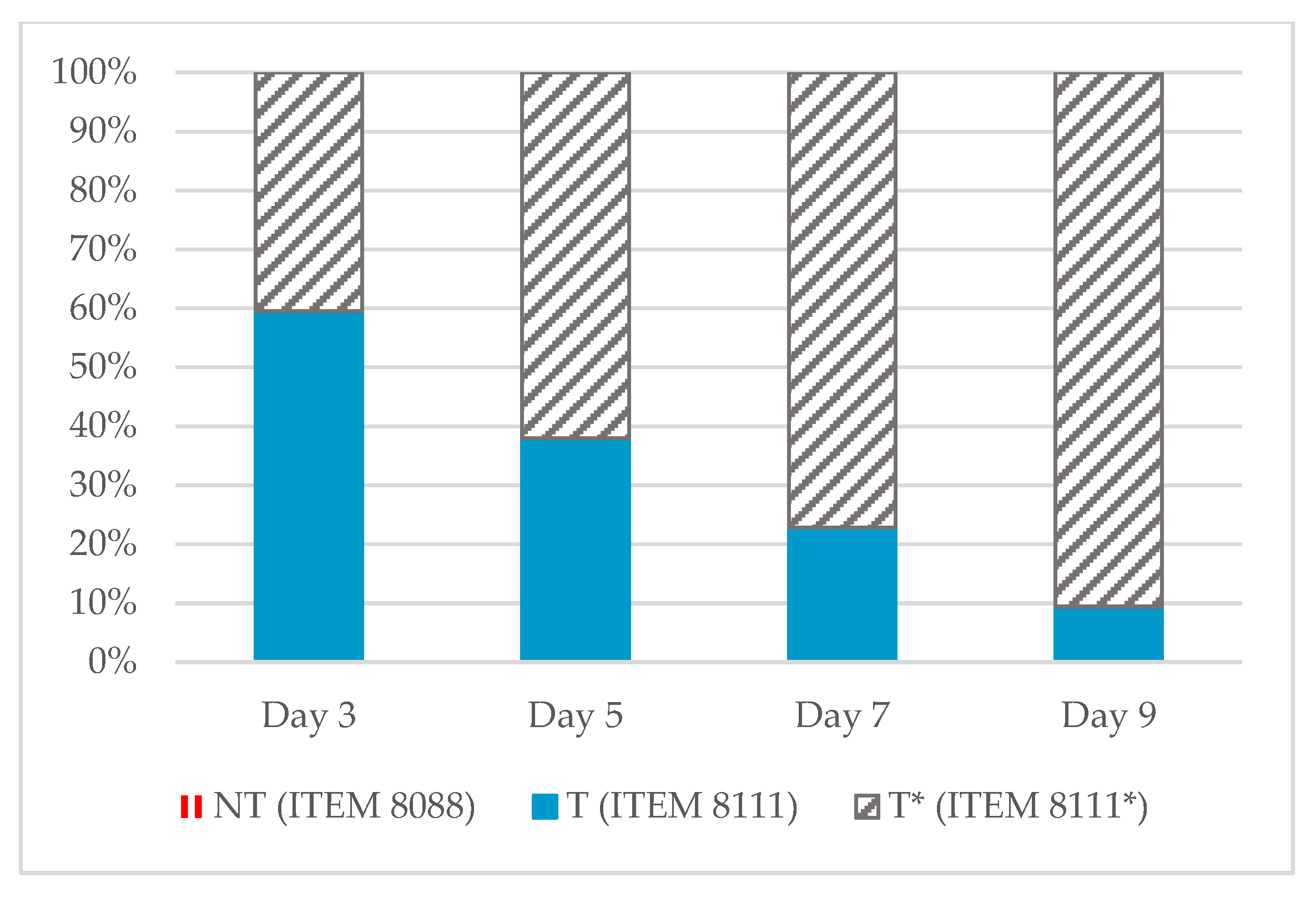
2.2. mVOCs Screening
- -
- On day 7: (E,Z)-1,2-diethylidenecyclopentane, NI 640, NI 729, NI 756;
- -
- On day 9: propan-2-ol with a large relative area of 72.3%;
- -
- Sno specific compound emission is recorded on day 3 and on day 5.
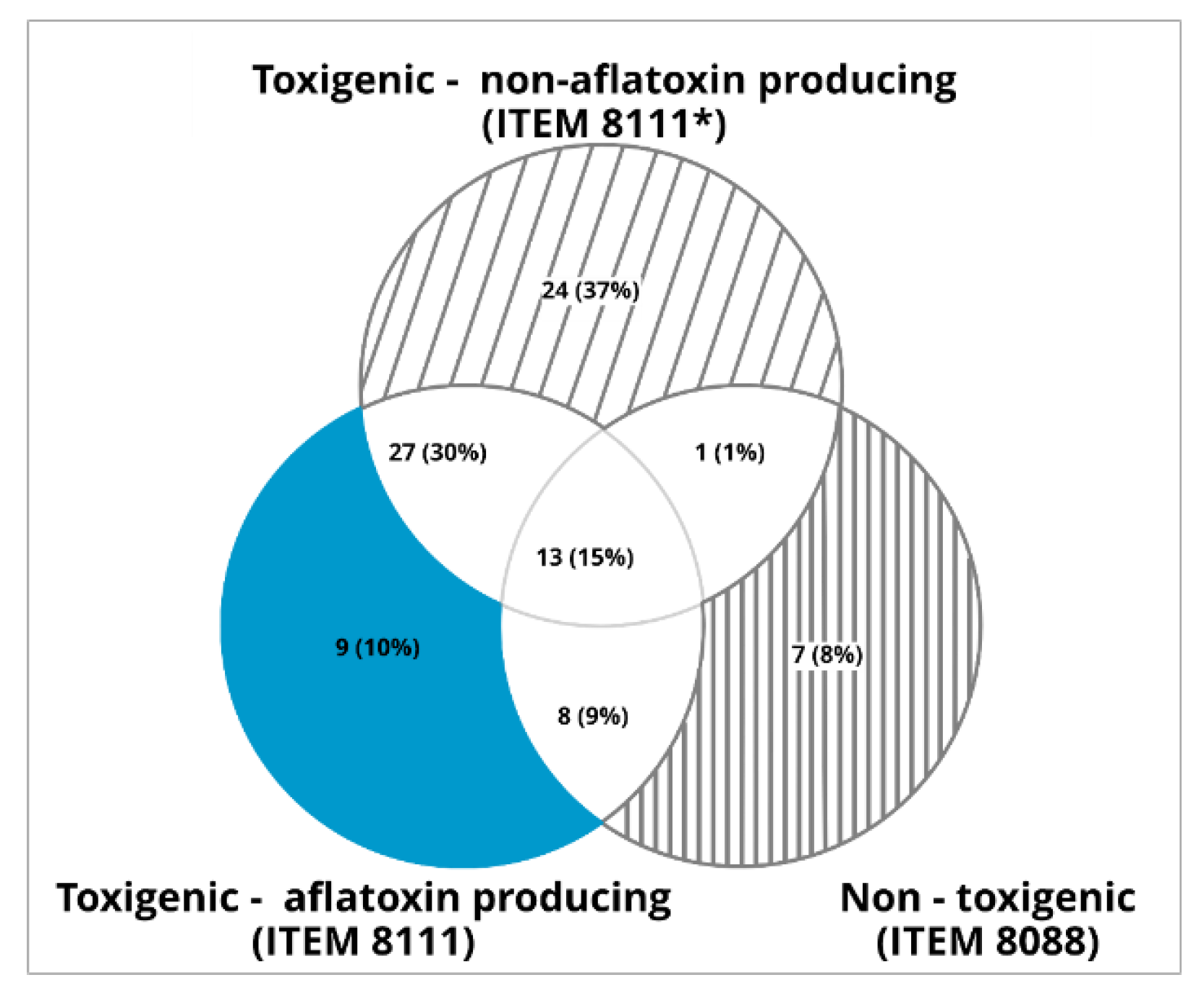
2.3. mVOCS Related to Toxigenic Characteristic
3. Discussion
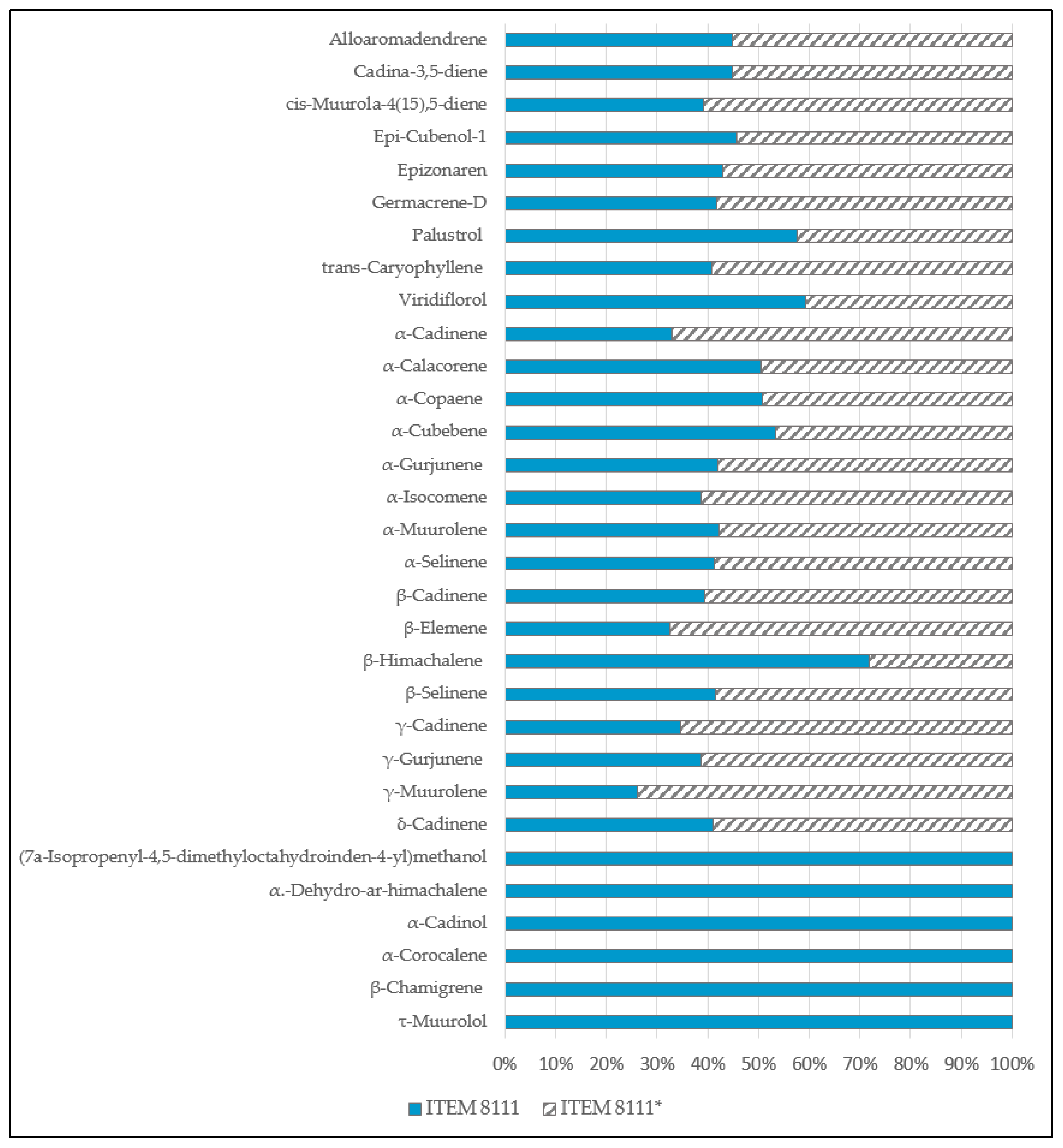
3.1. mVOCs
3.2. Potential mVOCs Markers
4. Materials and Methods
4.1. Fungal Strains
4.2. Fungi Inoculation
4.3. Aflatoxin Analysis
4.4. GC-MS Parameters
4.5. Identification of GC-MS Analysis
4.6. Statistical Model
4.7. Semi-Quantification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ekwomadu, T.I. Occurrence of filamentous fungi in maize destined for human consumption in South Africa. Food Sci. Nutr. 2018, 6, 884–890. [Google Scholar] [CrossRef]
- Laboratories, S.B.; Salleh, B.; Saad, B.; Abbas, H.K. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 2–26. [Google Scholar] [CrossRef]
- Enyiukwu, D.N.; Awurum, A.N.; Nwaneri, J.A. Mycotoxins in Stored Agricultural Products: Implications to Food Safety and Health and Prospects of Plant-derived Pesticides as Novel Approach to their Management. Greener J. Microbiol. Antimicrob. 2014, 2, 32–48. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Moretti, A.; Logrieco, A.F.; Susca, A. Chapter 1 Mycotoxins: An Underhand Food Problem. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1542, pp. 3–12. [Google Scholar] [CrossRef]
- Survey, A.T. Global Mycotoxin Occurrence in Feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.A.M.; Pereyra, M.L.G.; Keller, K.M.; Alonso, V.A.; Oliveira, A.A.; Almeida, T.X.; Barbosa, T.S.; Nunes, L.M.T.; Cavaglieri, L.R.; Rosa, C.A.R. Fungal and mycotoxins contamination in corn silage: Monitoring risk before and after fermentation Fungal and mycotoxins contamination in corn silage: Monitoring risk before and after fermentation. J. Stored Prod. Res. 2013, 52, 42–47. [Google Scholar] [CrossRef]
- Chauhan, Y.; Tatnell, J.; Krosch, S.; Karanja, J.; Gnonlonfin, B.; Wanjuki, I.; Wainaina, J.; Harvey, J. An improved simulation model to predict pre-harvest aflatoxin risk in maize. Field Crop. Res. 2015, 178, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered Pathway Genes in Aflatoxin Biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caceres, I.; Khoury, A.A.l.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Grasl-kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar] [CrossRef]
- Food, E.; Authority, S. Outcome of a Public Consultation on the Draft Risk Assessment of Aflatoxins in Food; EFSA Supporting Publications: Parma, Italy, 2020; Volume 17, Issue 3. [Google Scholar] [CrossRef] [Green Version]
- Laganà, A. LC-MS/MS Method for Mycotoxin Analysis; MDPI: Basel, Switzerland, 2017; ISBN 9783038426066. [Google Scholar]
- Matabaro, E.; Ishimwe, N.; Uwimbabazi, E.; Lee, B.H. Current Immunoassay Methods for the Rapid Detection of Aflatoxin in Milk and Dairy Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food mycotoxins: An update. J. Food Sci. 2006, 71, 51–65. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- Berthiller, F.; Cramer, B.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; MacDonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; et al. Developments in mycotoxin analysis: An update for 2016–2017. World Mycotoxin J. 2018, 10, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Gelderblom, W.C.; Shephard, G.S.; African, S.; Vismer, H. Mycotoxins Detection Methods, Management. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI Publishing: Manhattan, KS, USA, 2008; pp. 29–39. ISBN 9781845930820. [Google Scholar]
- Bennett, J.W.; Inamdar, A.A. Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins 2015, 7, 3785–3804. [Google Scholar] [CrossRef] [Green Version]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal Volatile Organic Compounds: More Than Just a Funky Smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five aspergillus species commonly found in problem buildings. Am. Ind. Hyg. Assoc. J. 2002, 63, 135–140. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; De Kimpe, N. Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. Sci. Total Environ. 2012, 414, 277–286. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [Green Version]
- Citron, C.A.; Gleitzmann, J.; Laurenzano, G.; Pukall, R.; Dickschat, J.S. Terpenoids are Widespread in Actinomycetes: A Correlation of Secondary Metabolism and Genome Data. ChemBioChem 2012, 13, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism–From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 86, 137–138. [Google Scholar]
- Magan, N.; Evans, P. Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J. Stored Prod. Res. 2000, 36, 319–340. [Google Scholar] [CrossRef]
- Pennerman, K.K.; Al-Maliki, H.S.; Lee, S.; Bennett, J.W. Fungal Volatile Organic Compounds (VOCs) and the Genus Aspergillus; Elsevier B.V.: Amsterdam, The Netherlands, 2016; ISBN 9780444635136. [Google Scholar]
- Sun, D.; Wood-Jones, A.; Wang, W.; Vanlangenberg, C.; Jones, D.; Gower, J.; Simmons, P.; Baird, R.E.; Mlsna, T.E. Monitoring MVOC Profiles over Time from Isolates of Aspergillus flavus Using SPME GC-MS. J. Agric. Chem. Environ. 2014, 3, 48–63. [Google Scholar] [CrossRef] [Green Version]
- Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. C15H24 Volatile Compounds Unique to Aflatoxigenic Strains of Aspergillus flavus. Appl. Environ. Microbiol. 1993, 59, 2264–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurjevic, Z.; Rains, G.C.; Wilson, D.M.; Lewis, W.J. Volatile metabolites associated with one aflatoxigenic and one nontoxigenic Aspergillus flavus strain grown on two different substrates. Phytopathol. Mediterr. 2008, 47, 266–271. [Google Scholar] [CrossRef]
- Heddergott, C.; Latgé, J.P.; Calvo, A.M. The volatome of Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 1014–1025. [Google Scholar] [CrossRef] [Green Version]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef] [Green Version]
- Polizzi, V.; Adams, A.; Malysheva, S.V.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; Picco, A.M.; De Kimpe, N. Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 2012, 116, 941–953. [Google Scholar] [CrossRef]
- Jeleń, H.; Wąsowicz, E. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 1998, 14, 391–426. [Google Scholar] [CrossRef]
- de Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.; Bhatnagar, D. Volatile profiles and aflatoxin production by toxigenic and non-toxigenic isolates of Aspergillus flavus grown on sterile and non-sterile cracked corn. Ann. Agric. Environ. Med. 2012, 19, 91–98. [Google Scholar] [PubMed]
- Dickschat, J.S.; Celik, E.; Brock, N.L. Volatiles from three genome sequenced fungi from the genus Aspergillus. Beilstein J. Org. Chem. 2018, 14, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; She, J.; Gower, J.L.; Stokes, C.E.; Windham, G.L.; Baird, R.E.; Mlsna, T.E. Effects of Growth Parameters on the Analysis of Aspergillus flavus Volatile Metabolites. Separations 2016, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, T.; Neubauer, P.; Sivelä, S.; Vatamo, S.; Silfverberg, P.; Salkinoja-Salonen, M. Volatile compounds produced by fungi grown in strawberry jam. LWT-Food Sci. Technol. 2008, 41, 2051–2056. [Google Scholar] [CrossRef]
- Schnurer, J. Volatile Metabolites Produced by Six Fungal Species Compared with Other Indicators of Fungal Growth on Cereal Grains. Appl. Environ. Microbiol. 1992, 58, 2599–2605. [Google Scholar]
- de Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.H.; Bland, J.M.; Bhatnagar, D.; Cleveland, T.E. Volatile profiles of toxigenic and non-toxigenic Aspergillus flavus using SPME for solid phase extraction. Ann. Agric. Environ. Med. 2010, 17, 301–308. [Google Scholar]
- Schulz-bohm, K.; Martín-sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ainane, A.; Benhima, R.; Khammour, F.; Elkouali, M. Composition Chimique et Activité Insecticide de cinq Huiles Essentielles: Composition Chimique et Activité Insecticide de cinq Huiles Essentielles: Cedrus Atlantica, Citrus Limonum, Eucalyptus Globules, Rosmarinus Officinalis et Syzygium Aromaticum. In Proceedings of the Biosune’1-2018. 2018, pp. 67–79. Available online: https://www.researchgate.net/publication/330637727_Composition_chimique_et_activite_anti_insecticide_des_huiles_essentielles_de_Thymus_du_Maroc_Thymus_Capitatus_Thymus_Bleicherianus_et_Thymus_Satureioides (accessed on 26 March 2021).
- Zhong, Y.; Han, X.; Li, S.; Qi, H.; Song, Y.; Qiao, X. Design, Synthesis, Antifungal Activity and Molecular Docking of Thiochroman-4-one Derivatives. Chem. Pharm. Bull. 2017, 65, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Micheluz, A.; Manente, S.; Rovea, M.; Slanzi, D.; Varese, G.C.; Ravagnan, G.; Formenton, G. Detection of volatile metabolites of moulds isolated from a contaminated library. J. Microbiol. Methods 2016, 128, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Lippolis, V.; Cervellieri, S.; Damascelli, A.; Pascale, M.; Di Gioia, A.; Longobardi, F.; De Girolamo, A. Rapid prediction of deoxynivalenol contamination in wheat bran by MOS-based electronic nose and characterization of the relevant pattern of volatile compounds. J. Sci. Food Agric. 2018, 98, 4955–4962. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Liu, Q. Gas Sensors Based on Molecular Imprinting Technology. Sensors 2017, 17, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, U.; Voigt, A.; Irschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Long-term stability of polymer-coated surface transversewave sensors for the detection of organic solvent vapors. Sensors 2017, 17, 2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, X.; Moni, P.; Liu, A.; Kim, D.H.; Jo, W.J.; Sojoudi, H.; Gleason, K.K. CVD Polymers for Devices and Device Fabrication. Adv. Mater. 2017, 29, 1604606. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D.W. Molecular biology of mycotoxin biosynthesis. FEMS Microbiol. Lett. 1999, 175, 149–163. [Google Scholar] [CrossRef]
- Kribii, R.; Soustre, I.; Karst, F. Biosynthèse des isoprénoïdes. Acta Bot. Gall. 1999, 146, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Spakowicz, D.J.; Strobel, S.A. Biosynthesis of hydrocarbons and volatile organic compounds by fungi: Bioengineering potential. Appl. Microbiol. Biotechnol. 2015, 99, 4943–4951. [Google Scholar] [CrossRef] [Green Version]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. Int. Microbiol. Off. J. Spanish Soc. Microbiol. 2019, 23, 89–96. [Google Scholar] [CrossRef]
- Srour, A.Y.; Fakhoury, A.M.; Brown, R.L. Targeting aflatoxin biosynthetic genes. Methods Mol. Biol. 2017, 1542, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bhatnagar, D.; Ehrlich, K.C. Aflatoxin biosynthesis. Toxin Rev. 2002, 10, 87–121. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [Green Version]
- Zeringue, H.J. Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J. Agric. Food Chem. 2000, 48, 921–925. [Google Scholar] [CrossRef]
- Monbaliu, S.; Van Poucke, C.; Detavernier, C.T.L.; Dumoultn, F.; Van Velde, M.D.E.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef]
- Jia, C.; Batterman, S.; Chernyak, S. Development and comparison of methods using MS scan and selective ion monitoring modes for a wide range of airborne VOCs. J. Environ. Monit. 2006, 8, 1029–1042. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiers, M.; Lognay, G.; Fauconnier, M.L.; Jijakli, M.H. Volatile Compound-Mediated Interactions between Barley and Pathogenic Fungi in the Soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Tuohy, M.G.; Ayyachamy, M.; Turner, K.M.; O’Donovan, A. (Eds.) Laboratory Protocol for Fungi; Springer: Berlin, Germany, 2013; ISBN 9781461423553. [Google Scholar]
- Ruiz-Hernández, V.; Roca, M.J.; Egea-Cortines, M.; Weiss, J. A comparison of semi-quantitative methods suitable for establishing volatile profiles. Plant Methods 2018, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5. [Google Scholar] [CrossRef]
- Bousova, K.; Godula, M.; Suman, M. SPME-GC-MS/MS for Identification and Quantification of Migration Contaminants in Paperboard Food Packaging. Thermo Sci. 2015, 1–7. Available online: https://tools.thermofisher.com/content/sfs/brochures/AN-10479-GC-MS-Migration-Contaminants-Paperboard-Food-Packaging-AN10479-EN.pdf (accessed on 26 March 2021).
- Davis, E.M.; Croteau, R. Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes. Biosynthesis 2000, 209, 53–95. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography 2015, 2, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Merck MilliporeSigma; Merck KGaA SPME for GC analysis. Supelco Anal. Prod. 2018, 1, 28. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/298/550/solid-phase-microextraction-for-gc-mk.pdf (accessed on 26 March 2021).
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding solid-phase microextraction: Key factors influencing the extraction process and trends in improving the technique. Chem. Rev. 2013, 113, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- de O. Dias, D.; Colombo, M.; Kelmann, R.G.; De Souza, T.P.; Bassani, V.L.; Teixeira, H.F.; Veiga, V.F.; Limberger, R.P.; Koester, L.S. Optimization of headspace solid-phase microextraction for analysis of β-caryophyllene in a nanoemulsion dosage form prepared with copaiba (Copaifera multijuga Hayne) oil. Anal. Chim. Acta 2012, 721, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dziadas, M.; Jeleń, H.H. Analysis of terpenes in white wines using SPE-SPME-GC/MS approach. Anal. Chim. Acta 2010, 677, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.J. Calibration of a Commercial Solid-Phase Microextraction Device for Measuring Headspace Concentrations of Organic Volatiles. Anal. Chem. 1997, 69, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.J.; Zilkowski, B.W. Volatile-Delivery System for Laboratory Bioassays. J. Chem. Ecol. 1998, 24, 535–558. [Google Scholar] [CrossRef]


| ITEM 8088 | ITEM 8111 | ITEM 8111* (Mutant) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | # Cas | RI (HP-5ms) | RI | Day 3 | Day 5 | Day 7 | Day 9 | Day 3 | Day 5 | Day 7 | Day 9 | Day 3 | Day 5 | Day 7 | Day 9 | ||
| Acetic acid | ac | Acid | 64-19-7 | - | 625 | - | - | - | - | - | - | - | - | - | - | 17.79 | - |
| 2-Methylpropanoic acid | 79-31-2 | 785 | 767 | - | - | - | - | - | - | - | - | - | - | - | 0.51 | ||
| 2-Butyloctan-1-ol * | Alcohol | 3913-02-8 | - | 1286 | - | - | - | - | 0.65 | - | - | - | 0.43 | - | - | - | |
| 2-Methylbutan-1-ol | ac | 137-32-6 | 736 | 720 | 13.2 | 7.84 | 9.32 | 17.8 | 16.25 | 8.87 | 10.5 | 10.5 | 32.21 | 16.84 | 11.34 | 11.35 | |
| 2-Methylpropan-1-ol | bc | 78-83-1 | 622 | 624 | 11.39 | 27.10 | 37.01 | 40.07 | 26.67 | 32.76 | 50.92 | 29.47 | 27.10 | 25.08 | 36.50 | 35.68 | |
| 3-Methylbutan-1-ol | ac | 123-51-3 | 734 | 724 | 13.3 | 11.6 | 11.9 | 14.1 | 13.09 | 13.04 | 8.48 | 16.6 | 21.18 | 11.54 | 8.57 | 9.11 | |
| Butan-1-ol | ab | 71-36-3 | 668 | 648 | - | - | - | - | - | - | - | - | - | 8.57 | 9.45 | 9.30 | |
| Butan-2,3-diol | abc | 513-85-9 | 804 | 809 | - | 0.37 | 0.43 | - | - | - | 1.04 | - | - | - | - | - | |
| Butan-2,3-diol (enantiomer) | abc | 24347-58-8 | - | 816 | - | - | - | - | - | - | 0.41 | - | - | - | - | - | |
| Decan-1-ol | b | 112-30-1 | 1272 | 1272 | - | - | - | - | 0.53 | - | - | - | 0.36 | - | - | - | |
| Ethanol | abc | 64-17-5 | - | 575 | 94.95 | 100 | 100 | 90.40 | 100 | 100 | 80.97 | 97.52 | 62.03 | 100 | 100 | 100 | |
| Propan-1-ol | abc | 71-23-8 | - | 595 | - | 26.2 | 31.4 | - | 36.42 | 39.75 | 13.4 | 33.04 | 51.76 | 14.77 | 37.05 | 34.78 | |
| Propan-2-ol | a | 67-63-0 | - | 584 | - | - | - | 72.3 | - | - | - | - | - | - | - | - | |
| 2-Methylbut-2-enal | Aldehyde | 497-03-0 | 737 * | 723 | 4.22 | 2.19 | 3.39 | - | 4.30 | 3.14 | 5.84 | 6.70 | 3.62 | - | - | - | |
| 2-Methylbutanal | abc | 96-14-0 | 660 | 649 | 12.54 | 12.64 | 15.16 | 22.1 | 10.26 | 10.99 | 10.03 | 17.09 | 9.77 | - | - | - | |
| 3-Methylbutanal | abc | 590-86-3 | 649 | 643 | 5.21 | 5.76 | - | - | - | 10.01 | 7.32 | 11.77 | - | - | - | - | |
| Acetaldehyde | ab | 75-07-0 | - | 566 | - | - | - | - | - | - | 6.82 | - | - | - | - | - | |
| (E,Z)-1,2-diethylidenecyclopentane* | Alkane | Not available | - | 975 | - | - | 0.36 | - | - | - | - | - | - | - | - | - | |
| 2,2,4,6,6-pentamethylheptane | b | 13475-82-6 | 997 | 984 | 1.22 | - | - | - | 0.52 | 0.63 | - | - | - | - | - | - | |
| 4,6-Dimethyldodecane * | 61141-72-8 | - | 1277 | - | - | - | - | 0.57 | - | - | - | - | - | - | - | ||
| Heptadecane | bc | 629-78-7 | 1700 | 1696 | - | - | - | - | 1.39 | - | - | - | 1.61 | 0.80 | 0.47 | 0.54 | |
| Heptane | ac | 142-82-5 | 700 | 677 | 1.57 | 0.26 | 0.10 | - | - | - | - | 0.48 | - | - | - | - | |
| Hexane | c | 110-54-3 | 600 | 612 | 4.68 | 17.18 | - | 8.62 | - | 17.97 | - | 13.93 | - | - | - | - | |
| Methyl-cyclooctane * | c | 1502-38-1 | - | 1386 | - | - | - | - | - | - | 1.49 | - | - | - | - | 0.33 | |
| Nonyl-cyclopropane * | 74663-85-7 | - | 1273 | - | - | - | - | 0.49 | - | - | - | - | - | - | - | ||
| Octane | c | 111-65-9 | 800 | 788 | - | - | - | - | - | - | - | 1.76 | - | - | - | - | |
| Bicyclo[2.2.0]hexa-2,5-diene * | Alkene | 7641-77-2 | - | 1380 | - | - | - | - | - | - | - | - | - | - | - | 0.33 | |
| Dodec-1-ene | 1120-36-1 | 1187 | 1188 | - | - | - | - | - | - | - | - | 0.50 | - | - | 0.27 | ||
| Styrene | abc | 100-42-5 | 898 | 882 | 8.31 | - | - | 6.10 | 3.73 | - | - | - | 63.64 | 42.46 | 32.57 | 29.06 | |
| Toluene | abc | 108-88-3 | 762 | 745 | - | - | - | - | - | - | - | 2.37 | - | - | - | - | |
| Ethyl 2-methylbutyrate | bc | Ester | 7452-79-1 | - | 840 | 2.28 | - | 0.60 | 2.84 | - | - | - | - | - | - | 0.31 | 0.35 |
| Ethyl acetate | bc | 141-78-6 | 612 | 618 | 5.87 | 12.9 | 18.7 | 17.1 | 3.62 | 14.5 | - | 12.7 | 11.93 | 9.00 | 13.08 | 11.67 | |
| Ethyl butyrate | c | 105-54-4 | 802 | 795 | - | - | - | - | - | - | - | 0.36 | - | - | - | - | |
| Ethyl isobutyrate | c | 97-62-1 | - | 740 | 1.01 | 0.42 | 1.20 | 2.09 | 0.41 | 0.31 | - | 1.28 | 1.51 | 0.62 | 0.25 | 0.43 | |
| Ethyl phenylethanoate | c | 101-97-3 | 1248 | 1242 | - | - | - | - | - | - | - | - | - | - | - | 0.39 | |
| Ethyl propanoate | c | 105-37-3 | 714 | 695 | 1.66 | - | - | 0.32 | 0.57 | - | - | 0.30 | - | 0.64 | 0.54 | 0.42 | |
| 2,5-Dimethylfuran | c | Furan | 625-86-5 | - | 689 | 0.75 | - | 0.14 | 0.21 | - | - | - | 0.15 | - | - | 0.25 | 0.27 |
| 2-Methylfuran | abc | 534-22-5 | 603 | 615 | - | - | - | - | - | - | 10.71 | - | - | - | - | - | |
| 3-Hydroxybutan-2-one | c | Ketone | 513-86-0 | - | 695 | - | 0.37 | 0.40 | - | - | 2.03 | 1.26 | - | - | - | - | - |
| Butan-2-one | a | 78-93-3 | 605 | 609 | - | - | - | - | - | - | - | - | 23.00 | 12.42 | - | 19.29 | |
| Thiochroman-4-one * | 3528-17-4 | - | 1124 | - | - | - | - | - | - | - | - | 0.56 | - | - | - | ||
| NI 640 | Other | - | - | 640 | - | - | 12.7 | - | - | - | - | - | - | - | - | - | |
| NI 729 | - | - | 729 | - | - | 1.74 | - | - | - | - | - | - | - | - | - | ||
| NI 756 | - | - | 756 | - | - | 0.11 | 0.47 | - | - | - | - | - | - | - | - | ||
| NI 1271 | - | - | 1271 | - | - | - | - | - | - | - | - | 0.41 | - | - | - | ||
| NI 1323 | - | - | 1323 | - | - | - | - | 0.46 | - | - | - | - | - | - | - | ||
| NI 1501 | - | - | 1501 | - | - | - | - | - | - | - | - | 0.45 | - | - | - | ||
| 2,4,5-Trimethyl-1,3-dioxolane * | 3299-32-9 | 752 * | 708 | - | - | 0.32 | - | - | - | 0.31 | - | - | - | - | - | ||
| Trichloromethane | c | 67-66-3 | - | 623 | 3.76 | - | - | - | 8.20 | - | - | - | - | - | - | - | |
| 4a,8-Dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene * | Terpene | 103827-22-1 | - | 1476 | - | - | - | - | - | - | - | - | - | 0.46 | 0.68 | 0.57 | |
| (7a-Isopropenyl-4,5-dimethyloctahydroinden-4-yl)methanol * | Not available | - | 1738 | - | - | - | - | - | - | - | - | 0.54 | - | - | - | ||
| Di-epi-1,10-cubenol | 73365-77-2 | 1623 | 1611 | - | - | - | - | - | - | - | - | - | - | - | 0.16 | ||
| Aromadendrene | c | 109119-91-7 | 1444 | 1443 | - | - | - | - | 0.86 | - | - | - | 0.70 | - | - | - | |
| cis-Muurola-3,5-diene * | b | 157374-44-2 | 1447 * | 1448 | - | - | - | - | 2.43 | - | - | - | 1.97 | - | 0.34 | 0.26 | |
| Epi-bicyclosesquiphellandrene | abc | 54274-73-5 | 1478 | 1463 | - | - | - | - | 6.27 | - | - | - | 4.04 | 1.25 | 0.85 | 0.68 | |
| Epi-cubeno-1-ol * | 19912-67-5 | 1619 * | 1611 | - | - | - | - | 0.94 | - | - | - | 0.79 | - | - | - | ||
| Epizonaren | abc | 41702-63-0 | 1497 | 1494 | - | - | - | - | 23.22 | 6.38 | 4.22 | 1.27 | 17.54 | 7.17 | 5.15 | 4.50 | |
| Germacrene-D | ab | 23986-74-5 | 1480 | 1480 | - | - | - | - | 4.24 | 0.63 | - | - | 3.03 | 0.92 | 0.59 | 0.46 | |
| Palustrol | 5986-49-2 | 1569 | 1565 | - | - | - | - | 0.34 | - | - | - | 0.45 | - | - | - | ||
| trans-Caryophyllene | abc | 87-44-5 | 1418 | 1414 | - | - | - | - | 1.63 | - | - | - | 1.12 | - | - | - | |
| Valencene | abc | 997297 | 1490 | 1491 | - | - | - | - | - | - | - | - | - | - | - | 0.69 | |
| Viridiflorol | 552-02-3 | 1589 | 1589 | - | - | - | - | 0.51 | - | - | - | 0.74 | - | - | - | ||
| α.-Dehydro-ar-himachalene | 78204-62-3 | 1522 | 1537 | - | - | - | - | - | - | - | - | 0.36 | - | - | - | ||
| α-Cadinene | b | 24406-05-1 | 1538 | 1534 | - | - | - | - | 1.59 | - | - | - | 0.78 | 0.24 | - | 0.09 | |
| α-Cadinol | 481-34-5 | 1656 | 1654 | - | - | - | - | - | - | - | - | 0.53 | - | - | - | ||
| α-Calacorene | b | 21391-99-1 | 1540 | 1540 | - | - | - | - | 0.55 | - | - | - | 0.56 | - | - | - | |
| α-Copaene | ac | 3856-25-5 | 1372 | 1365 | - | - | - | - | 1.16 | - | - | - | 1.20 | - | - | - | |
| α-Corocalene | bc | 20129-39-9 | 1629 | 1620 | - | - | - | - | - | - | - | - | 0.32 | - | - | - | |
| α-Cubebene | a | 17699-14-8 | 1348 | 1342 | - | - | - | - | 0.69 | - | - | - | 0.79 | - | - | - | |
| α-Gurjunene | ac | 489-40-7 | 1408 | 1401 | - | - | - | - | 2.02 | - | - | - | 1.45 | - | 0.26 | 0.22 | |
| α-Isocomene | 65372-78-3 | 1392 | 1380 | - | - | - | - | 3.51 | 0.47 | - | - | 2.21 | 0.65 | 0.40 | - | ||
| α-Muurolene | bc | 31983-22-9 | 1472 | 1471 | - | - | - | - | 0.88 | - | - | - | 0.64 | - | - | - | |
| α-Selinene | ab | 473-13-2 | 1494 | 1491 | - | - | - | - | 6.73 | - | - | - | 4.73 | 1.42 | 0.88 | - | |
| β-Cadinene | c | 523-47-7 | - | 1489 | - | - | - | - | 1.11 | - | - | - | 0.72 | - | - | - | |
| β-Chamigrene | a | 18431-82-8 | 1472 | 1476 | - | - | - | - | - | - | - | - | 1.05 | - | - | - | |
| β-Elemene (E) | abc | Terpene | 515-13-9 | 1382 | 1376 | - | - | - | - | 1.85 | - | - | - | 0.93 | - | 3.05 | 1.83 |
| β-Elemene (Z) | abc | 515-13-9 | 1382 | 1384 | - | - | - | - | 35.71 | 4.15 | - | - | 17.10 | 5.13 | - | - | |
| β-Himachalene | abc | 1461-03-6 | 1498 | 1497 | 0.54 | - | - | - | 2.53 | 1.47 | - | - | 6.41 | 5.24 | 4.46 | 5.61 | |
| β-Selinene | a | 17066-67-0 | 1479 | 1483 | - | - | - | - | 3.30 | - | - | - | 2.35 | 0.65 | 0.42 | 0.28 | |
| γ-Cadinene | abc | 39029-41-9 | 1513 | 1508 | - | - | - | - | 18.85 | 1.93 | - | - | 9.95 | 3.30 | 2.09 | 1.57 | |
| γ-Gurjunene | ac | 22567-17-5 | 1476 | 1472 | - | - | - | - | 10.06 | - | - | - | 6.37 | 1.66 | 1.17 | 0.95 | |
| γ-Muurolene | abc | 30021-74-0 | 1477 | 1477 | - | - | - | - | 2.80 | - | - | - | 0.99 | - | - | - | |
| δ-Cadinene | abc | 483-76-1 | 1524 | 1520 | - | - | - | - | 26.06 | 5.91 | 2.92 | 0.92 | 18.11 | 6.58 | 4.43 | 3.68 | |
| τ-Muurolol | 19912-62-0 | 1641 | 1644 | - | - | - | - | - | - | - | - | 0.38 | - | - | - | ||
| Compound | ITEM 8111 | ITEM 8111* |
|---|---|---|
| α-Cadinene | 0.432 | 0.277 |
| α-Cadinol | - | 0.175 |
| α-Isocomene | 0.950 | 0.720 |
| α-Muurolene | 0.282 | 0.209 |
| α-Selinene | 1.817 | 1.565 |
| β-Chamigrene | - | 0.370 |
| β-Elemene | 8.897 | 5.181 |
| β-Himachalene | 0.737 | 2.590 |
| δ-Cadinene | 6.042 | 7.874 |
| γ-Gurjunene | 2.615 | 1.895 |
| γ-Muurolene | 0.769 | 0.381 |
| τ-Muurolol | - | 0.105 |
| Aromadendrene | 0.205 | 0.255 |
| Epi-cuben-1-ol | 0.311 | 0.360 |
| Epizonaren | 7.128 | 5.948 |
| Germacrene-D | 1.132 | 0.996 |
| Styrene | 261.75 | 29.8 × 106 |
| 2-Methylbutan-1-ol | 2.223 | 0.888 |
| 3-Methylbutan-1-ol | 0.934 | 0.440 |
| Samples | Aflatoxin Mixture 2.5 ng·µL−1 | Zearalanone 10 ng·µL−1 | Deepoxydeoxynivalenol 50 ng·µL−1 |
|---|---|---|---|
| Blank | - | 20 | 10 |
| Spike 0.5 X | 10 | 20 | 10 |
| Spike 1 X | 20 | 20 | 10 |
| Spike 1.5 X | 30 | 20 | 10 |
| Spike 2 X | 40 | 20 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Jijakli, M.H.; Soyeurt, H.; Fauconnier, M.-L. Volatile Organic Compounds Emitted by Aspergillus flavus Strains Producing or Not Aflatoxin B1. Toxins 2021, 13, 705. https://doi.org/10.3390/toxins13100705
Josselin L, De Clerck C, De Boevre M, Moretti A, Jijakli MH, Soyeurt H, Fauconnier M-L. Volatile Organic Compounds Emitted by Aspergillus flavus Strains Producing or Not Aflatoxin B1. Toxins. 2021; 13(10):705. https://doi.org/10.3390/toxins13100705
Chicago/Turabian StyleJosselin, Laurie, Caroline De Clerck, Marthe De Boevre, Antonio Moretti, M. Haïssam Jijakli, Hélène Soyeurt, and Marie-Laure Fauconnier. 2021. "Volatile Organic Compounds Emitted by Aspergillus flavus Strains Producing or Not Aflatoxin B1" Toxins 13, no. 10: 705. https://doi.org/10.3390/toxins13100705






