Adsorption Behavior of Crystal Violet and Congo Red Dyes on Heat-Treated Brazilian Palygorskite: Kinetic, Isothermal and Thermodynamic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Heat Treatment
2.3. Characterizations
2.4. Batch Adsorption Experiments
3. Results and Discussion
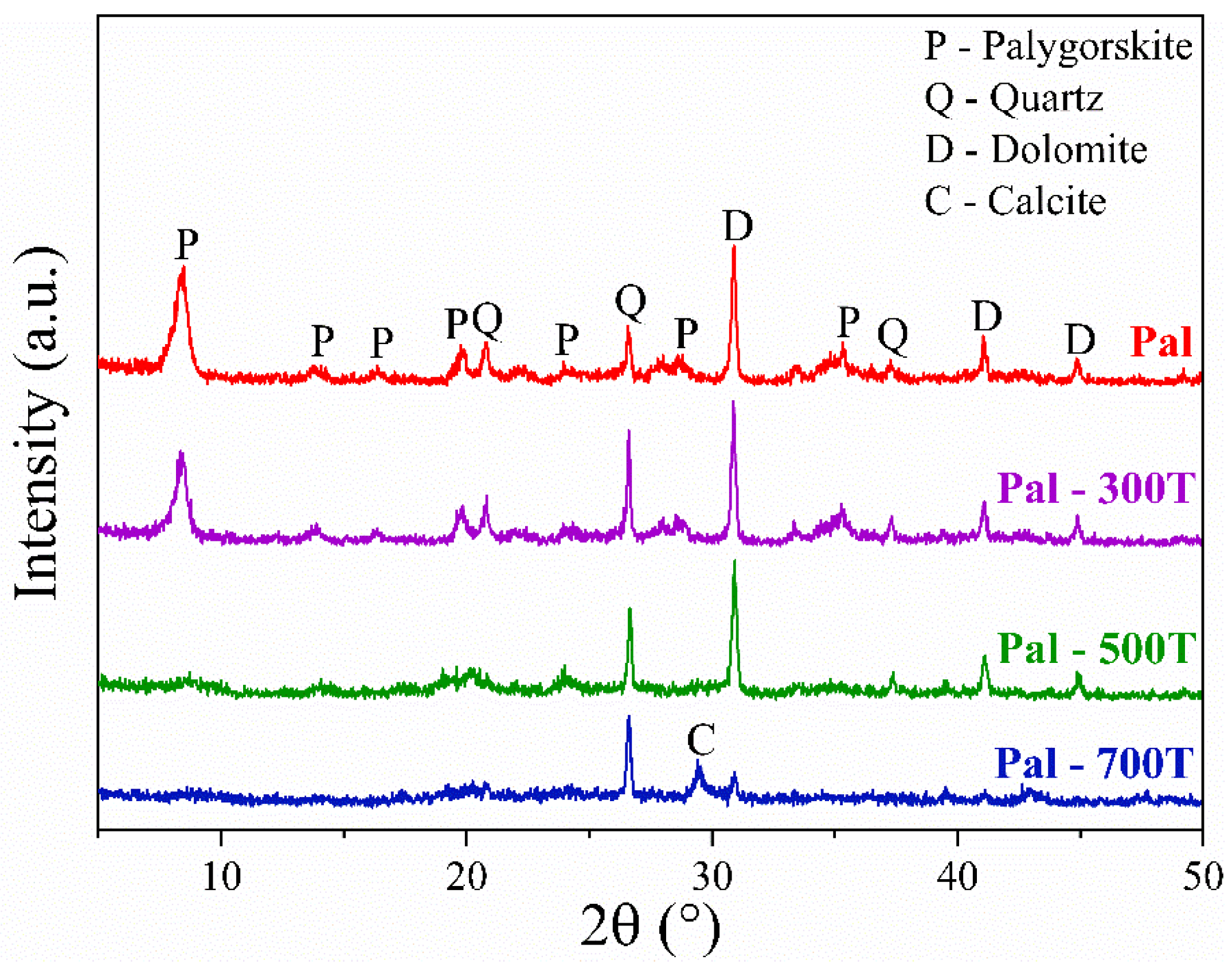
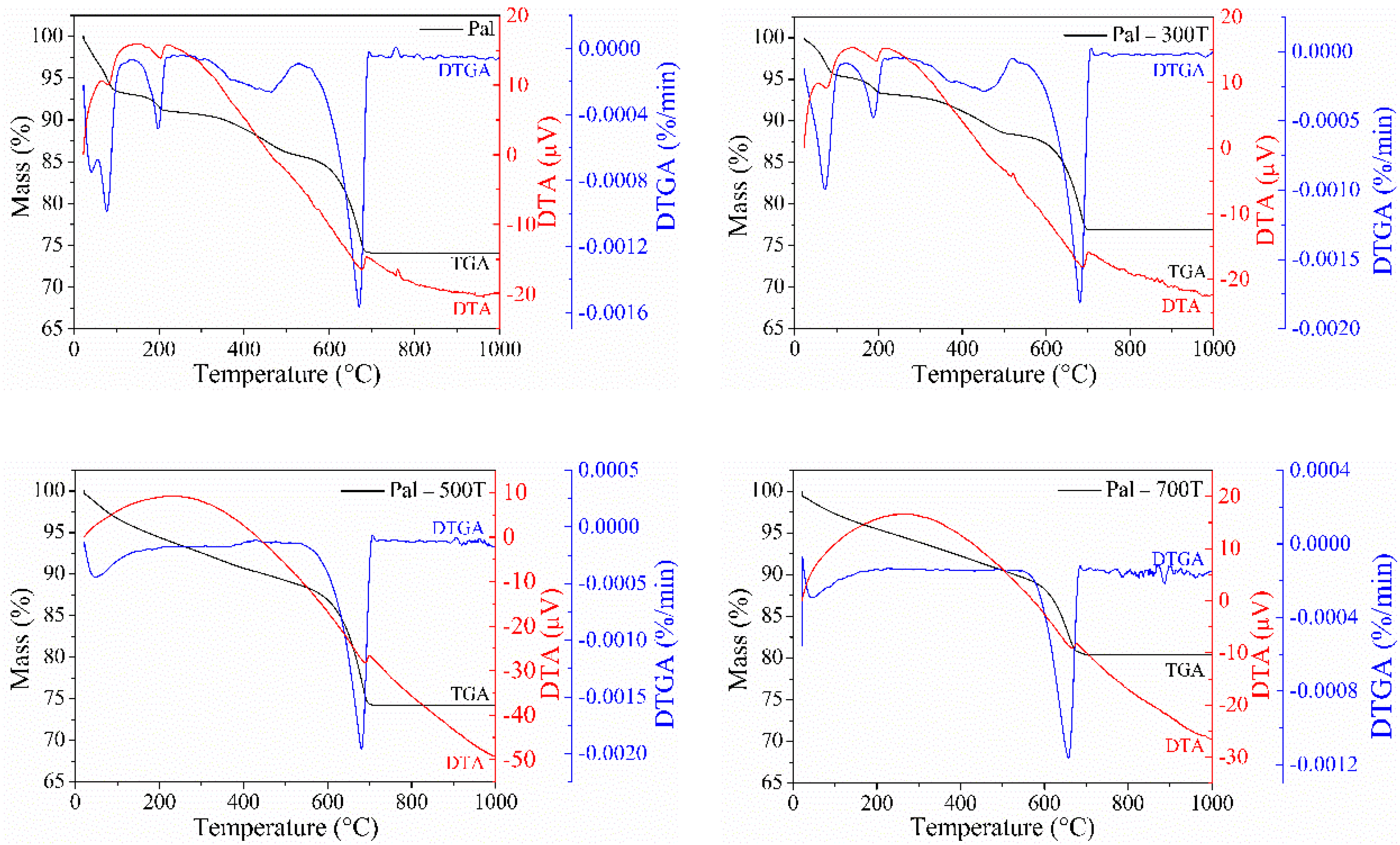
3.1. Characterization of Natural and Heat-Treated Palygorskite
3.2. Adsorption Experiments
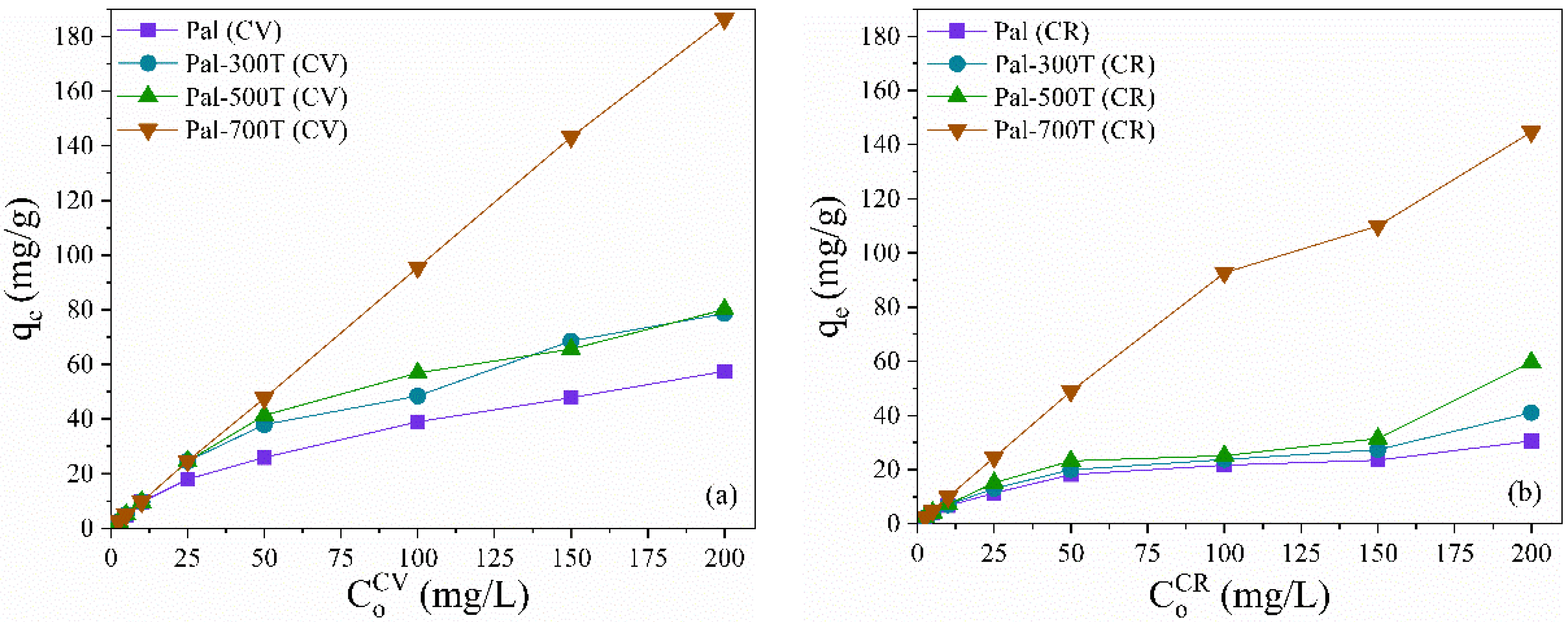
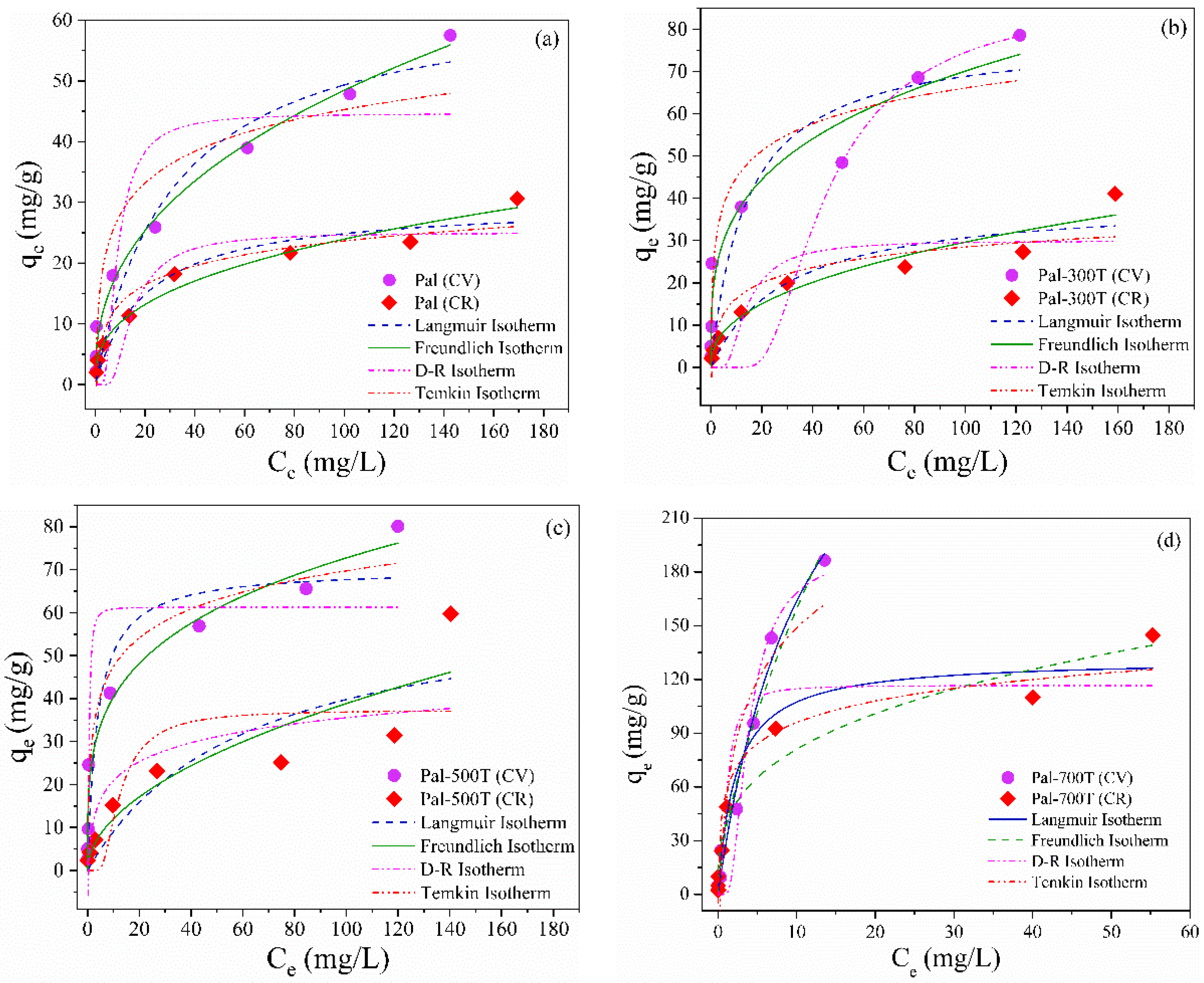
3.2.1. Effect of Initial Concentration and Adsorption Isotherms
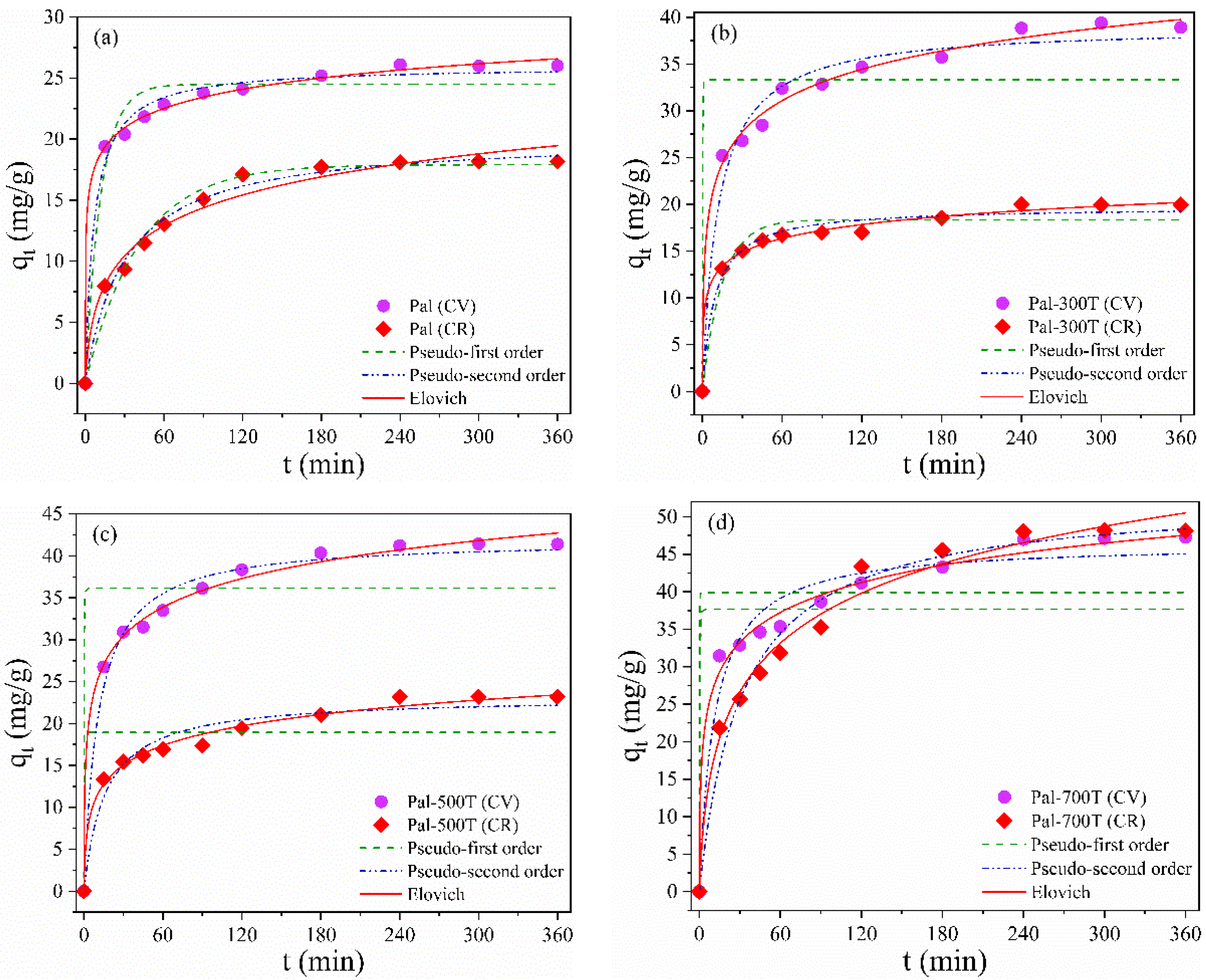
3.2.2. Effect of Contact Time and Kinetic Study
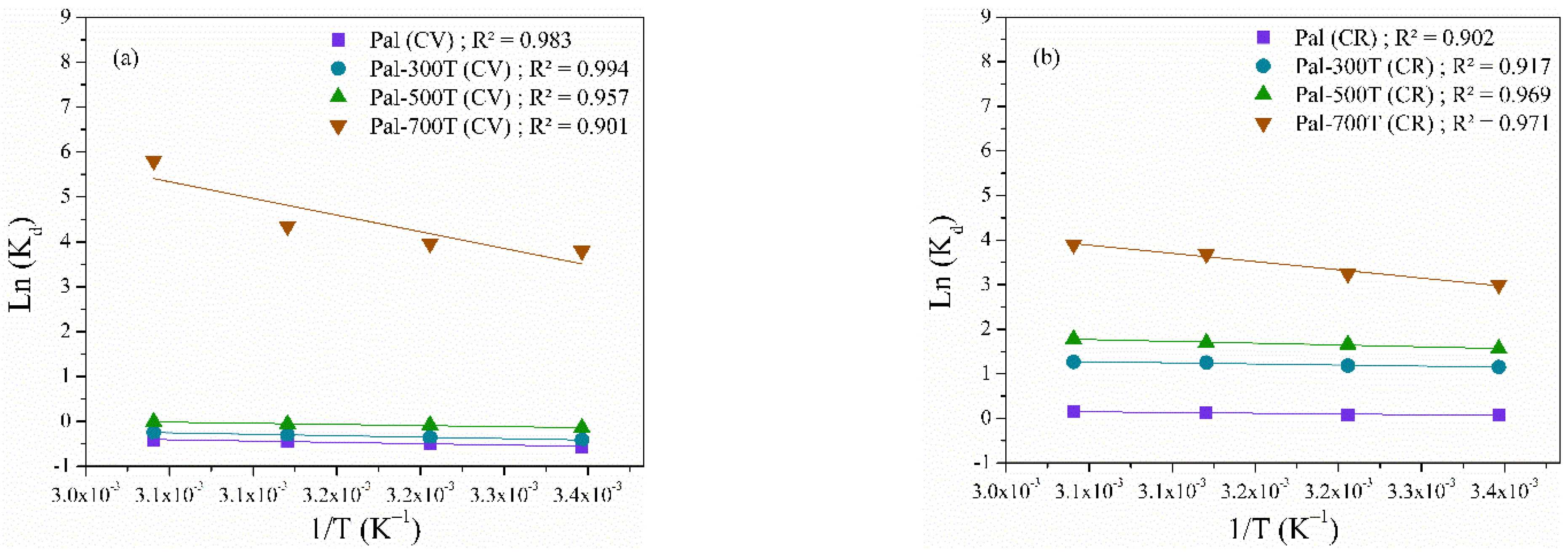
3.2.3. Effect of Temperature and Thermodynamics
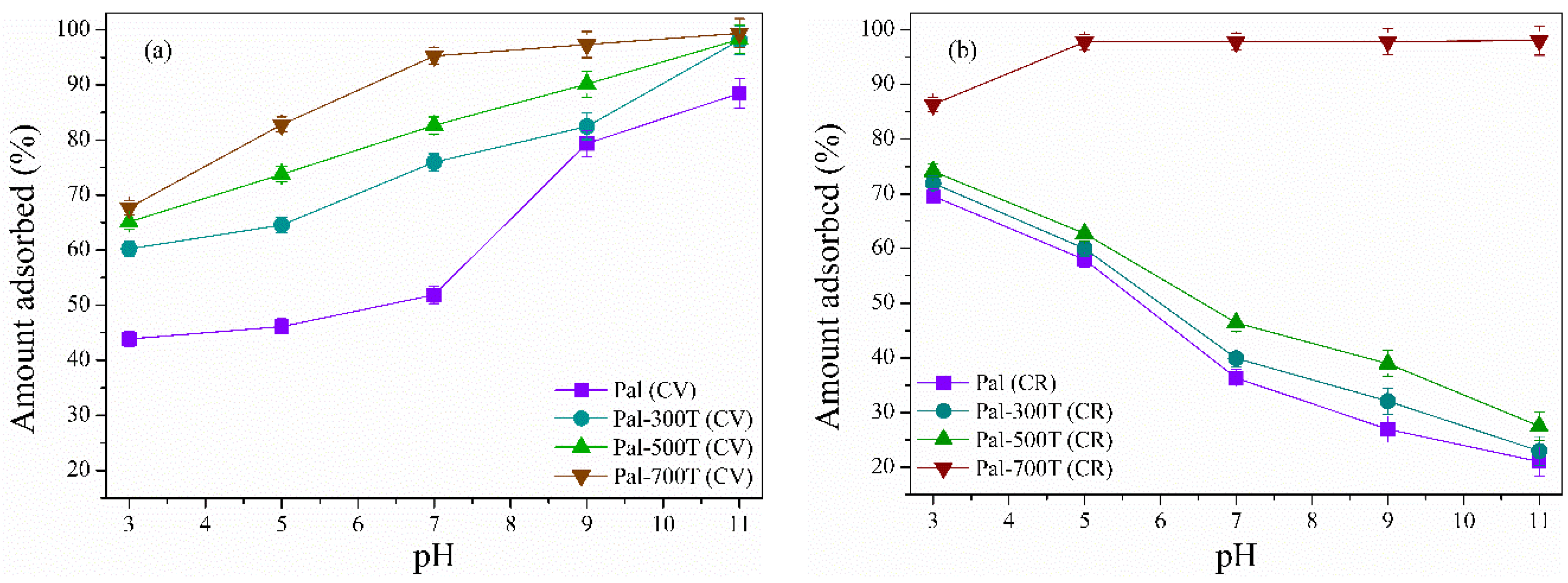
3.2.4. Effect of PH Variation
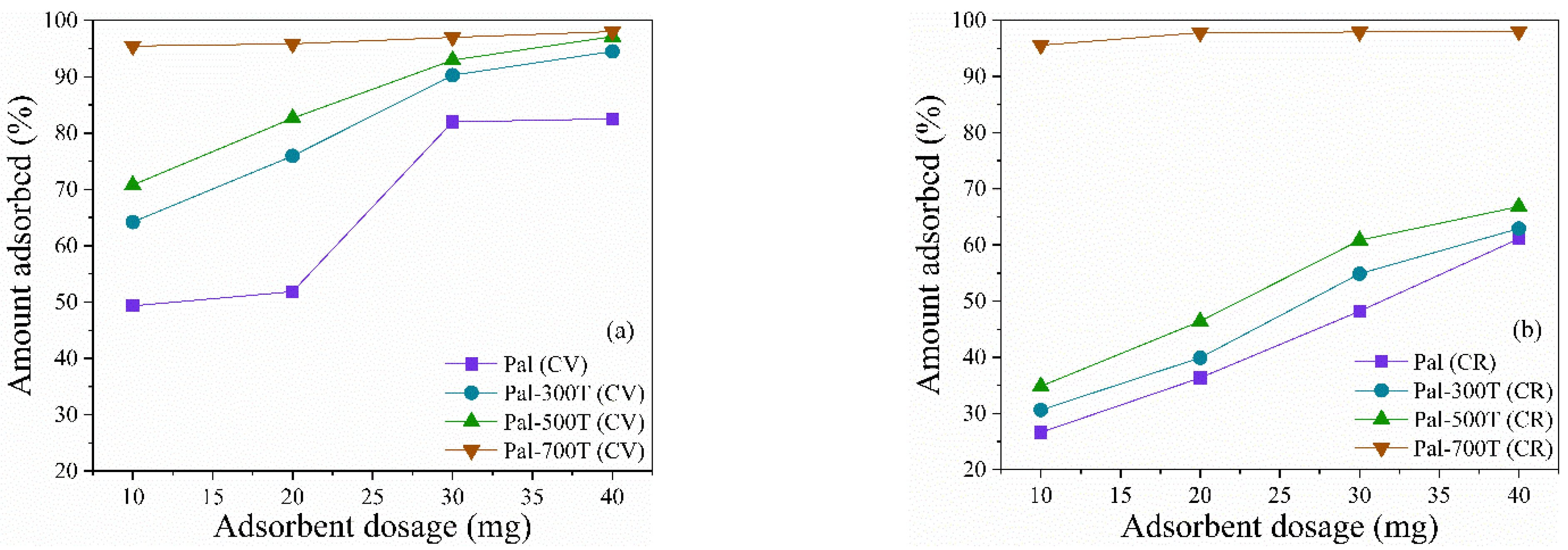
3.2.5. Effect of the Amount of Adsorbent
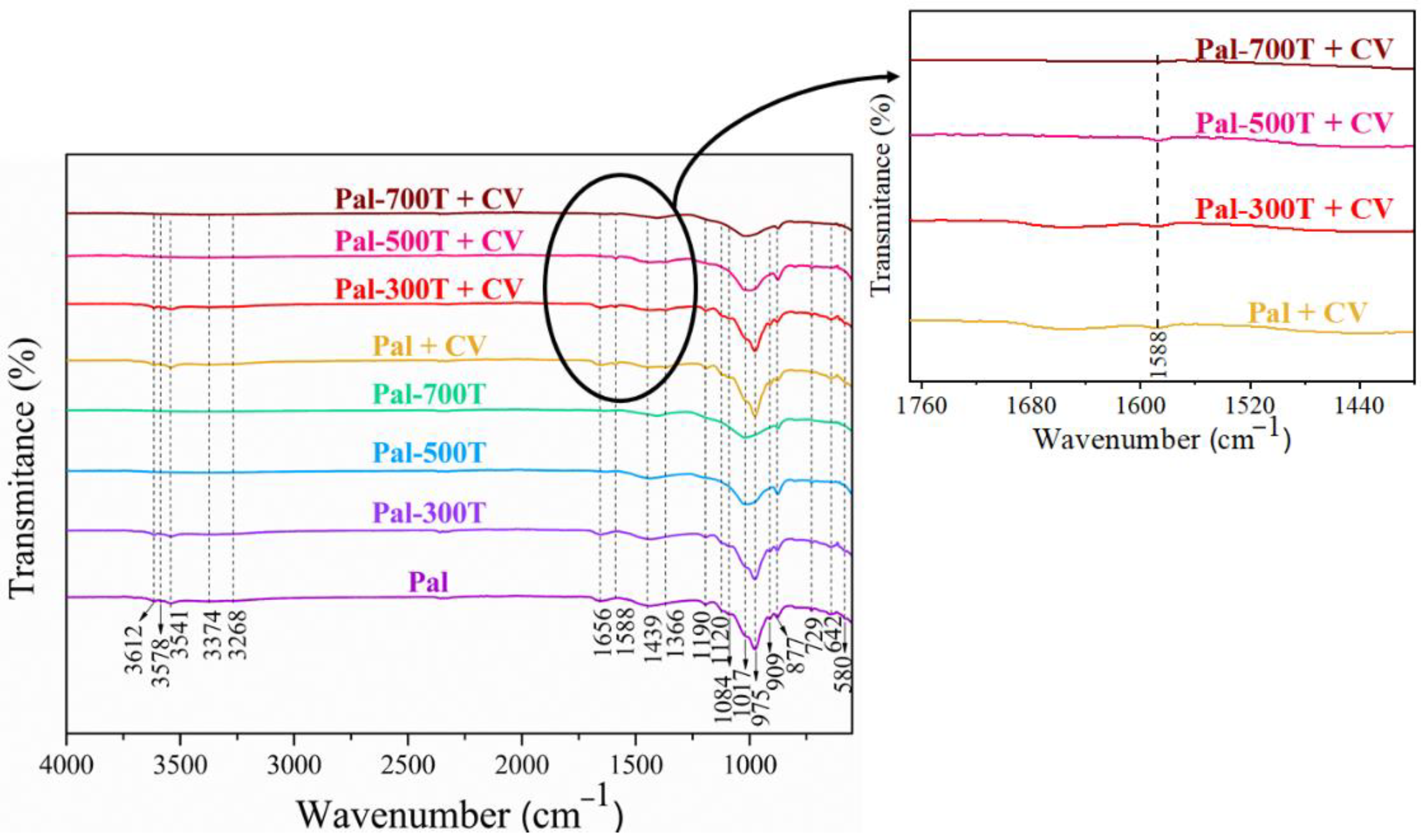
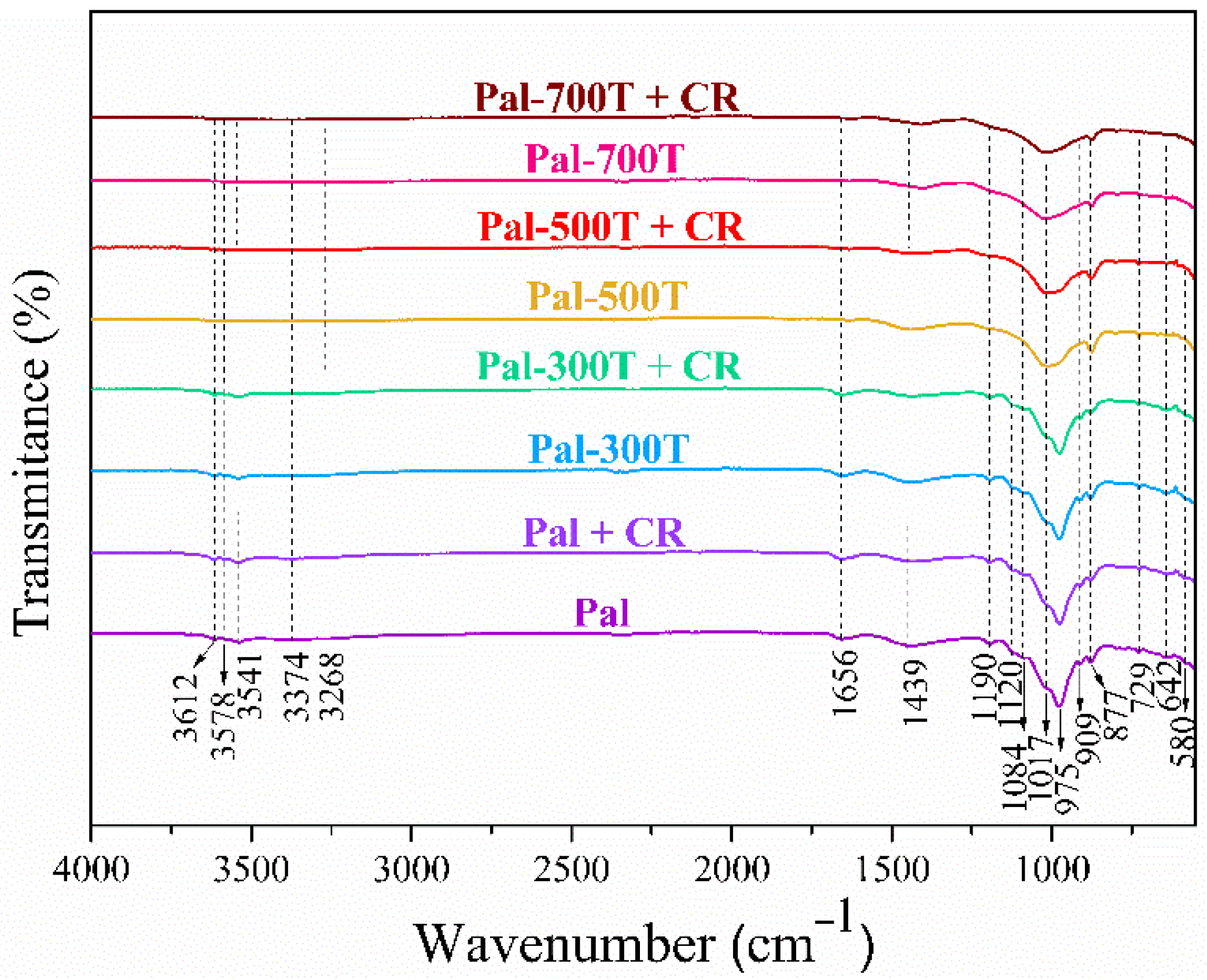
3.3. FTIR before and after Adsorption
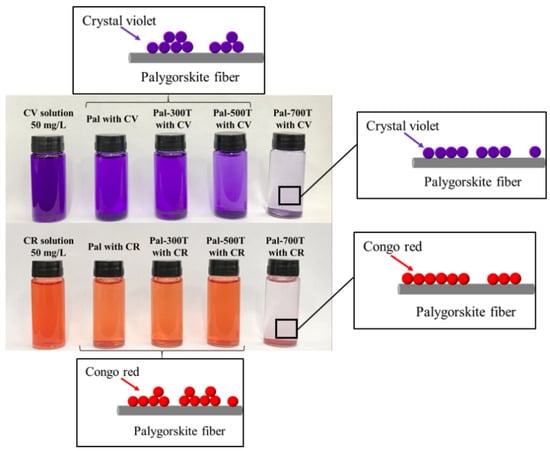
3.4. Recyclability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Khan, M.R. Synthesis of Schiff’s base magnetic crosslinked chitosan-glyoxal/ZnO/Fe3O4 nanoparticles for enhanced adsorption of organic dye: Modeling and mechanism study. Sustain. Chem. Pharm. 2021, 20, 100379. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Ahmad, R. An efficient sequestration of toxic crystal violet dye from aqueous solution by Alginate/Pectin nanocomposite: A novel and ecofriendly adsorbent. Groundw. Sustain. Dev. 2020, 11, 100373. [Google Scholar] [CrossRef]
- Tyagi, U. Adsorption of dyes using activated carbon derived from pyrolysis of vetiveria zizanioides in a fixed bed reactor. Groundw. Sustain. Dev. 2020, 10, 100303. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Su, Y.; Yu, H.; Liu, H.; Qian, S.; Zheng, W.; Zhao, Y. Zr-MOFs loaded on polyurethane foam by polydopamine for enhanced dye adsorption. J. Environ. Sci. 2021, 101, 177–188. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F.; Radwan, E.K.; Abdel Mageid, R.E.; Khattab, T.A.; Olson, M.A. A bipyridinium-based polyhydrazone adsorbent that exhibits ultrahigh adsorption capacity for the anionic azo dye, direct blue 71. Chem. Eng. J. 2021, 409, 128195. [Google Scholar] [CrossRef]
- He, T.; Hua, J.; Chen, R.; Yu, L. Adsorption characteristics of methylene blue by a dye-degrading and extracellular polymeric substance -producing strain. J. Environ. Manag. 2021, 288, 112446. [Google Scholar] [CrossRef]
- Mohanty, S.; Moulick, S.; Maji, S.K. Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J. Water Process Eng. 2020, 37, 101428. [Google Scholar] [CrossRef]
- Uddin, M.K.; Mashkoor, F.; AlArifi, I.M.; Nasar, A. Simple one-step synthesis process of novel MoS2@bentonite magnetic nanocomposite for efficient adsorption of crystal violet from aqueous solution. Mater. Res. Bull. 2021, 139, 111279. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Hassaninejad-Darzi, S.K.; Zavvar Mousavi, H. Adsorption of ternary toxic crystal violet, malachite green and methylene blue onto synthesised SBA-15 mesoporous nanoparticles. Int. J. Environ. Anal. Chem. 2020, 1–24. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Lamine, A.B.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Chahinez, H.-O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M.; Diaconu, M.; Cimpeanu, C.; Teodorescu, R.; Bucur, D. Crystal violet dye removal from aqueous solution by nanohydroxyapatite. J. Food Agric. Environ. 2014, 12, 499–502. [Google Scholar]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2016; Volume 237, pp. 71–104. [Google Scholar]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Abbasi, F.; Tavakkoli Yaraki, M.; Farrokhnia, A.; Bamdad, M. Keratin nanoparticles obtained from human hair for removal of crystal violet from aqueous solution: Optimized by Taguchi method. Int. J. Biol. Macromol. 2020, 143, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Ahmad, R.; Mirza, A.; Mittal, J. Sequestration of toxic congo red dye from aqueous solution using ecofriendly guar gum/activated carbon nanocomposite. Int. J. Biol. Macromol. 2020, 158, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Borth, K.W.; Galdino, C.W.; de Carvalho Teixeira, V.; Anaissi, F.J. Iron oxide nanoparticles obtained from steel waste recycling as a green alternative for Congo red dye fast adsorption. Appl. Surf. Sci. 2021, 546, 149126. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Review on nickel-based adsorption materials for Congo red. J. Hazard. Mater. 2021, 403, 123559. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Kumar, R.; Barakat, M.A.; Basheer, C.; Aburiazaiza, A.S.; Nizami, A.S.; Rehan, M. Untapped conversion of plastic waste char into carbon-metal LDOs for the adsorption of Congo red. J. Colloid Interface Sci. 2018, 511, 402–410. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Kelishami, A.R. Enhanced adsorption of congo red using cationic surfactant functionalized zeolite particles. Microchem. J. 2020, 153, 104281. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr(VI) and Congo red dye. J. Environ. Manag. 2021, 290, 112615. [Google Scholar] [CrossRef] [PubMed]
- Valadi, F.M.; Ekramipooya, A.; Gholami, M.R. Selective separation of Congo Red from a mixture of anionic and cationic dyes using magnetic-MOF: Experimental and DFT study. J. Mol. Liq. 2020, 318, 114051. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. Copper and cerium co-doped cobalt ferrite nanoparticles: Structural, morphological, optical, magnetic, and photocatalytic properties. Environ. Sci. Pollut. Res. 2019, 26, 19189–19206. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Mohallem, N.D.S. Comparative adsorption mechanism of doxycycline and Congo red using synthesized kaolinite supported CoFe2O4 nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Sabaa, M.W.; Abd ElHafeez, E.; Mohamed, R.R. Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 2019, 137, 1086–1101. [Google Scholar] [CrossRef]
- Ain, Q.U.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb (II), Cd (II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Shanmugavel, M.; Manikandan, E.; Nguyen-Tri, P.; Brindhadevi, K.; Pugazhendhi, A. Facile synthesis and characterization of hydroxyapatite from fish bones: Photocatalytic degradation of industrial dyes (crystal violet and Congo red). Prog. Org. Coatings 2020, 148, 105890. [Google Scholar] [CrossRef]
- An, S.; Liu, X.; Yang, L.; Zhang, L. Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: Study in single- and binary-solute systems. Chem. Eng. Res. Des. 2015, 94, 726–735. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Joseph, J.; Radhakrishnan, R.C.; Johnson, J.K.; Joy, S.P.; Thomas, J. Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Mater. Chem. Phys. 2020, 242, 122488. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Xu, Z.; Jin, C.; Fyda, T.J.; Zhang, J.X.J. Microfluidics-enabled acceleration of Fenton oxidation for degradation of organic dyes with rod-like zero-valent iron nanoassemblies. J. Colloid Interface Sci. 2020, 559, 254–262. [Google Scholar] [CrossRef]
- Pereira, L.A.; Couto, A.B.; Almeida, D.A.L.; Ferreira, N.G. Singular properties of boron-doped diamond/carbon fiber composite as anode in Brilliant Green dye electrochemical degradation. Diam. Relat. Mater. 2020, 103, 107708. [Google Scholar] [CrossRef]
- Achour, Y.; Bahsis, L.; Ablouh, E.-H.; Yazid, H.; Laamari, M.R.; Haddad, M. El Insight into adsorption mechanism of Congo red dye onto Bombax Buonopozense bark Activated-carbon using Central composite design and DFT studies. Surf. Interfaces 2021, 23, 100977. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Khnifira, M.; Boumya, W.; Abdennouri, M.; Sadiq, M.; Achak, M.; Serdaroğlu, G.; Kaya, S.; Şimşek, S.; Barka, N. A combined molecular dynamic simulation, DFT calculations, and experimental study of the eriochrome black T dye adsorption onto chitosan in aqueous solutions. Int. J. Biol. Macromol. 2021, 166, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Xu, M.-Y.; Xie, Z.-W.; Hai, W.; Xie, X.-L.; He, F.-A. Selective adsorption of anionic dyes from aqueous solution by a novel β-cyclodextrin-based polymer. J. Mol. Struct. 2020, 1203, 127373. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of acid activation of palygorskite on their toluene adsorption behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water. Sustainability 2021, 13, 3954. [Google Scholar] [CrossRef]
- El Berrichi, F.Z.; Ayari, F. Kaolin-issued zeolite A as efficient adsorbent for Bezanyl Yellow and Nylomine Green anionic dyes. Microporous Mesoporous Mater. 2017, 243, 91–101. [Google Scholar]
- Chaari, I.; Medhioub, M.; Jamoussi, F.; Hamzaoui, A.H. Acid-treated clay materials (Southwestern Tunisia) for removing sodium leuco-vat dye: Characterization, adsorption study and activation mechanism. J. Mol. Struct. 2021, 1223, 128944. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Azha, S.F.; Sellaoui, L.; Yunus, E.H.E.; Yee, C.J.; Bonilla-Petriciolet, A.; Lamine, A.B.; Ismail, S. Iron-modified composite adsorbent coating for azo dye removal and its regeneration by photo-Fenton process: Synthesis, characterization and adsorption mechanism interpretation. Chem. Eng. J. 2019, 361, 31–40. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Piri, F.; Mollahosseini, A.; Hosseini, M.M. Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Xinguo, X.; Jiling, Z.; Ruiyu, J.; Qi, X. Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization. China Pet. Process. Petrochemical Technol. 2014, 16, 63–68. [Google Scholar]
- Wang, W.; Tian, G.; Zhang, Z.; Wang, A. A simple hydrothermal approach to modify palygorskite for high-efficient adsorption of methylene blue and Cu (II) ions. Chem. Eng. J. 2015, 265, 228–238. [Google Scholar] [CrossRef]
- Câmara, A.B.F.; Sales, R.V.; Bertolino, L.C.; Furlanetto, R.P.P.; Rodríguez-Castellón, E.; De Carvalho, L.S. Novel application for palygorskite clay mineral: A kinetic and thermodynamic assessment of diesel fuel desulfurization. Adsorption 2020, 26, 267–282. [Google Scholar] [CrossRef]
- de Souza, C.G.; de Jesus, T.C.L.; dos Santos, R.C.; Bomfim, L.M.; Bertolino, L.C.; de Andrade, D.F.; Spinelli, L.S. Characterization of Brazilian palygorskite (Guadalupe region) and adsorptive behaviour for solvatochromic dyes. Clay Miner. 2021, 1–10. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, K.; Wu, H.; Yuan, P.; Liu, D.; Du, P.; Chen, P.; Wei, L.; Chen, W. Highly regenerative and efficient adsorption of phosphate by restructuring natural palygorskite clay via alkaline activation and co-calcination. Chem. Commun. 2021, 57, 1639–1642. [Google Scholar] [CrossRef]
- Kotti, M.; Papafilippaki, A.; Prassa, P.; Xirouhaki, A. Removal of Cationic Surfactants from Water by Adsorption on Attapulgite. Comput. Water Energy Environ. Eng. 2018, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, Y.; Feng, X.; Lv, C.; Liu, X.; Zhao, Y.; Chen, L. Adsorption removal of Ni (II) and phenol from aqueous solution by modified attapulgite and its composite hydrogel. Environ. Technol. 2019, 1–15. [Google Scholar] [CrossRef]
- Zhdanyuk, N.V. Adsorption of Cr (VI) and Co (II) by palygorskite modified with cationic surfactants. Вісник Одеськoгo націoнальнoгo університету. Хімія 2017, 22, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhao, J.; Zhong, A.; Jin, Y. Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue. Chem. Eng. J. 2011, 174, 143–150. [Google Scholar] [CrossRef]
- Gan, F.; Zhou, J.; Wang, H.; Du, C.; Chen, X. Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Res. 2009, 43, 2907–2915. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef]
- da Costa, F.P.; da Silva Morais, C.R.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Pereira da Costa, F.; Rodrigues da Silva Morais, C.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- da Silva, A.L.; Luna, C.B.B.; de Farias, A.F.F.; de Medeiros, S.A.S.L.; Meneghetti, S.M.P.; Rodrigues, A.M.; Costa, A.C.F.D.M. From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability 2020, 12, 10159. [Google Scholar] [CrossRef]
- da Silva, A.L.; Farias, A.F.F.; Pontes, J.R.M.; Rodrigues, A.M.; de M. Costa, A.C.F. Synthesis of the ZnO-Ni0.5Zn0.5Fe2O4-Fe2O3 magnetic catalyst in pilot-scale by combustion reaction and its application on the biodiesel production process from oil residual. Arab. J. Chem. 2020, 13, 7665–7679. [Google Scholar] [CrossRef]
- Dong, W.; Lu, Y.; Wang, W.; Zhang, M.; Jing, Y.; Wang, A. A sustainable approach to fabricate new 1D and 2D nanomaterials from natural abundant palygorskite clay for antibacterial and adsorption. Chem. Eng. J. 2020, 382, 122984. [Google Scholar] [CrossRef]
- Ryan, B.H.; Kaczmarek, S.E.; Rivers, J.M. Dolomite dissolution: An alternative diagenetic pathway for the formation of palygorskite clay. Sedimentology 2019, 66, 1803–1824. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, G.; Guo, Y. Thermal modified palygorskite: Preparation, characterization, and application for cationic dye-containing wastewater purification. Desalin. Water Treat. 2011, 30, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.F.; Lin, L.J.; Xi, Y.M.; Han, Z.Y. Effects of raw and heated palygorskite on rumen fermentation in vitro. Appl. Clay Sci. 2017, 138, 125–130. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Naidu, R. Bacterial mineralization of phenanthrene on thermally activated palygorskite: A 14C radiotracer study. Sci. Total Environ. 2017, 579, 709–717. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.S.F.; Osajima, J.A.; Luz, A.B.; Fonseca, M.G.; Silva Filho, E.C. Thermally activated palygorskites as agents to clarify soybean oil. Appl. Clay Sci. 2016, 119, 338–347. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Zhang, Y.; Zhang, Y.; Liang, J. Microstructural evolution and sintering properties of palygorskite nanofibers. Int. J. Appl. Ceram. Technol. 2020, 17, 1833–1842. [Google Scholar] [CrossRef]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Galai, H.; Pijolat, M.; Nahdi, K.; Trabelsi-Ayadi, M. Mechanism of growth of MgO and CaCO3 during a dolomite partial decomposition. Solid State Ion. 2007, 178, 1039–1047. [Google Scholar] [CrossRef]
- Rat’ko, A.I.; Ivanets, A.I.; Kulak, A.I.; Morozov, E.A.; Sakhar, I.O. Thermal decomposition of natural dolomite. Inorg. Mater. 2011, 47, 1372–1377. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, T.; Yin, S. Properties of thermally treated granular montmorillonite-palygorskite adsorbent (GMPA) and use to remove Pb2+ and Cu2+ from aqueous solutions. Clays Clay Miner. 2017, 65, 184–192. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qin, Z.; Li, S.; Zhang, T. Preparation of three-dimensional palygorskite based carrier. MethodsX 2020, 7, 100815. [Google Scholar] [CrossRef] [PubMed]
- Frinisrasra, N.; Srasra, E. Effect of heating on palygorskite and acid treated palygorskite properties. Электрoнная oбрабoтка материалoв 2008, 44, 43–49. [Google Scholar]
- Ogorodova, L.; Vigasina, M.; Melchakova, L.; Krupskaya, V.; Kiseleva, I. Thermochemical study of natural magnesium aluminum phyllosilicate: Palygorskite. J. Chem. Thermodyn. 2015, 89, 205–211. [Google Scholar] [CrossRef]
- Bayram, H.; Önal, M.; Üstünışık, G.; Sarıkaya, Y. Some thermal characteristics of a mineral mixture of palygorskite, metahalloysite, magnesite and dolomite. J. Therm. Anal. Calorim. 2007, 89, 169–174. [Google Scholar] [CrossRef]
- Amorim, K.B.; Angélica, R.S. Mineralogy and geochemistry of occurrence of palygorskite of Alcântara, S. Luís-Grajaú basin, Maranhão, Brazil. Cerâmica 2011, 57, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Bouna, L.; Rhouta, B.; Amjoud, M.; Maury, F.; Lafont, M.-C.; Jada, A.; Senocq, F.; Daoudi, L. Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers. Appl. Clay Sci. 2011, 52, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Kadir, S.; Eren, M.; Atabey, E. Dolocretes and associated palygorskite occurrences in siliciclastic red mudstones of the Sariyer formation (Middle Miocene), southeastern side of the Çanakkale strait, Turkey. Clays Clay Miner. 2010, 58, 205–219. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Qing, C.; Peng, S.; Song, Y.; Guo, Y. Effect of heat treatment on structure, morphology and surface properties of palygorskite. J. -Chin. Ceram. Soc. 2006, 34, 1406. [Google Scholar]
- Boudriche, L.; Calvet, R.; Hamdi, B.; Balard, H. Surface properties evolution of attapulgite by IGC analysis as a function of thermal treatment. Colloids Surf. A Physicochem. Eng. Asp. 2012, 399, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Mu, B.; Zong, L.; Wang, A. From waste hot-pot oil as carbon precursor to development of recyclable attapulgite/carbon composites for wastewater treatment. J. Environ. Sci. 2019, 75, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.D.A. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Nebaghe, K.C.; El Boundati, Y.; Ziat, K.; Naji, A.; Rghioui, L.; Saidi, M. Comparison of linear and non-linear method for determination of optimum equilibrium isotherm for adsorption of copper (II) onto treated Martil sand. Fluid Phase Equilib. 2016, 430, 188–194. [Google Scholar] [CrossRef]
- Garba, Z.N. The Relevance of Isotherm and Kinetic Models to Chlorophenols Adsorption: A Review. Avicenna J. Environ. Heal. Eng. 2019, 6, 55–65. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Zhang, T.; Li, Q.; Xing, J.; Liu, H. Improvement in diesel desulfurization capacity by equilibrium isotherms analysis. Sep. Purif. Technol. 2011, 78, 352–356. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Shigaki, N.; Mogi, Y.; Haraoka, T.; Furuya, E. Measurements and calculations of the equilibrium adsorption amounts of CO2–N2, CO–N2, and CO2–CO mixed gases on 13X zeolite. SN Appl. Sci. 2020, 2, 488. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [Green Version]
- Al-Futaisi, A.; Jamrah, A.; Al-Hanai, R. Aspects of cationic dye molecule adsorption to palygorskite. Desalination 2007, 214, 327–342. [Google Scholar] [CrossRef]
- Liu, M.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Enhanced fluoride adsorption from aqueous solution by zirconium (IV)-impregnated magnetic chitosan graphene oxide. Int. J. Biol. Macromol. 2021, 182, 1759–1768. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C.; Ok, Y.S.; Vithanage, M. Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Ashiq, A.; Adassooriya, N.M.; Sarkar, B.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Municipal solid waste biochar-bentonite composite for the removal of antibiotic ciprofloxacin from aqueous media. J. Environ. Manag. 2019, 236, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Romdhane, D.F.; Satlaoui, Y.; Nasraoui, R.; Charef, A.; Azouzi, R. Adsorption, modeling, thermodynamic, and kinetic studies of methyl red removal from textile-polluted water using natural and purified organic matter rich clays as low-cost adsorbent. J. Chem. 2020, 2020, 4376173. [Google Scholar] [CrossRef]
- Sevim, F.; Lacin, O.; Ediz, E.F.; Demir, F. Adsorption capacity, isotherm, kinetic, and thermodynamic studies on adsorption behavior of malachite green onto natural red clay. Environ. Prog. Sustain. Energy 2021, 40, e13471. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Far, H.S. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020, 81, 405–414. [Google Scholar] [CrossRef]
- Youcef, L.D.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and continuous studies for adsorption of anionic dye onto waste tea residue: Kinetic, equilibrium, breakthrough and reusability studies. J. Clean. Prod. 2020, 252, 119778. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]
- Dong, W.; Lu, Y.; Wang, W.; Zong, L.; Zhu, Y.; Kang, Y.; Wang, A. A new route to fabricate high-efficient porous silicate adsorbents by simultaneous inorganic-organic functionalization of low-grade palygorskite clay for removal of Congo red. Microporous Mesoporous Mater. 2019, 277, 267–276. [Google Scholar] [CrossRef]
- Sousa, H.R.; Silva, L.S.; Sousa, P.A.A.; Sousa, R.R.M.; Fonseca, M.G.; Osajima, J.A.; Silva-Filho, E.C. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Omer, A.M.; Elgarhy, G.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. Fabrication of novel iminodiacetic acid-functionalized carboxymethyl cellulose microbeads for efficient removal of cationic crystal violet dye from aqueous solutions. Int. J. Biol. Macromol. 2020, 148, 1072–1083. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Yang, H.; Tang, A.; Zuo, X. Magnetic carbon-coated palygorskite loaded with cobalt nanoparticles for Congo Red removal from waters. Appl. Clay Sci. 2020, 198, 105856. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, Y.; Zhang, Z.; Quan, Q.; Qiang, X. Synergistic effect of hydrophilic palygorskite and hydrophobic zein particles on the properties of chitosan films. Mater. Des. 2020, 185, 108229. [Google Scholar] [CrossRef]
- Wong, S.; Abd Ghafar, N.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [Green Version]
- Madejova, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Boudriche, L.; Calvet, R.; Hamdi, B.; Balard, H. Effect of acid treatment on surface properties evolution of attapulgite clay: An application of inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Suarez, M.; Garcia-Romero, E. FTIR spectroscopic study of palygorskite: Influence of the composition of the octahedral sheet. Appl. Clay Sci. 2006, 31, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Tian, G.; Wang, W.; Kang, Y.; Wang, A. Palygorskite in sodium sulphide solution via hydrothermal process for enhanced methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2016, 58, 417–423. [Google Scholar] [CrossRef]
- Frost, R.L.; Locos, O.B.; Ruan, H.; Kloprogge, J.T. Near-infrared and mid-infrared spectroscopic study of sepiolites and palygorskites. Vib. Spectrosc. 2001, 27, 1–13. [Google Scholar] [CrossRef]
- Zou, H.; Cao, Q.; Liu, D.; Yu, X.; Lai, H. Surface Features of Fluorapatite and Dolomite in the Reverse Flotation Process Using Sulfuric Acid as a Depressor. Miner 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, M.; Taheran, M.; Brar, K.; Galvez-Cloutier, R.; Martel, R. Hydrophobic dolomite sorbent for oil spill clean-ups: Kinetic modeling and isotherm study. Fuel 2019, 251, 57–72. [Google Scholar] [CrossRef]
- Tian, G.; Wang, W.; Mu, B.; Kang, Y.; Wang, A. Facile fabrication of carbon/attapulgite composite for bleaching of palm oil. J. Taiwan Inst. Chem. Eng. 2015, 50, 252–258. [Google Scholar] [CrossRef]
- Rhouta, B.; Zatile, E.; Bouna, L.; Lakbita, O.; Maury, F.; Daoudi, L.; Lafont, M.C.; Amjoud, M.; Senocq, F.; Jada, A. Comprehensive physicochemical study of dioctahedral palygorskite-rich clay from Marrakech High Atlas (Morocco). Phys. Chem. Miner. 2013, 40, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, W.; Zhang, J.; Liu, P.; Wang, A. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, X.; Cheng, H.; Frost, R.L. An infrared spectroscopic comparison of four Chinese palygorskites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 784–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Zhao, Z.; Suib, S.L.; Yang, H. Degradation of Congo Red dye by a Fe2O3@ CeO2-ZrO2/Palygorskite composite catalyst: Synergetic effects of Fe2O3. J. Colloid Interface Sci. 2019, 539, 135–145. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of triphenylmethane dyes by bacterial consortium. Sci. World J. 2012, 2012, 512454. [Google Scholar] [CrossRef] [Green Version]
- Abdi, M.; Balagabri, M.; Karimi, H.; Hossini, H.; Rastegar, S.O. Degradation of crystal violet (CV) from aqueous solutions using ozone, peroxone, electroperoxone, and electrolysis processes: A comparison study. Appl. Water Sci. 2020, 10, 168. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A.; Alabi, K.A.; Ahmed, I.B. Kinetics and mechanisms of congo-red dye removal from aqueous solution using activated Moringa oleifera seed coat as adsorbent. Appl. Water Sci. 2020, 10, 136. [Google Scholar] [CrossRef]



| Sample | Pal | Pal-300T | Pal-500T | Pal-700T | |
|---|---|---|---|---|---|
| Oxides (%) | |||||
| SiO2 | 52.78 | 51.80 | 52.72 | 52.02 | |
| MgO | 13.91 | 14.54 | 14.43 | 15.27 | |
| Al2O3 | 13.48 | 12.70 | 12.89 | 12.92 | |
| CaO | 11.92 | 12.77 | 12.11 | 12.09 | |
| Fe2O3 | 5.29 | 5.56 | 5.33 | 5.25 | |
| K2O | 0.90 | 0.93 | 0.94 | 0.96 | |
| Other Oxides | 1.72 | 1.70 | 1.58 | 1.49 | |
| Sample | Specific Surface Area (m2/g) | Average Pore Diameter (nm) |
|---|---|---|
| Pal | 80.4 | 14.3 |
| Pal-300T | 79.8 | 14.4 |
| Pal-500T | 67.6 | 16.6 |
| Pal-700T | 63.2 | 18.1 |
| Sample | Dye | Model | ||
|---|---|---|---|---|
| Langmuir Isotherm | ||||
| qmax (mg/g) | KL (L/mg) | R2 | ||
| Pal | CV CR | 64.7 29.9 | 0.03 0.05 | 0.93 0.94 |
| Pal-300T | CV CR | 78.7 39.7 | 0.07 0.03 | 0.83 0.88 |
| Pal-500T | CV CR | 70.3 63.9 | 0.26 0.02 | 0.88 0.75 |
| Pal-700T | CV CR | 189.3 136.1 | 0.87 0.46 | 0.98 0.96 |
| Freundlich Isotherm | ||||
| 1/n | KF (mg/g)(L/mg)1/n | R2 | ||
| Pal | CV CR | 0.26 0.42 | 11.0 4.1 | 0.99 0.97 |
| Pal-300T | CV CR | 0.26 0.43 | 19.1 4.2 | 0.95 0.94 |
| Pal-500T | CV CR | 0.28 0.51 | 22.4 4.9 | 0.97 0.81 |
| Pal-700T | CV CR | 0.65 0.71 | 35.5 39.1 | 0.97 0.94 |
| D-R Isotherm | ||||
| qD (mg/g) | KDR (mol2/J2) | R2 | ||
| Pal | CV CR | 44.6 25.1 | 1.1 × 10−5 2.9 × 10−5 | 0.79 0.82 |
| Pal-300T | CV CR | 61.3 29.9 | 2.6 × 10−4 2.6 × 10−5 | 0.57 0.75 |
| Pal-500T | CV CR | 87.3 37.4 | 1.2 × 10−7 2.1 × 10−5 | 0.84 0.61 |
| Pal-700T | CV CR | 191.5 116.7 | 2.3 × 10−6 2.9 × 10−7 | 0.96 0.91 |
| Temkin Isotherm | ||||
| bT (J/mol) | AT (L/mg) | R2 | ||
| Pal | CV CR | 329.6 551.3 | 4.2 1.9 | 0.89 0.93 |
| Pal-300T | CV CR | 267.9 468.2 | 12.8 2.2 | 0.91 0.84 |
| Pal-500T | CV CR | 255.2 389.2 | 13.2 2.7 | 0.95 0.66 |
| Pal-700T | CV CR | 57.6 143.1 | 25.5 3.2 | 0.90 0.94 |
| Sample | Dye | Model | |||
|---|---|---|---|---|---|
| Pseudo-first order | |||||
| qexp (mg/g) | qcal (mg/g) | k1 (min−1) | R2 | ||
| Pal | CV CR | 26 18.2 | 24.5 20.1 | 0.08 0.02 | 0.95 0.97 |
| Pal-300T | CV CR | 38.9 20 | 33.3 18.4 | 4.23 0.07 | 0.79 0.94 |
| Pal-500T | CV CR | 41.4 23.2 | 36.2 18.9 | 5.10 2.04 | 0.81 0.71 |
| Pal-700T | CV CR | 47.3 48.1 | 39.9 37.7 | 8.04 2.68 | 0.79 0.54 |
| Pseudo-second order | |||||
| qexp (mg/g) | qcal (mg/g) | k2 (g/(mg min)) | R2 | ||
| Pal | CV CR | 26 18.2 | 25.9 17.9 | 0.001 0.002 | 0.98 0.98 |
| Pal-300T | CV CR | 38.9 20 | 39.0 19.7 | 0.002 0.006 | 0.97 0.98 |
| Pal-500T | CV CR | 41.4 23.2 | 41.9 23.1 | 0.002 0.003 | 0.98 0.95 |
| Pal-700T | CV CR | 47.3 48.1 | 46.4 52.6 | 0.002 0.005 | 0.96 0.97 |
| Elovich | |||||
| α (mg·g−1/min) | β (g/mg) | R2 | |||
| Pal | CV CR | 7.7 1.9 | 0.4 0.3 | 0.99 0.99 | |
| Pal-300T | CV CR | 49.3 5.2 | 0.2 0.5 | 0.99 0.99 | |
| Pal-500T | CV CR | 61.9 9.6 | 0.2 0.3 | 0.99 0.99 | |
| Pal-700T | CV CR | 78.1 4.8 | 0.2 0.1 | 0.99 0.99 | |
| Sample | Dye | ∆G (kJ/mol) | ∆H (kJ/mol) | ΔS (J/K mol) | |||
|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | ||||
| Pal | CV | −1.41 | −1.56 | −1.69 | −1.83 | 0.49 | 1.11 |
| Pal-300T | CV | −0.15 | −0.20 | −0.29 | −0.35 | 0.54 | 1.39 |
| Pal-500T | CV | −0.66 | −0.79 | −0.90 | −1.01 | 0.41 | 1.24 |
| Pal-700T | CV | −9.40 | −10.16 | −11.49 | −15.83 | 6.19 | 24.32 |
| Pal | CR | −0.17 | −0.20 | −0.32 | −0.41 | 0.27 | 0.97 |
| Pal-300T | CR | −2.85 | −3.02 | −3.31 | −3.45 | 0.41 | 2.53 |
| Pal-500T | CR | −3.87 | −4.23 | −4.47 | −4.83 | 0.68 | 3.85 |
| Pal-700T | CR | −7.43 | −8.29 | −9.76 | −10.64 | 3.08 | 13.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Vitorino, M.d.B.C.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption Behavior of Crystal Violet and Congo Red Dyes on Heat-Treated Brazilian Palygorskite: Kinetic, Isothermal and Thermodynamic Studies. Materials 2021, 14, 5688. https://doi.org/10.3390/ma14195688
Silva VC, Araújo MEB, Rodrigues AM, Vitorino MdBC, Cartaxo JM, Menezes RR, Neves GA. Adsorption Behavior of Crystal Violet and Congo Red Dyes on Heat-Treated Brazilian Palygorskite: Kinetic, Isothermal and Thermodynamic Studies. Materials. 2021; 14(19):5688. https://doi.org/10.3390/ma14195688
Chicago/Turabian StyleSilva, Vanderlane Cavalcanti, Maria Eduarda Barbosa Araújo, Alisson Mendes Rodrigues, Maria do Bom Conselho Vitorino, Juliana Melo Cartaxo, Romualdo Rodrigues Menezes, and Gelmires Araújo Neves. 2021. "Adsorption Behavior of Crystal Violet and Congo Red Dyes on Heat-Treated Brazilian Palygorskite: Kinetic, Isothermal and Thermodynamic Studies" Materials 14, no. 19: 5688. https://doi.org/10.3390/ma14195688