Carbonization Temperature and Its Effect on the Mechanical Properties, Wear and Corrosion Resistance of Aluminum Reinforced with Eggshell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reinforcement Preparations
2.2. Composites Fabrication
2.3. Experimental Methodology
3. Results and Discussion
3.1. Microstructure
3.2. Density and Porosity
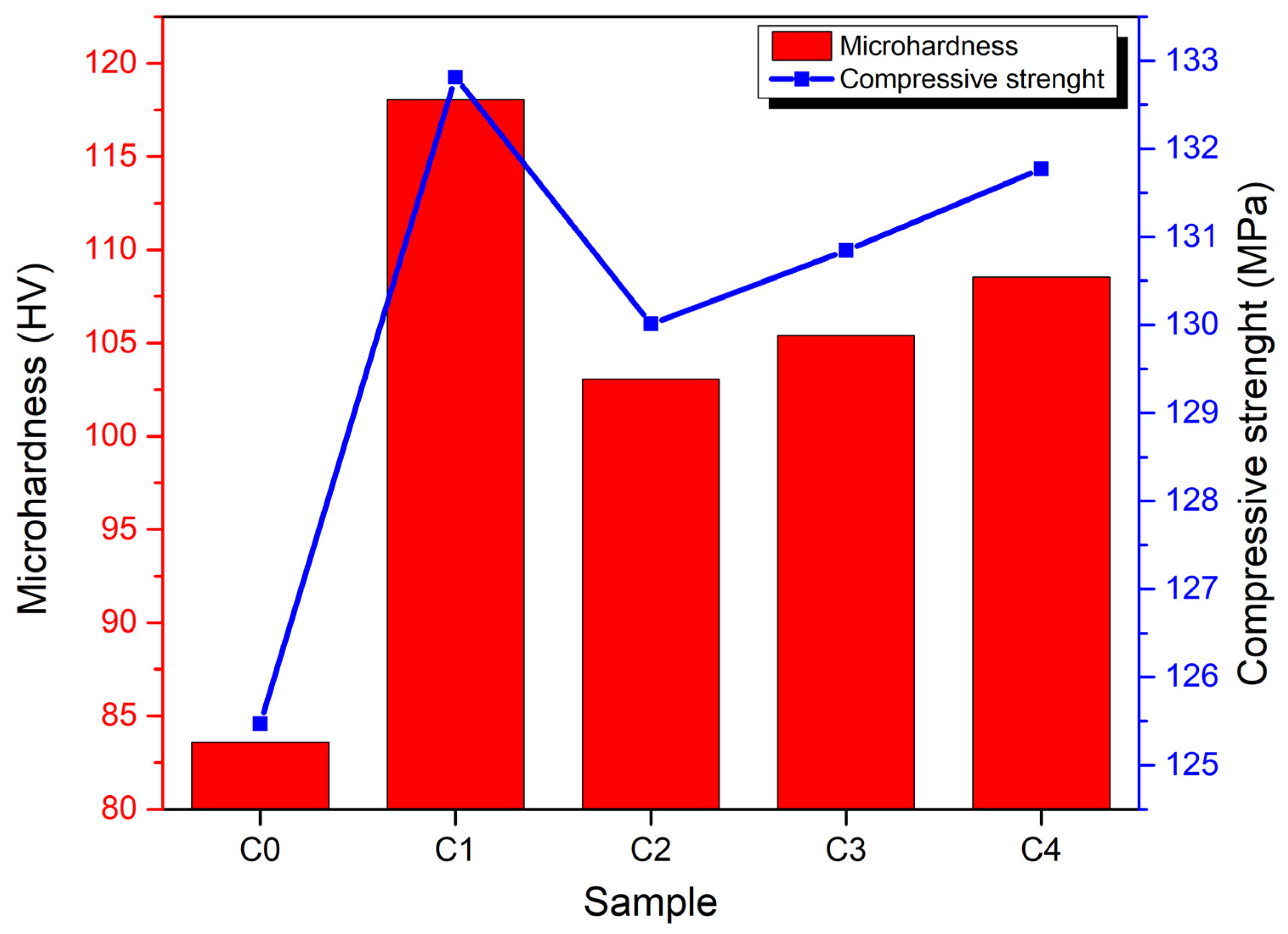
3.3. Mechanical Properties
3.4. Wear
3.5. Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Durowoju, M.O.; Asafa, T.B.; Sadiku, E.R.; Diouf, S.; Shongwe, M.B. Improving mechanical and thermal properties of graphite–aluminium composite using Si, SiC and eggshell particles. J. Compos. Mater. 2020, 54, 2365–2376. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Ozoegwu, C.G.; Madushele, N.; Akinlabi, E.T. Characterization, machinability studies and multi-response optimization of AA 6082 hybrid metal matrix composite. Int. J. Adv. Manuf. Technol. 2021, 116, 1555–1573. [Google Scholar] [CrossRef]
- Haghshenas, M. Metal–Matrix Composites. Ref. Modul. Mater. Sci. Mater. Eng. 2016, 35, 1–28. [Google Scholar]
- Jose, J.; Christy, T.V.; Peter, P.E.; Feby, J.A.; George, A.J.; Joseph, J.; Chandra, R.G.; Benjie, N.M. Manufacture and characterization of a novel agro-waste based low-cost metal matrix composite (MMC) by compocasting. Mater. Res. Express 2018, 5, 066530. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Akinlabi, E.T.; Ozoegwu, C.G.; Aigbodion, V.S. A Concise Review of the Effects of Hybrid Particulate Reinforced Aluminium Metal Matrix Composites on the Microstructure, Density and Mechanical Properties in Lecture Notes in Mechanical Engineering: Advances in Manufacturing Engineering; Seyed, S.E., Mokhotar, A., Yusof, F., Eds.; Springer: Singapore, 2020; pp. 433–443. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Ozoegwu, C.G.; Madushele, N.; Akinlabi, T. Evaluation of particle size distribution, mechanical properties, microstructure and electrochemical studies of AA1050/fly ash metal matrix composite. Adv. Mater. Process. Technol. 2021, 1–15. [Google Scholar] [CrossRef]
- Quader, S.M.; Murthy, B.S.; Reddy, P.R. Processing and Mechanical Properties of Al2O3 and Red Mud Particle Reinforced AA6061 Hybrid Composites. J. Miner. Mater. Charact. Eng. 2016, 4, 135. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.B.N.; Arif, S.; Aziz, T.; Waseem, A.; Shaikh, M.A.N.; Ali, M. Microstructural, mechanical and tribological behaviour of powder metallurgy processed SiC and RHA reinforced Al-based composites. Surf. Interfaces 2018, 15, 166–179. [Google Scholar] [CrossRef]
- Raju, R.S.S.; Panigrahi, M.K.; Ganguly, R.I.; Rao, G.S. Tribological behaviour of Al-1100-coconut shell ash (CSA) composite at elevated temperature. Tribol. Int. 2019, 129, 55–66. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Sharma, S.; Mishra, R.K. Characterization of waste eggshells and CaCO3 reinforced AA2014 green metal matrix composites: A green approach in the synthesis of composites. Int. J. Precis. Eng. Manuf. 2016, 17, 1383–1393. [Google Scholar] [CrossRef]
- Sharma, S.; Dwivedi, S.P. Effects of waste eggshells and SiC addition on specific strength and thermal expansion of hybrid green metal matrix composite. J. Hazard. Mater. 2017, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asuke, F.; Aigbodion, V.S.; Abdulwahab, M.; Fayomi, O.S.I.; Popoola, A.P.I.; Nwoyi, C.I.; Garba, B. Effects of bone particle on the properties and microstructure of polypropylene/bone ash particulate composites. Results Phys. 2012, 2, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Oghenevweta, J.E.; Aigbodion, V.S.; Nyior, G.B.; Asuke, F. Mechanical properties and microstructural analysis of Al–Si–Mg/carbonized maize stalk waste particulate composites. J. King Saud Univ. Eng. Sci. 2014, 28, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.P.; Sharma, S.; Mishra, R.K. Mechanical and Metallurgical Characterizations of AA2014 / Eggshells Waste Particulate Metal Matrix Composite. Int. J. Precis. Eng. Manuf. Green Technol. 2016, 3, 281–288. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Ozoegwu, C.G.; Madushele, N.; Akinlabi, E.T. Effects of carbonised eggshells on the mechanical properties, microstructure and corrosion resistance of AA1050 of metal matrix composites. Adv. Mater. Process. Technol. 2021, 1–13. [Google Scholar] [CrossRef]
- Hassan, S.B.; Aigbodion, V.S. Effects of eggshell on the microstructures and properties of Al–Cu–Mg/eggshell particulate composites. J. King Saud Univ. Eng. Sci. 2013, 27, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Ononiwu, N.H.; Akinlabi, E.T.; Ozoegwu, C.G. Optimization techniques applied to machinability studies for turning aluminium metal matrix composites: A literature review. Mater. Today Proc. 2020, 441, 1124–1129. [Google Scholar] [CrossRef]
- Ononiwu, N.H.; Akinlabi, E.T.; Ozoegwu, C.G. Sustainability in Production and Selection of Reinforcement particles in Aluminium Alloy Metal Matrix Composites: A Review. J. Phys. Conf. Ser. 2019, 1378, 1–10. [Google Scholar] [CrossRef]


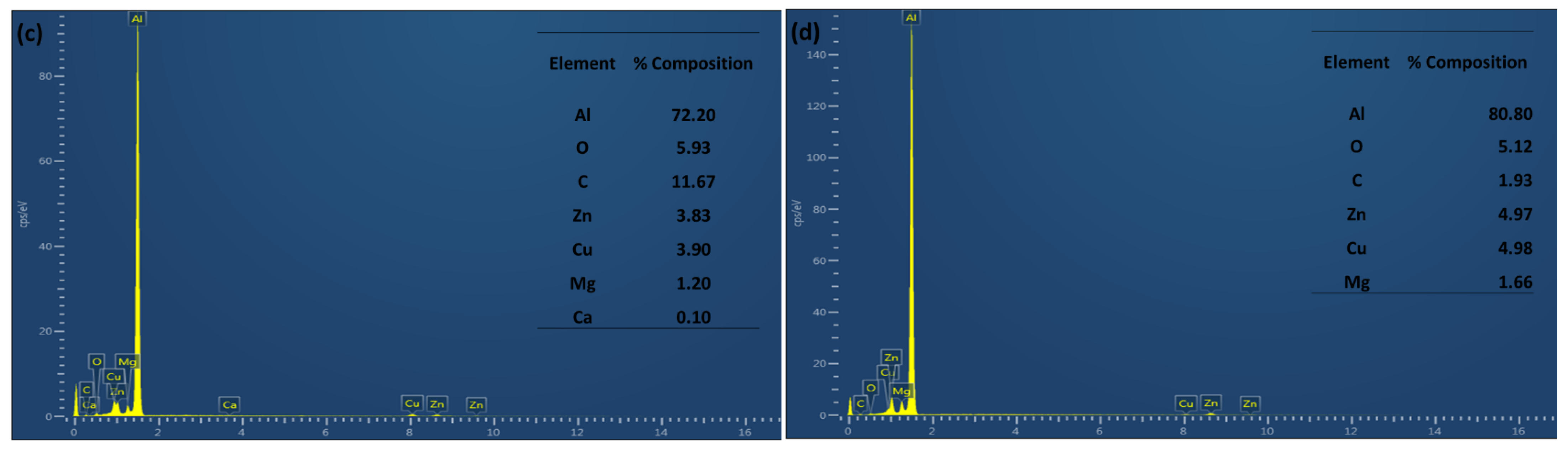





| Composition | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| % | 0.5 | 0.07 | 0.45 | 0.02 | 1.02 | 0.14 | 0.6 | 0.02 | Bal |
| Sample | Designation |
|---|---|
| AA 6063 | C0 |
| AA6063/5 wt.% (900 °C) | C1 |
| AA6063/5 wt.% (1000 °C) | C2 |
| AA6063/5 wt.% (1100 °C) | C3 |
| AA6063/5 wt.% (1200 °C) | C4 |
| Sample | Linear Polarization Resistance (Ω) | Corrosion Rate (g/hr) | Corrosion Density (Icoor) | Potential (Ecoor) |
|---|---|---|---|---|
| C0 | 1144 | 5.53 × 10−6 | 1.64 × 10−5 | −0.471 |
| C1 | 331,405 | 4.08 × 10−8 | 1.22 × 10−7 | −1.144 |
| C2 | 5461 | 1.74 × 10−6 | 5.17 × 10−6 | −0.259 |
| C3 | 165 | 6.92 × 10−5 | 2.06 × 10−4 | −0.208 |
| C4 | 574 | 2.59 × 10−5 | 7.72 × 10−5 | −0.481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ononiwu, N.H.; Ozoegwu, C.G.; Madushele, N.; Akinlabi, E.T. Carbonization Temperature and Its Effect on the Mechanical Properties, Wear and Corrosion Resistance of Aluminum Reinforced with Eggshell. J. Compos. Sci. 2021, 5, 262. https://doi.org/10.3390/jcs5100262
Ononiwu NH, Ozoegwu CG, Madushele N, Akinlabi ET. Carbonization Temperature and Its Effect on the Mechanical Properties, Wear and Corrosion Resistance of Aluminum Reinforced with Eggshell. Journal of Composites Science. 2021; 5(10):262. https://doi.org/10.3390/jcs5100262
Chicago/Turabian StyleOnoniwu, Ndudim H., Chigbogu G. Ozoegwu, Nkosinathi Madushele, and Esther T. Akinlabi. 2021. "Carbonization Temperature and Its Effect on the Mechanical Properties, Wear and Corrosion Resistance of Aluminum Reinforced with Eggshell" Journal of Composites Science 5, no. 10: 262. https://doi.org/10.3390/jcs5100262







