Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Dual Analysis of Absolute and Relative Alternative Splicing
2.2. Functional Enrichment
2.3. Gene and Transcription Factor Network Reconstruction
3. Results
3.1. Absolute Differential Expression of Isoforms Associated with Opioid-Induced Hyperalgesia
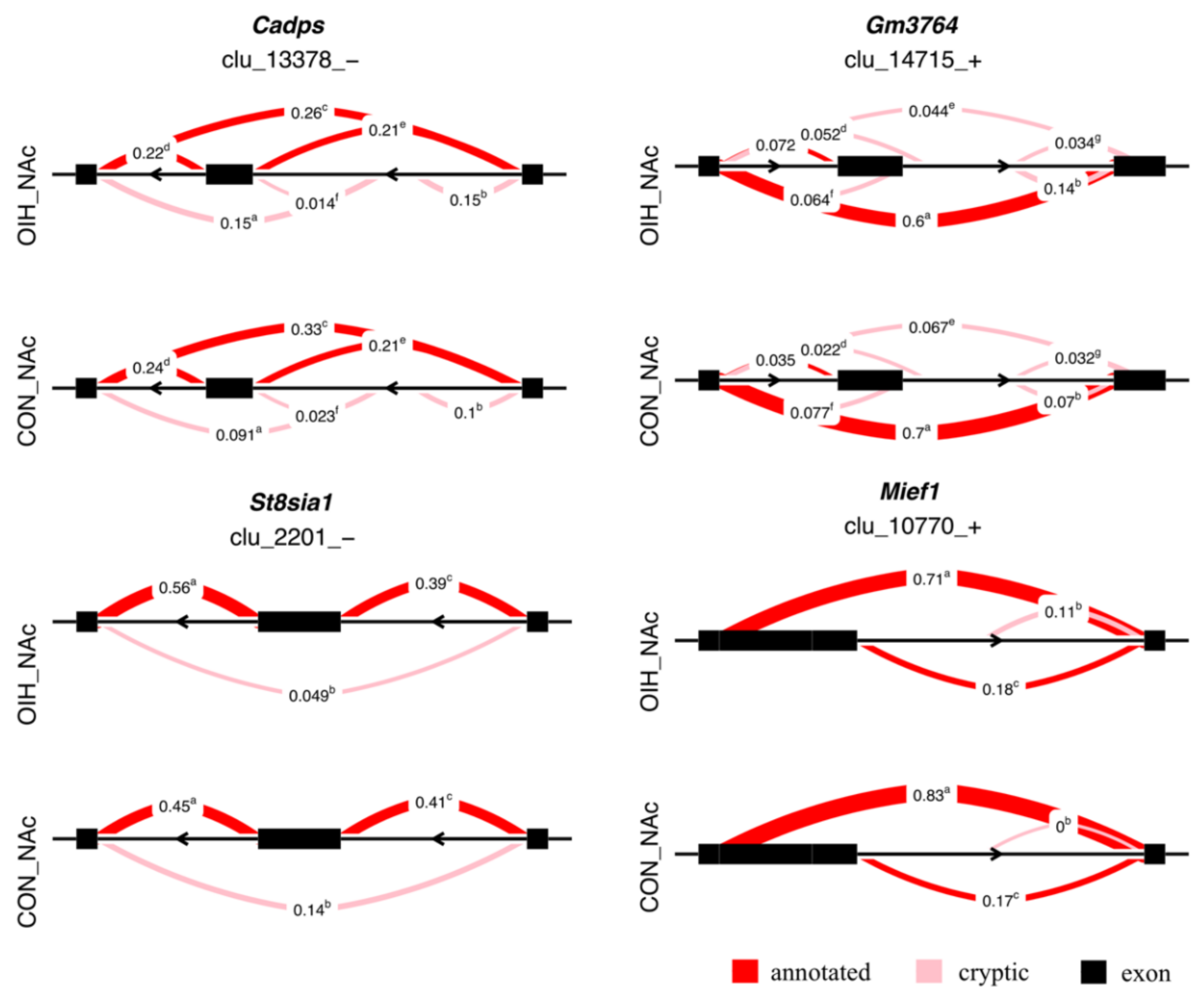
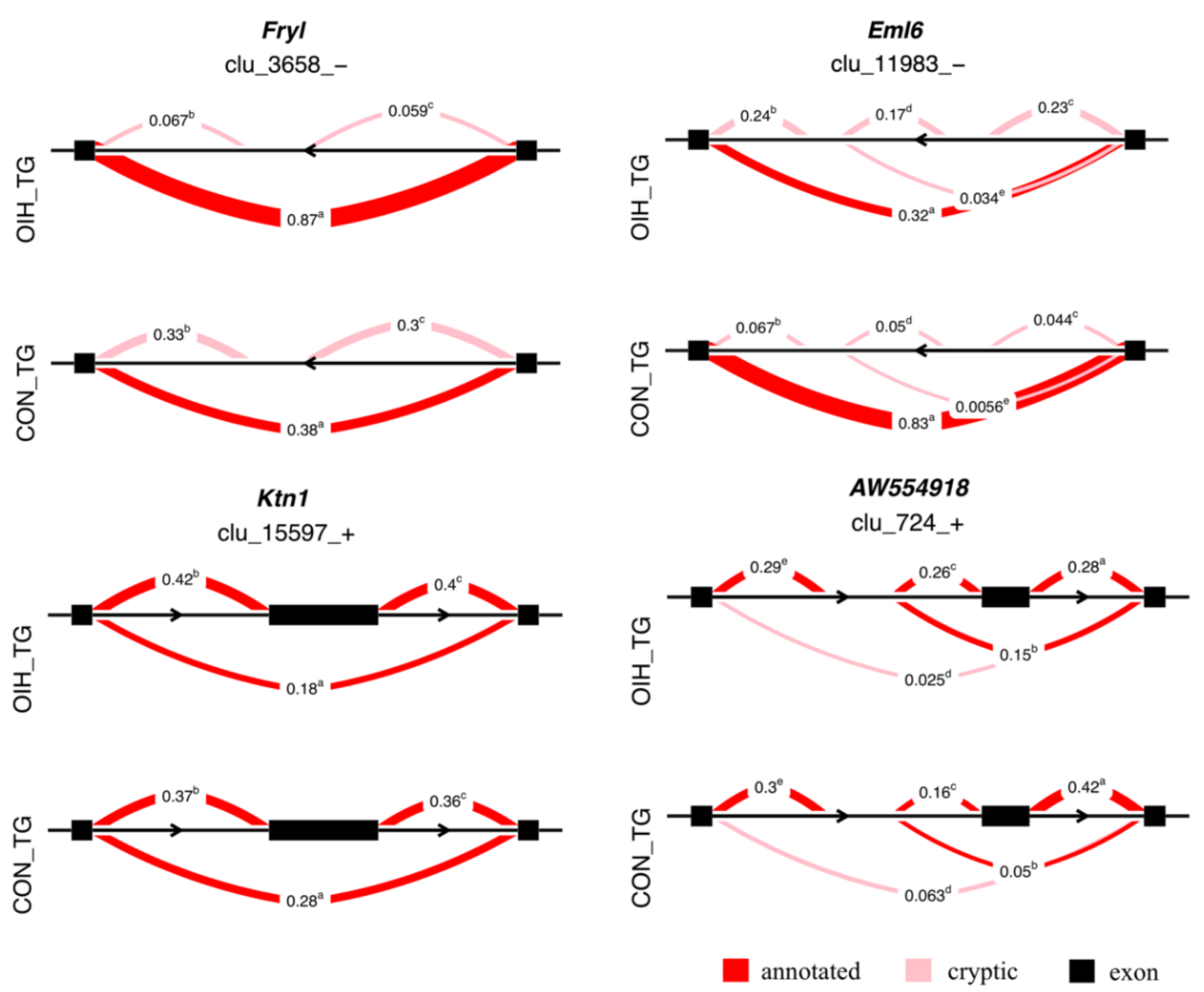
3.2. Relative Differential Expression of Isoform Associated with Opioid-Induced Hyperalgesia
3.3. Functional Analysis of Absolute and Relative Differential Isoform Expression Associated with Opioid-Induced Hyperalgesia
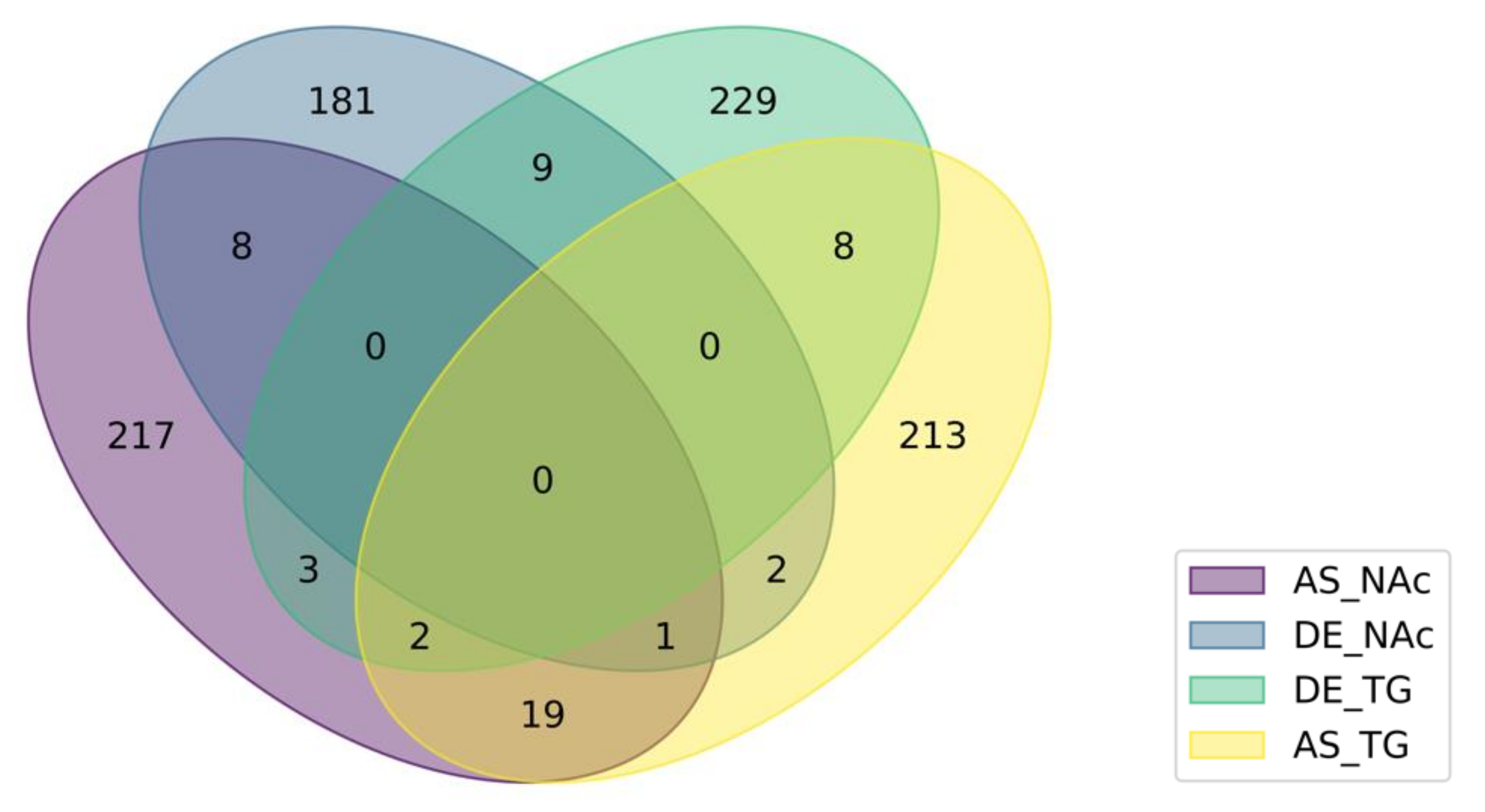
3.4. Integrated Analysis of Absolute and Relative Differential Isoform Expression Associated with Opioid-Induced Hyperalgesia
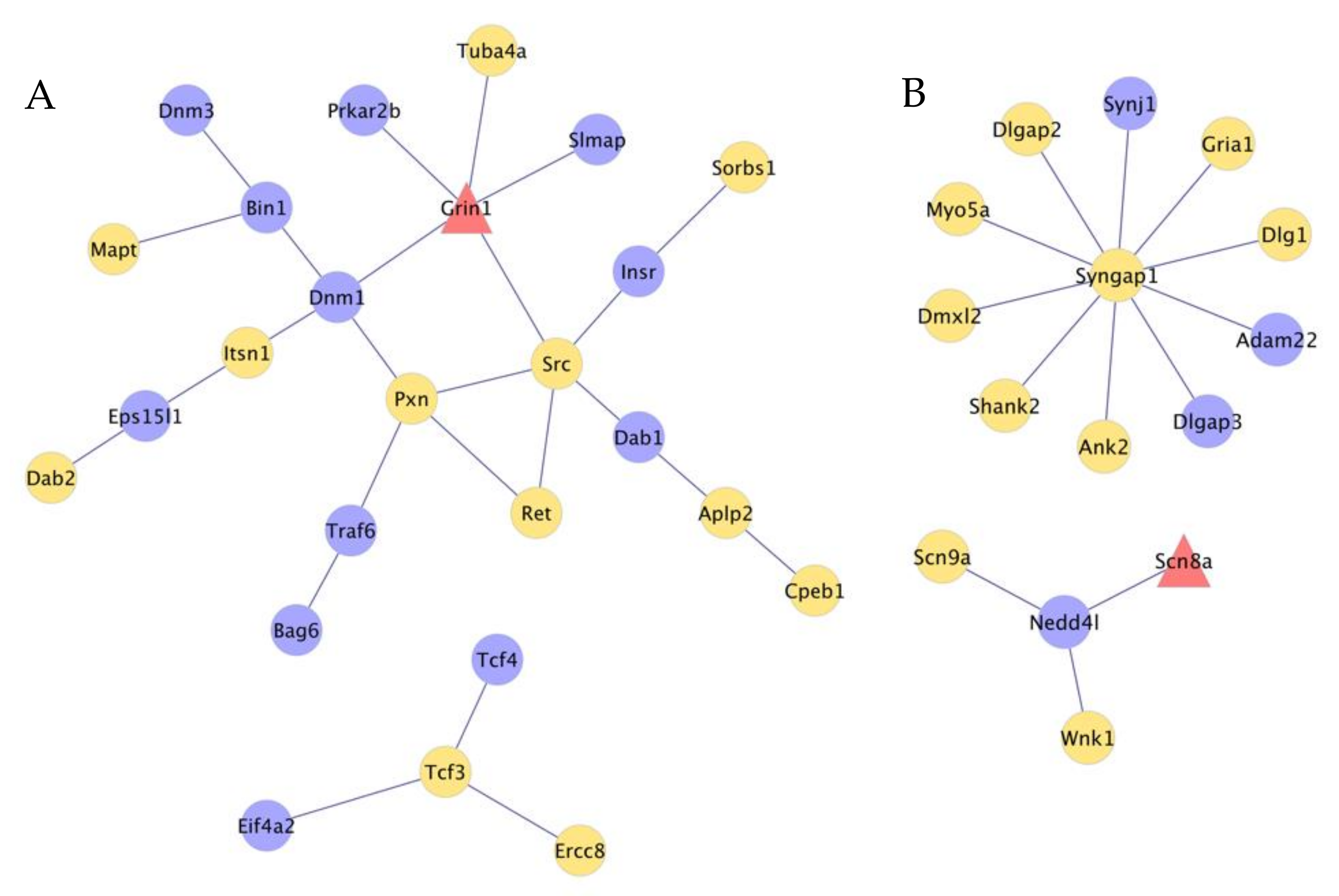
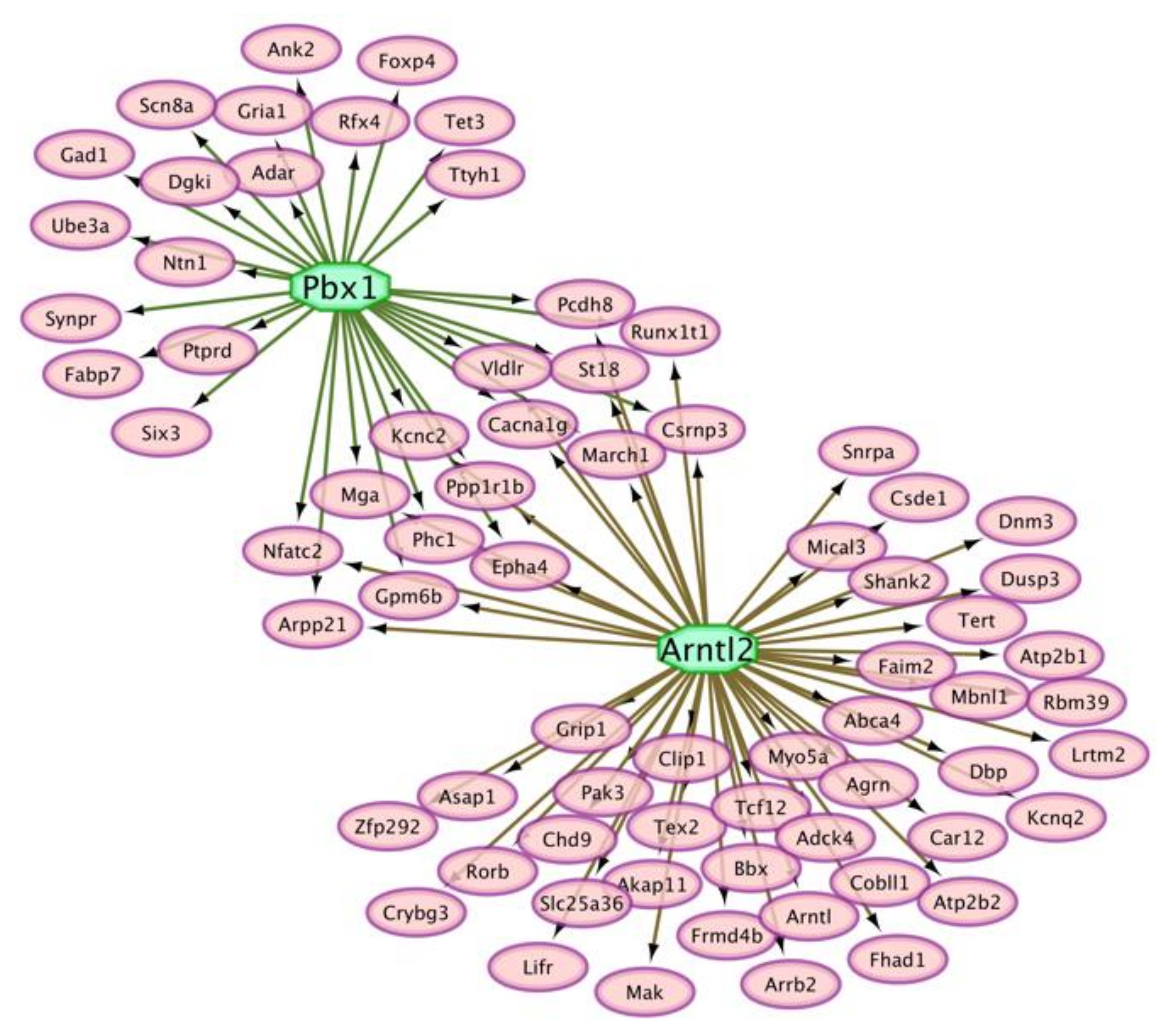
3.5. Interactions between Genes That Present Absolute and Relative Differential Isoform Expression Associated with Opioid-Induced Hyperalgesia
4. Discussion
4.1. Absolute Differential Expression of Transcript Isoforms Associated with Opioid-Induced Hyperalgesia in the Nucleus Accumbens
4.2. Absolute Differential Expression of Transcript Isoforms Associated with Opioid-Induced Hyperalgesia in the Trigeminal Ganglia
4.3. Relative Differential Expression of Transcript Isoforms Associated with Opioid-Induced Hyperalgesia in the Nucleus Accumbens
4.4. Relative Differential Expression of Transcript Isoforms Associated with Opioid-Induced Hyperalgesia in the Trigeminal Ganglia
4.5. Functional Analysis of Genes Presenting Alternative Splicing in Association with Opioid-Induced Hyperalgesia
4.6. Insights from the Integrated Study of Absolute and Relative Splicing Associated with Opioid-Induced Hyperalgesia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Kaur, K.; Sharma, S.; Goyal, S.; Arora, S.; Murthy, R.S.R. Clinical aspects of acute post-operative pain management & its assessment. J. Adv. Pharm. Technol. Res. 2010, 1, 97–108. [Google Scholar]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Angst, M.S.; Clark, J.D. Opioid-induced Hyperalgesia. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef]
- Chu, L.; Angst, M.S.; Clark, D. Opioid-induced Hyperalgesia in Humans. Clin. J. Pain 2008, 24, 479–496. [Google Scholar] [CrossRef]
- Stoicea, N.; Russell, D.; Weidner, G.; Durda, M.; Joseph, N.; Yu, J.; Bergese, S. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front. Pharmacol. 2015, 6, 104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anapindi, K.D.B.; Yang, N.; Romanova, E.V.; Rubakhin, S.S.; Tipton, A.; Dripps, I.; Sheets, Z.; Sweedler, J.V.; Pradhan, A.A. PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models. Mol. Cell. Proteom. 2019, 18, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Dripps, I.J.; Bertels, Z.; Moye, L.S.; Tipton, A.F.; Siegersma, K.; Baca, S.M.; Kieffer, B.L.; Pradhan, A.A. Forebrain delta opioid receptors regulate the response of delta agonist in models of migraine and opioid-induced hyperalgesia. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Moye, L.S.; Tipton, A.F.; Dripps, I.; Sheets, Z.; Crombie, A.; Violin, J.D.; Pradhan, A.A. Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology 2018, 148, 77–86. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Smith, M.L.; Zyuzin, J.; Charles, A. δ-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br. J. Pharmacol. 2014, 171, 2375–2384. [Google Scholar] [CrossRef]
- Zhang, P.; Moye, L.S.; Southey, B.R.; Dripps, I.; Sweedler, J.V.; Pradhan, A.; Rodriguez-Zas, S.L. Opioid-Induced Hyperalgesia Is Associated with Dysregulation of Circadian Rhythm and Adaptive Immune Pathways in the Mouse Trigeminal Ganglia and Nucleus Accumbens. Mol. Neurobiol. 2019, 56, 7929–7949. [Google Scholar] [CrossRef]
- Zhang, P.; Southey, B.R.; Sweedler, J.V.; Pradhan, A.; Rodriguez-Zas, S.L. Enhanced Understanding of Molecular Interactions and Function Underlying Pain Processes Through Networks of Transcript Isoforms, Genes, and Gene Families. Adv. Appl. Bioinform. Chem. 2021, 14, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Southey, B.; Rodriguez-Zas, S. Co-expression networks uncover regulation of splicing and transcription markers of disease. EPiC Series Comput. 2020, 70, 119–128. [Google Scholar] [CrossRef]
- Silverman, S.M. Opioid induced hyperalgesia: Clinical implications for the pain practitioner. Pain Physician 2009, 12, 679–684. [Google Scholar] [CrossRef]
- Brush, D.E. Complications of Long-Term Opioid Therapy for Management of Chronic Pain: The Paradox of Opioid-Induced Hyperalgesia. J. Med. Toxicol. 2012, 8, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.; Graveley, B.R. RNA structure and the mechanisms of alternative splicing. Curr. Opin. Genet. Dev. 2011, 21, 373–379. [Google Scholar] [CrossRef]
- Donaldson, L.F.; Beazley-Long, N. Alternative RNA splicing: Contribution to pain and potential therapeutic strategy. Drug Discov. Today 2016, 21, 1787–1798. [Google Scholar] [CrossRef]
- Pasternak, G.W.; Childers, S.R.; Pan, Y.-X. Emerging Insights into Mu Opioid Pharmacology. Subst. Use Disord. 2019, 89–125. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Z.; Narayan, A.; Le Rouzic, V.P.; Xu, M.; Hunkele, A.; Brown, T.; Hoefer, W.F.; Rossi, G.C.; Rice, R.C.; et al. Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J. Clin. Investig. 2017, 127, 1561–1573. [Google Scholar] [CrossRef]
- Sengar, A.S.; Li, H.; Zhang, W.; Leung, C.; Ramani, A.K.; Saw, N.M.; Wang, Y.; Tu, Y.; Ross, P.J.; Scherer, S.; et al. Control of Long-Term Synaptic Potentiation and Learning by Alternative Splicing of the NMDA Receptor Subunit GluN1. Cell Rep. 2019, 29, 4285–4294.e5. [Google Scholar] [CrossRef]
- Evsyukova, I.; Somarelli, J.A.; Gregory, S.; Garcia-Blanco, M.A. Alternative splicing in multiple sclerosis and other autoimmune diseases. RNA Biol. 2010, 7, 462–473. [Google Scholar] [CrossRef]
- Goncalves, T.M.; Southey, B.R.; Rodriguez-Zas, S.L. Interplay Between Amphetamine and Activity Level in Gene Networks of the Mouse Striatum. Bioinform. Biol. Insights 2018, 12. [Google Scholar] [CrossRef]
- Jeong, H.; Moye, L.S.; Southey, B.R.; Hernandez, A.G.; Dripps, I.; Romanova, E.V.; Rubakhin, S.S.; Sweedler, J.V.; Pradhan, A.A.; Rodriguez-Zas, S.L. Gene Network Dysregulation in the Trigeminal Ganglia and Nucleus Accumbens of a Model of Chronic Migraine-Associated Hyperalgesia. Front. Syst. Neurosci. 2018, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.; Smyth, G. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Li, Y.I.; Knowles, D.A.; Humphrey, J.; Barbeira, A.N.; Dickinson, S.P.; Im, H.K.; Pritchard, J.K. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 2017, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S. Alternative splicing, RNA-seq and drug discovery. Drug Discov. Today 2019, 24, 1258–1267. [Google Scholar] [CrossRef]
- Raj, T.; Li, Y.I.; Wong, G.; Humphrey, J.; Wang, M.; Ramdhani, S.; Wang, Y.-C.; Ng, B.; Gupta, I.; Haroutunian, V.; et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat. Genet. 2018, 50, 1584–1592. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.-M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2018, 47, D766–D773. [Google Scholar] [CrossRef]
- Mi, H.; Thomas, P. PANTHER Pathway: An Ontology-Based Pathway Database Coupled with Data Analysis Tools. Methods Mol Biol. 2009, 563, 123–140. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Nixon, S.E.; O’Connor, J.C.; Southey, B.R.; Lawson, M.A.; McCusker, R.H.; Borras, T.; Machuca, D.; Hernandez, A.G.; Dantzer, R.; et al. Microglia Transcriptome Changes in a Model of Depressive Behavior after Immune Challenge. PLoS ONE 2016, 11, e0150858. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pena, D.; Nixon, S.E.; Southey, B.R.; Lawson, M.A.; McCusker, R.H.; Hernandez, A.G.; Dantzer, R.; Kelley, K.W.; Rodriguez-Zas, S.L. Differential Transcriptome Networks between IDO1-Knockout and Wild-Type Mice in Brain Microglia and Macrophages. PLoS ONE 2016, 11, e0157727. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef]
- Martin, A.; Ochagavia, M.E.; Rabasa, L.C.; Miranda, J.; Fernandez-De-Cossio, J.; Bringas, R. BisoGenet: A new tool for gene network building, visualization and analysis. BMC Bioinform. 2010, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for Visualization and Analysis of Biological Networks. In Data Mining in Proteomics; Humana Press: Totowa, NJ, USA, 2010; Volume 696, pp. 291–303. [Google Scholar] [CrossRef]
- Salwinski, L. The Database of Interacting Proteins: 2004 update. Nucleic Acids Res. 2004, 32, 449D–451D. [Google Scholar] [CrossRef]
- Alfarano, C. The Biomolecular Interaction Network Database and related tools 2005 update. Nucleic Acids Res. 2004, 33, D418–D424. [Google Scholar] [CrossRef] [PubMed]
- Stark, C. BioGRID: A general repository for interaction datasets. Nucleic Acids Res. 2006, 34, D535–D539. [Google Scholar] [CrossRef]
- Licata, L.; Briganti, L.; Peluso, D.; Perfetto, L.; Iannuccelli, M.; Galeota, E.; Sacco, F.; Palma, A.; Nardozza, A.P.; Santonico, E.; et al. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 2011, 40, D857–D861. [Google Scholar] [CrossRef] [PubMed]
- Kerrien, S.; Alam-Faruque, Y.; Aranda, B.; Bancarz, I.; Bridge, A.; Derow, C.; Dimmer, E.; Feuermann, M.; Friedrichsen, A.; Huntley, R.; et al. IntAct—Open source resource for molecular interaction data. Nucleic Acids Res. 2006, 35, D561–D565. [Google Scholar] [CrossRef]
- Mishra, G.R. Human protein reference database—2006 update. Nucleic Acids Res. 2006, 34, D411–D414. [Google Scholar] [CrossRef]
- Janky, R.; Verfaillie, A.; Imrichova, H.; Van de Sande, B.; Standaert, L.; Christiaens, V.; Hulselmans, G.; Herten, K.; Sanchez, M.N.; Potier, D.; et al. iRegulon: From a Gene List to a Gene Regulatory Network Using Large Motif and Track Collections. PLoS Comput. Biol. 2014, 10, e1003731. [Google Scholar] [CrossRef] [PubMed]
- Ventéo, S.; Bourane, S.; Méchaly, I.; Sar, C.; Samad, O.A.; Puech, S.; Blostein, R.; Valmier, J.; Pattyn, A.; Carroll, P. Regulation of the Na,K-ATPase Gamma-Subunit FXYD2 by Runx1 and Ret Signaling in Normal and Injured Non-Peptidergic Nociceptive Sensory Neurons. PLoS ONE 2012, 7, e29852. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Ruan, Y.; Gegen, T.; Yu, L.; Zhu, C.; Yang, Y.; Zhou, Y.; Yu, G.; Tang, Z. Pirt Together with TRPV1 Is Involved in the Regulation of Neuropathic Pain. Neural Plast. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Hurt, J.K.; Zylka, M.J. PAPupuncture Has Localized and Long-Lasting Antinociceptive Effects in Mouse Models of Acute and Chronic Pain. Mol. Pain 2012, 8, 28. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lee, C.-H.; Sun, W.-H.; Chen, C.-C. Involvement of advillin in somatosensory neuron subtype-specific axon regeneration and neuropathic pain. Proc. Natl. Acad. Sci. USA 2018, 115, E8557–E8566. [Google Scholar] [CrossRef]
- Royds, J.; Cassidy, H.; Conroy, M.J.; Dunne, M.R.; Lysaght, J.; McCrory, C. Examination and characterisation of the effect of amitriptyline therapy for chronic neuropathic pain on neuropeptide and proteomic constituents of human cerebrospinal fluid. Brain Behav. Immun. Health 2020, 10, 100184. [Google Scholar] [CrossRef]
- Ramirez, J.D.; Barnes, P.R.; Mills, K.R.; Bennett, D.L. Intermediate Charcot-Marie-Tooth disease due to a novel Trp101Stop myelin protein zero mutation associated with debilitating neuropathic pain. Pain 2012, 153, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.-P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998. [Google Scholar] [CrossRef]
- Petrenko, A.B.; Yamakura, T.; Baba, H.; Shimoji, K. The Role of N-Methyl-d-Aspartate (NMDA) Receptors in Pain: A Review. Anesthesia Analg. 2003, 97, 1108–1116. [Google Scholar] [CrossRef]
- Oort, P.J.; Warden, C.H.; Baumann, T.K.; Knotts, T.A.; Adams, S.H. Characterization of Tusc5, an adipocyte gene co-expressed in peripheral neurons. Mol. Cell. Endocrinol. 2007, 276, 24–35. [Google Scholar] [CrossRef]
- Garzón, J.; López-Fando, A.; Sánchez-Blázquez, P. The R7 Subfamily of RGS Proteins Assists Tachyphylaxis and Acute Tolerance at μ-Opioid Receptors. Neuropsychopharmacology 2003, 28, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Hrstka, S.C.; Ankam, S.; Agac, B.; Klein, J.P.; Moore, R.A.; Narapureddy, B.; Schneider, I.; Hrstka, R.F.; Dasari, S.; Staff, N.P. Proteomic analysis of human iPSC-derived sensory neurons implicates cell stress and microtubule dynamics dysfunction in bortezomib-induced peripheral neurotoxicity. Exp. Neurol. 2020, 335, 113520. [Google Scholar] [CrossRef]
- Nair, H.K.; Hain, H.; Quock, R.M.; Philip, V.M.; Chesler, E.J.; Belknap, J.K.; Lariviere, W.R. Genomic loci and candidate genes underlying inflammatory nociception. Pain 2011, 152, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Eun-Sung, P.; Jung-Mo, A.; Sang-Min, J.; Hee-Jung, C.; Ki-Myung, C.; Je-Yoel, C.; Dong-Ho, Y. Proteomic analysis of the dorsal spinal cord in the mouse model of spared nerve injury-induced neuropathic pain. J. Biomed. Res. 2017, 31, 494–502. [Google Scholar] [CrossRef]
- Pearse, D.D.; Hughes, Z.A. PDE4B as a microglia target to reduce neuroinflammation. Glia 2016, 64, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, J.; Mogil, J.S. Genetics of Opioid Actions. In The Opiate Receptors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 457–497. [Google Scholar] [CrossRef]
- O’Leary, H.; Vanderlinden, L.; Southard, L.; Castano, A.; Saba, L.M.; Benke, T.A. Transcriptome analysis of rat dorsal hippocampal CA1 after an early life seizure induced by kainic acid. Epilepsy Res. 2020, 161, 106283. [Google Scholar] [CrossRef]
- Ko, H.-G.; Oh, S.-B.; Zhuo, M.; Kaang, B.-K. Reduced acute nociception and chronic pain inShank2−/−mice. Mol. Pain 2016, 12, 1744806916647056. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Kwon, S.-G.; Kim, Y.H.; Yeo, J.-H.; Ko, H.-G.; Roh, D.-H.; Kaang, B.-K.; Beitz, A.J.; Lee, J.-H.; Oh, S.B. A critical role of spinal Shank2 proteins in NMDA-induced pain hypersensitivity. Mol. Pain 2017, 13, 1744806916688902. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Li, X.; Fei, Z.; Poon, W. Scaffold protein Homer 1: Implications for neurological diseases. Neurochem. Int. 2012, 61, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Schwarz, T.L. O-GlcNAc Transferase Is Essential for Sensory Neuron Survival and Maintenance. J. Neurosci. 2017, 37, 2125–2136. [Google Scholar] [CrossRef]
- Carter, B.; Justin, H.S.; Gulick, D.; Gamsby, J.J. The Molecular Clock and Neurodegenerative Disease: A Stressful Time. Front. Mol. Biosci. 2021, 8, 175. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Haro-Acuña, V.; Momboisse, F.; Caviedes, P.; Bevilacqua, J.A.; Cárdenas, A.M. Dynamin-2 in nervous system disorders. J. Neurochem. 2013, 128, 210–223. [Google Scholar] [CrossRef]
- Shimojima, K.; Komoike, Y.; Tohyama, J.; Takahashi, S.; Páez, M.T.; Nakagawa, E.; Goto, Y.; Ohno, K.; Ohtsu, M.; Oguni, H.; et al. TULIP1 (RALGAPA1) haploinsufficiency with brain development delay. Genomics 2009, 94, 414–422. [Google Scholar] [CrossRef]
- Kozaki, Y.; Umetsu, R.; Mizukami, Y.; Yamamura, A.; Kitamori, K.; Tsuchikura, S.; Ikeda, K.; Yamori, Y. Peripheral gene expression profile of mechanical hyperalgesia induced by repeated cold stress in SHRSP5/Dmcr rats. J. Physiol. Sci. 2015, 65, 417–425. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; A Salazar, G.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Cai, Y.; Ma, L.; Ma, C.; Luo, A.; Huang, Y. Glutaminase 1 is a potential biomarker for chronic post-surgical pain in the rat dorsal spinal cord using differential proteomics. Amino Acids 2015, 48, 337–348. [Google Scholar] [CrossRef]
- Xian, H.; Liou, Y.-C. Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy. Autophagy 2019, 15, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, S.; Ceylan, S.; Etus, V.; Yasa, N.; Ceylan, S. Morinda citrifolia L. (noni) and memantine attenuate periventricular tissue injury of the fourth ventricle in hydrocephalic rabbits. Neural Regen. Res. 2013, 8, 773–782. [Google Scholar] [CrossRef]
- Chew, L.; Bellampalli, S.S.; Dustrude, E.T.; Khanna, R. Mining the Nav1.7 interactome: Opportunities for chronic pain therapeutics. Biochem. Pharmacol. 2019, 163, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-H.; Ji, F.-T.; Liu, L.; Li, F. Expression changes of parvalbumin and microtubule-associated protein 2 induced by chronic constriction injury in rat dorsal root ganglia. Chin. Med. J. 2011, 124, 2184–2190. [Google Scholar]
- Santos, H.P.; Bhattacharya, A.; Martin, E.M.; Addo, K.; Psioda, M.; Smeester, L.; Joseph, R.M.; Hooper, S.R.; Frazier, J.A.; Kuban, K.C.; et al. Epigenome-wide DNA methylation in placentas from preterm infants: Association with maternal socioeconomic status. Epigenetics 2019, 14, 751–765. [Google Scholar] [CrossRef]
- Damez-Werno, D.M.; Sun, H.; Scobie, K.N.; Shao, N.-Y.; Rabkin, J.; Dias, C.; Calipari, E.; Maze, I.; Pena, C.J.; Walker, D.M.; et al. Histone arginine methylation in cocaine action in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2016, 113, 9623–9628. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Mao, Q.; Shi, J.; Wang, X.; Li, C.-S.R. Putamen gray matter volumes in neuropsychiatric and neurodegenerative disorders. World J. Psychiatry Ment. Health Res. 2019, 3, 1020. [Google Scholar]
- Patel, C.A.; Ghiselli, G. Hinderin, a five-domains protein including coiled-coil motifs that binds to SMC3. BMC Cell Biol. 2005, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.E.; Wang, M.; Brown, E.; Fones, L.; Cave, J.W. Selective repression of gene expression in neuropathic pain by the neuron-restrictive silencing factor/repressor element-1 silencing transcription (NRSF/REST). Neurosci. Lett. 2015, 625, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.-J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kadowaki, Y.; Fukazawa, Y.; Saika, F.; Kishioka, S. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J. Neurochem. 2013, 129, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Llorián-Salvador, M.; González-Rodríguez, S. Painful Understanding of VEGF. Front. Pharmacol. 2018, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Barkai, O.; Puig, S.; Lev, S.; Title, B.; Katz, B.; Eli-Berchoer, L.; Gutstein, H.B.; Binshtok, A.M. Platelet-derived growth factor activates nociceptive neurons by inhibiting M-current and contributes to inflammatory pain. Pain 2019, 160, 1281–1296. [Google Scholar] [CrossRef]
- Masuda, J.; Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Intrathecal Delivery of PDGF Produces Tactile Allodynia through its Receptors in Spinal Microglia. Mol. Pain 2009, 5, 23. [Google Scholar] [CrossRef]
- Dina, O.A.; Parada, C.A.; Yeh, J.; Chen, X.; McCarter, G.C.; Levine, J.D. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur. J. Neurosci. 2004, 19, 634–642. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, Y.; Chen, B.; Song, G.; Peng, M.; Hu, H.; Zheng, Y.; Chen, C.; Yang, J.; Chen, P.; et al. Network and pathway-based analysis of microRNA role in neuropathic pain in rat models. J. Cell. Mol. Med. 2019, 23, 4534–4544. [Google Scholar] [CrossRef]
- Watkins, L.; Suberg, S.; Thurston, C.; Culhane, E. Role of spinal cord neuropeptides in pain sensitivity and analgesia: Thyrotropin releasing hormone and vasopressin. Brain Res. 1986, 362, 308–317. [Google Scholar] [CrossRef]
- Lee, P.-T.; Chao, P.-K.; Ou, L.-C.; Chuang, J.-Y.; Lin, Y.-C.; Chen, S.-C.; Chang, H.-F.; Law, P.-Y.; Loh, H.H.; Chao, Y.-S.; et al. Morphine drives internal ribosome entry site-mediated hnRNP K translation in neurons through opioid receptor-dependent signaling. Nucleic Acids Res. 2014, 42, 13012–13025. [Google Scholar] [CrossRef] [PubMed]
- Groisman, R.; Kuraoka, I.; Chevallier, O.; Gaye, N.; Magnaldo, T.; Tanaka, K.; Kisselev, A.F.; Harel-Bellan, A.; Nakatani, Y. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 2006, 20, 1429–1434. [Google Scholar] [CrossRef]
- Wilczynska, A.; Gillen, S.L.; Schmidt, T.; Meijer, H.A.; Jukes-Jones, R.; Langlais, C.; Kopra, K.; Lu, W.-T.; Godfrey, J.D.; Hawley, B.; et al. eIF4A2 drives repression of translation at initiation by Ccr4-Not through purine-rich motifs in the 5′UTR. Genome Biol. 2019, 20, 1–21. [Google Scholar] [CrossRef]
- Meijer, H.A.; Kong, Y.W.; Lu, W.T.; Wilczynska, A.; Spriggs, R.V.; Robinson, S.W.; Godfrey, J.D.; Willis, A.E.; Bushell, M. Translational Repression and eIF4A2 Activity Are Critical for MicroRNA-Mediated Gene Regulation. Science 2013, 340, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.A.; Schmidt, T.; Gillen, S.L.; Langlais, C.; Jukes-Jones, R.; De Moor, C.H.; Cain, K.; Wilczynska, A.; Bushell, M. DEAD-box helicase eIF4A2 inhibits CNOT7 deadenylation activity. Nucleic Acids Res. 2019, 47, 8224–8238. [Google Scholar] [CrossRef]
- Alvarado, S.; Tajerian, M.; Millecamps, M.; Suderman, M.; Stone, L.S.; Szyf, M. Peripheral Nerve Injury is Accompanied by Chronic Transcriptome-Wide Changes in the Mouse Prefrontal Cortex. Mol. Pain 2013, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Qiu, Y.; Loh, H.H.; Law, P.-Y. GRIN1 Regulates μ-Opioid Receptor Activities by Tethering the Receptor and G Protein in the Lipid Raft. J. Biol. Chem. 2009, 284, 36521–36534. [Google Scholar] [CrossRef]
- Lynch, W.J.; Girgenti, M.J.; Breslin, F.J.; Newton, S.S.; Taylor, J.R. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: A focus on circadian genes. Brain Res. 2008, 1213, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Khasabov, S.G.; Rogness, V.M.; Beeson, M.B.; Vulchanova-Hart, L.; Yuan, L.-L.; Simone, D.A.; Tran, P.V. The nAChR Chaperone TMEM35a (NACHO) Contributes to the Development of Hyperalgesia in Mice. Neuroscience 2021, 457, 74–87. [Google Scholar] [CrossRef] [PubMed]
| Gene Symbol | Gene Name | Transcript Identifier 1 | LFC 2 | FDR P-Value 3 |
|---|---|---|---|---|
| Fxyd2 | FXYD domain-containing ion transport reg. 2 | 213158.1 | –2.08 | 2.0 × 10–15 |
| Tusc5 | tumor suppressor candidate 5 | 62024.2 | –1.40 | 2.3 × 10–14 |
| Tmem233 | transmembrane protein 233 | 111997.1 | –1.47 | 1.7 × 10–12 |
| Zbtb7b | zinc finger and BTB domain containing 7B | 107433.7 | –8.55 | 1.2 × 10–11 |
| Pirt | phosphoinositide–interacting regulator of trans. | 123434.2 | –1.29 | 1.8 × 10–9 |
| Sncg | synuclein, gamma | 23826.4 | –1.27 | 1.5 × 10–8 |
| Mpz | myelin protein zero | 70758.9 | –1.30 | 1.5 × 10–8 |
| Acpp | acid phosphatase, prostate | 62723.13 | –1.64 | 2.5 × 10–8 |
| Avil | advillin | 26500.11 | –1.15 | 4.1 × 10–8 |
| Rab2b | RAB2B, member RAS oncogene family | 100631.1 | –9.26 | 5.7 × 10–8 |
| Tspan8 | tetraspanin 8 | 80630.1 | –3.74 | 9.3 × 10–8 |
| Ntng1 | netrin G1 | 128219.8 | –0.62 | 6.4 × 10–7 |
| Cdh1 | cadherin 1 | 312.11 | –1.08 | 9.0 × 10–7 |
| Pmp22 | peripheral myelin protein 22 | 108702.7 | –1.41 | 1.1 × 10–6 |
| Mpz | myelin protein zero | 111334.1 | –1.24 | 2.9 × 10–6 |
| Hnrnpa1 | heterogeneous nuclear ribonucleoprotein A1 | 36004.15 | –1.82 | 3.2 × 10–6 |
| Prr13 | proline rich 13(Prr13) | 164688.1 | –7.54 | 1.3 × 10–5 |
| Prrxl1 | paired related homeobox protein–like 1 | 189022.7 | –1.29 | 1.3 × 10–5 |
| Dysf | dysferlin | 204591.2 | –8.72 | 1.3 × 10–5 |
| Prph | peripherin | 24249.4 | –1.11 | 1.3 × 10–5 |
| Rab3d | RAB3D, member RAS oncogene family | 122211.7 | –6.99 | 1.3 × 10–5 |
| Brap | BRCA1 associated protein | 111765.7 | –6.95 | 1.7 × 10–5 |
| Rgs7 | regulator of G protein signaling 7 | 27812.1 | 8.41 | 1.9 × 10–5 |
| Ppp1r1c | protein phosphatase 1, regulatory subunit 1C | 111780.2 | –2.83 | 1.9 × 10–5 |
| Shroom3 | shroom family member 3 | 113055.8 | –6.96 | 2.6 × 10–5 |
| Gene Symbol | Gene Name | Transcr. Identity 1 | LFC 2 | FDR P-Value 3 |
|---|---|---|---|---|
| Akap11 | A kinase (PRKA) anchor protein 11 | 227722.1 | –9.04 | 7.8 × 10–37 |
| Dbp | D site albumin promoter binding protein | 80885.11 | 1.23 | 5.8 × 10–21 |
| Zfp871 | zinc finger protein 871 | 159086.8 | 1.48 | 9.3 × 10–19 |
| Dmxl2 | Dmx-like 2 | 118600.7 | 1.29 | 1.0 × 10–18 |
| RP24-200D3.4 | predicted gene, 38394 | 179598.3 | 1.47 | 1.2 × 10–10 |
| Dmxl2 | Dmx-like 2 | 118163.7 | 2.29 | 1.2 × 10–10 |
| Bdp1 | B double prime 1, transcription initiation fact. | 38104.11 | 1.43 | 2.5 × 10–10 |
| Ralgapa1 | Ral GTPase activating protein, alpha subunit 1 | 220367.1 | –0.89 | 6.9 × 10–10 |
| Ciart | circadian associated repressor of transcription | 36418.9 | 1.28 | 1.8 × 10–9 |
| RP23-423B1.6 | predicted gene, 38020 | 193066.1 | 1.43 | 1.8 × 10–9 |
| RP23-243E17.1 | RIKEN cDNA 4932438A13 | 152564.7 | 1.25 | 6.2 × 10–9 |
| Mbnl1 | muscleblind-like 1 | 192394.5 | 1.19 | 6.2 × 10–9 |
| Atp13a5 | ATPase type 13A5 | 75806.1 | 1.04 | 1.1 × 10–7 |
| Arntl | aryl hydrocarbon receptor nuclear translo.-like | 47321.8 | –1.15 | 1.7 × 10–7 |
| Ralgapa1 | Ral GTPase activating protein, alpha subunit 1 | 226244.1 | 1.07 | 2.8 × 10–7 |
| Map2 | microtubule-associated protein 2 | 114013.7 | 1.06 | 3.3 × 10–7 |
| Grip1 | glutamate receptor interacting protein 1 | 105261.8 | 4.95 | 7.2 × 10–7 |
| Tert | telomerase reverse transcriptase | 22104.8 | 3.28 | 9.0 × 10–7 |
| Lcor | ligand dependent nuclear receptor corepressor | 67795.11 | 1.23 | 1.1 × 10–6 |
| Psd3 | pleckstrin and Sec7 domain containing 3 | 98696.9 | 1.72 | 1.6 × 10–6 |
| Rfx4 | regulatory factor X, 4 | 166696.8 | –5.58 | 2.7 × 10–6 |
| Fabp7 | fatty acid binding protein 7, brain | 165013.1 | –1.00 | 3.0 × 10–6 |
| Scn9a | sodium channel, voltage-gated, type IX, alpha | 169900.7 | 1.68 | 3.0 × 10–6 |
| Psd3 | pleckstrin and Sec7 domain containing 3 | 93468.11 | 2.48 | 8.2 × 10–6 |
| Tia1 | cytotoxic granule-associat. RNA bind. prot. 1 | 136387.1 | 1.00 | 1.2 × 10–5 |
| Map2 | microtubule-associated protein 2 | 114018.9 | 1.01 | 1.5 × 10–5 |
| Wnk3 | WNK lysine deficient protein kinase 3 | 184730.7 | 2.03 | 1.6 × 10–5 |
| Snrpa | small nuclear ribonucleoprotein polypep. A | 163311.8 | 7.63 | 2.9 × 10–5 |
| Region Gene Symbol | Gene Name | ΔPSI Range 1 | Count 2 | FDR P-Value | |
|---|---|---|---|---|---|
| NAc | |||||
| Cadps | Ca2+-dependent secretion activator | –0.080 | 0.108 | 6 | 3.2 × 10–2 |
| Rab28 | RAB28, member RAS oncogene family | –0.060 | 0.067 | 4 | 3.2 × 10–2 |
| Trak2 | trafficking protein, kinesin binding 2 | –0.026 | 0.013 | 4 | 8.0 × 10–2 |
| Grin1 | glutamate receptor, ionotropic, NMDA1 | –0.011 | 0.010 | 6 | 9.9 × 10–2 |
| Pex5l | peroxisomal biogenesis factor 5-like | –0.040 | 0.044 | 14 | 9.9 × 10–2 |
| Gm3764 | predicted gene 3764 | –0.099 | 0.066 | 7 | 9.9 × 10–2 |
| St8sia1 | ST8 sialyltransferase 1 | –0.097 | 0.119 | 3 | 9.9 × 10–2 |
| Xiap | X-linked inhibitor of apoptosis | –0.081 | 0.052 | 6 | 9.9 × 10–2 |
| Uxs1 | UDP-glucuronate decarboxylase 1 | –0.034 | 0.051 | 3 | 9.9 × 10–2 |
| Mbp | myelin basic protein | –0.012 | 0.023 | 3 | 9.9 × 10–2 |
| Mief1 | mitochondrial elongation factor 1 | –0.127 | 0.116 | 3 | 9.9 × 10–2 |
| TG | |||||
| Fryl | FRY like transcription coactivator | –0.331 | 0.634 | 3 | 1.1 × 10–4 |
| S100a6 | S100 calcium binding protein A6 | –0.004 | 0.007 | 5 | 8.4 × 10–4 |
| Map2 | microtubule-associated protein 2 | –0.088 | 0.065 | 13 | 8.4 × 10–4 |
| Eml6 | echinoderm microtubule protein like 6 | –0.663 | 0.231 | 5 | 2.4 × 10–3 |
| Ktn1 | kinectin 1 | –0.100 | 0.059 | 3 | 3.7 × 10–3 |
| AW554918 | expressed sequence (hinderin) | –0.148 | 0.103 | 5 | 4.1 × 10–2 |
| Clasp2 | CLIP associating protein 2 | –0.025 | 0.033 | 6 | 4.3 × 10–2 |
| Sdccag8 | serologic. defined colon cancer antigen 8 | –0.054 | 0.101 | 6 | 7.7 × 10–2 |
| Region, Type, Analysis 1 | Path 2 | Pathway Name | Size 3 | Enr. Score 4 | P-Value |
|---|---|---|---|---|---|
| NAc absolute differential isoform expression | |||||
| ORA | P00047 | PDGF signaling pathway | 115 | 2.39 | 5.1 × 10–3 |
| ORA | P00049 | Parkinson disease | 85 | 2.65 | 5.8 × 10–3 |
| ORA | P00034 | Integrin signalling pathway | 154 | 2.11 | 6.9 × 10–3 |
| NAc relative differential isoform expression | |||||
| ORA | P05918 | p38 MAPK pathway | 35 | 9.59 | 3.3 × 10–3 |
| ORA | P00043 | Muscarinic acetylcholine recep. 2–4 signal. | 50 | 6.71 | 9.1 × 10–3 |
| GSEA | P06664 | Gonadotropin-releasing hormone receptor | 119 | –2.31 | 0.0E+00 |
| TG absolute differential isoform expression | |||||
| ORA | P00015 | Circadian clock system | 9 | 13.73 | 1.1 × 10–5 |
| ORA | P00047 | PDGF signaling pathway | 115 | 2.58 | 1.8 × 10–3 |
| ORA | P00009 | Axon guidance mediated by netrin | 28 | 4.41 | 4.5 × 10–3 |
| ORA | P00008 | Axon guidance mediated by Slit/Robo | 18 | 5.49 | 4.9 × 10–3 |
| ORA | P00034 | Integrin signalling pathway | 154 | 2.09 | 7.6 × 10–3 |
| ORA | P00003 | Alzheimer disease-amyloid secretase | 60 | 2.88 | 9.4 × 10–3 |
| TG relative differential isoform expression | |||||
| GSEA | P00056 | VEGF signaling pathway | 40 | 2.04 | 3.1 × 10–3 |
| GSEA | P05730 | Endogenous cannabinoid signaling | 12 | −1.96 | 4.1 × 10–3 |
| GSEA | P00041 | Metabotropic glutamate receptor group I | 13 | −1.85 | 7.1 × 10–3 |
| Group 1 | Genes Detected in Two Differential Splicing-Region Categories 2 |
|---|---|
| NAc | Prpf40b,Grin1,Ablim2,Rbm3,Hebp2,Ciz1,Dhx30,Fam219a,Hnrnpa1 |
| TG | Asap1,Scn8a,Eef1d,Map2,Rbm39,Cnot1,Fabp7,Ciart,Fam222b,Peg3 |
| DE | Slc38a4,Pak3,Prg4,Csde1,Celf3,Cacna1g,Ndst3,AC166328.1,Mapk8ip3 |
| AS | 4933431E20Rik,Rasl10a,Fabp7,Ssbp2,Hnrnpk,Plce1,Tenm4,Cdon,Zfp948, Rbm3,Clasp2,Spag9,Ptpn23,Mprip,Cadps,Mink1,Prune2,Asap1,Fam184a, Podxl2,Sema4c,Smpdl3a |
| Category | Category Name | Count 1 | Enrich. Score 2 | P-Value | FDR P-Value |
|---|---|---|---|---|---|
| GO:0050767 | regulation of neurogenesis | 11 | 5.99 | 1.2 × 10–6 | 1.1 × 10–2 |
| GO:0016071 | mRNA metabolic process | 9 | 7.29 | 2.8 × 10–6 | 1.2 × 10–2 |
| GO:0008380 | RNA splicing | 6 | 8.35 | 7.4 × 10–5 | 6.3 × 10–2 |
| GO:0043410 | positive regulation of MAPK cascade | 7 | 6.46 | 8.8 × 10–5 | 6.3 × 10–2 |
| Transcription Factor | NES 1 | Target Gene Count 2 | Motif 3 | P-Value |
|---|---|---|---|---|
| Pbx1 | 4.00 | 31 | 2 | 1.0 × 10–3 |
| Arntl2 | 3.73 | 55 | 4 | 3.8 × 10–3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Perez, O.C.; Southey, B.R.; Sweedler, J.V.; Pradhan, A.A.; Rodriguez-Zas, S.L. Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia. Genes 2021, 12, 1570. https://doi.org/10.3390/genes12101570
Zhang P, Perez OC, Southey BR, Sweedler JV, Pradhan AA, Rodriguez-Zas SL. Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia. Genes. 2021; 12(10):1570. https://doi.org/10.3390/genes12101570
Chicago/Turabian StyleZhang, Pan, Olivia C. Perez, Bruce R. Southey, Jonathan V. Sweedler, Amynah A. Pradhan, and Sandra L. Rodriguez-Zas. 2021. "Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia" Genes 12, no. 10: 1570. https://doi.org/10.3390/genes12101570
APA StyleZhang, P., Perez, O. C., Southey, B. R., Sweedler, J. V., Pradhan, A. A., & Rodriguez-Zas, S. L. (2021). Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia. Genes, 12(10), 1570. https://doi.org/10.3390/genes12101570






