Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update
Abstract
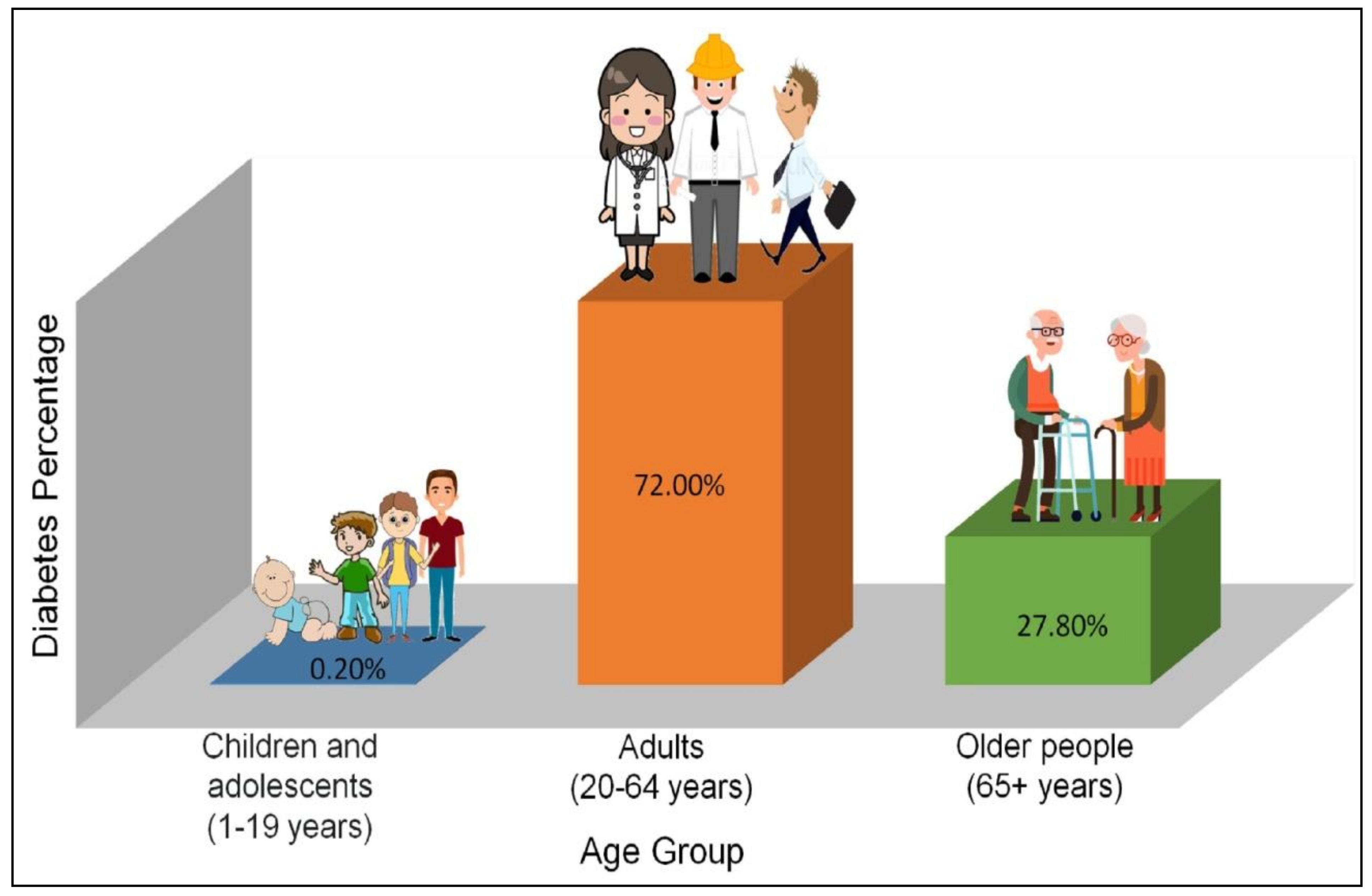
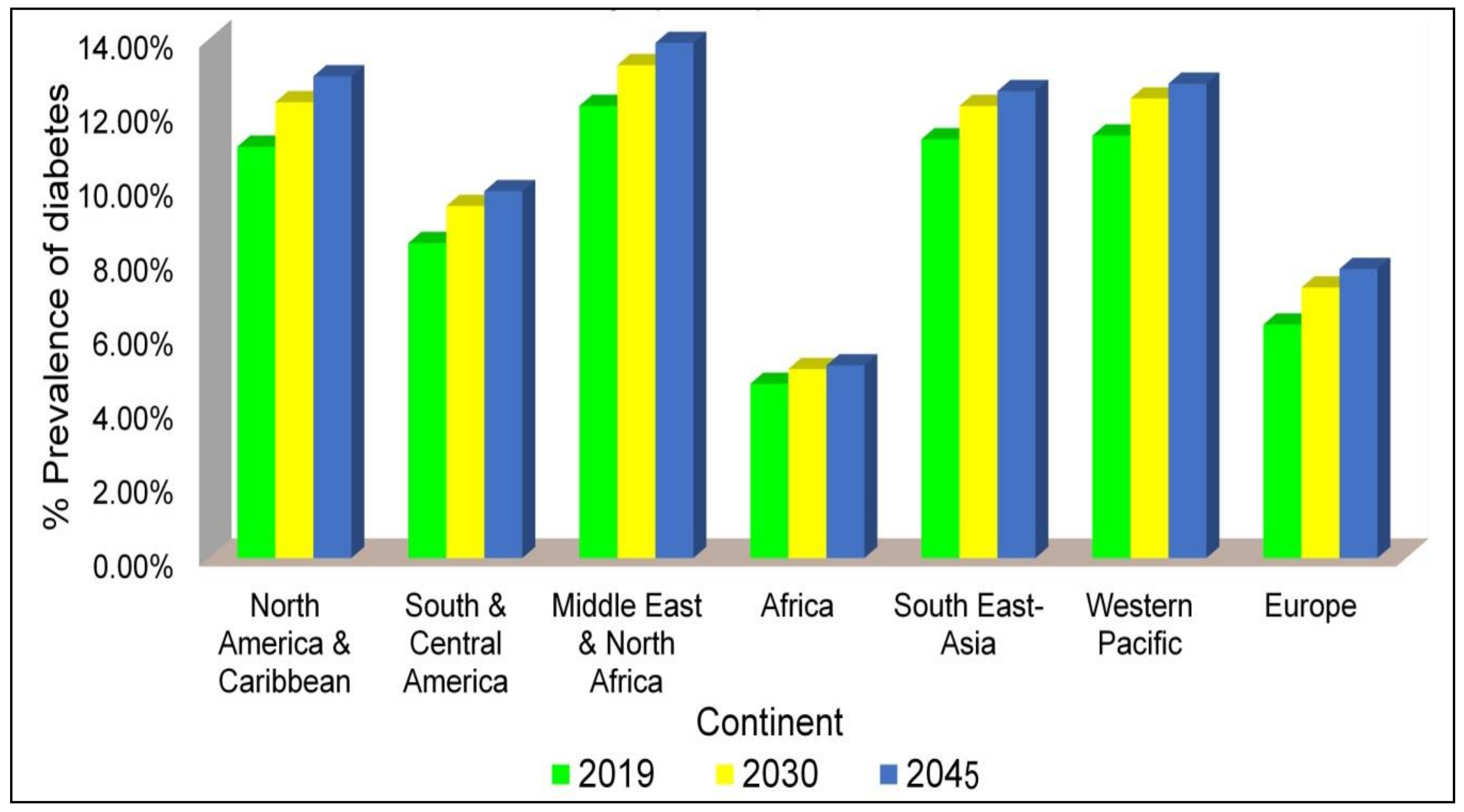
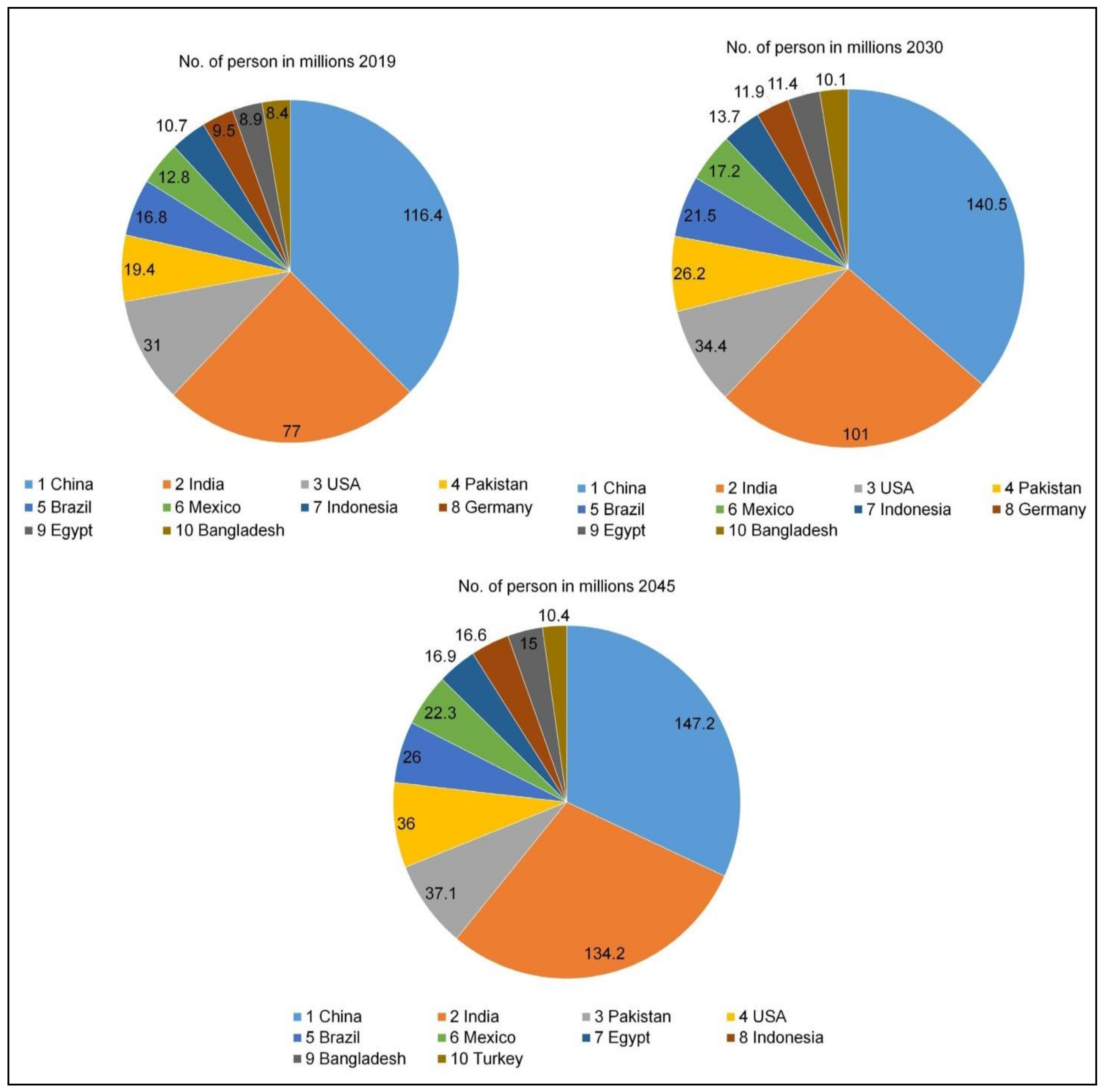
1. Introduction
Diabetes Mellitus: An Insight
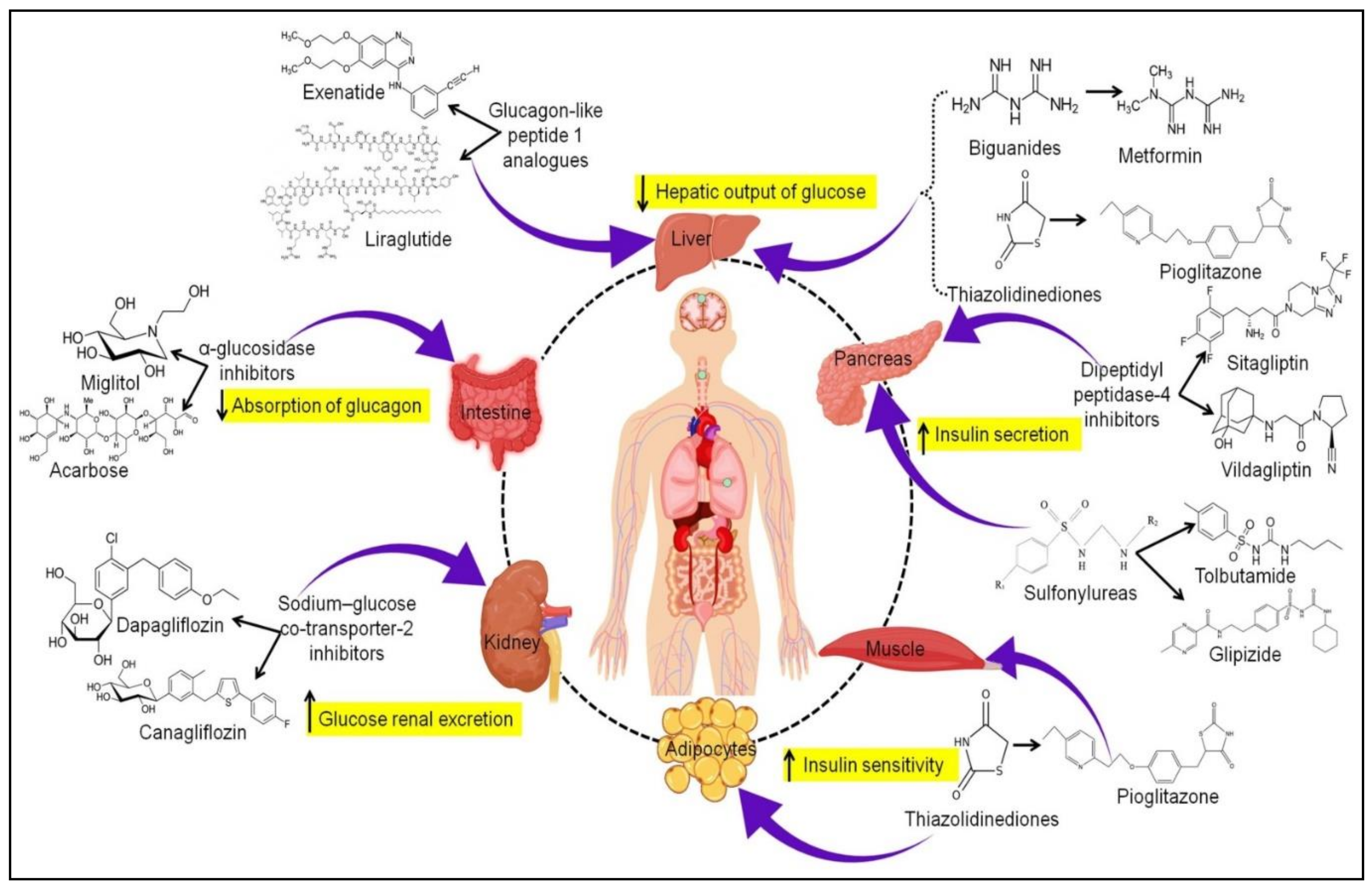
2. Oral Antidiabetics: Currently Available Therapy
2.1. Biguanides
2.2. Sulfonylureas
2.3. Thiazolidinediones
2.4. Dipeptidyl Peptidase-4 Inhibitors
2.5. Glucagon-like Peptide 1 Analogs
2.6. Sodium-Glucose Co-Transporter-2 Inhibitors
3. Emerging Treatment Strategies
3.1. Oral Hypoglycemics Incorporated Nanocarrier-Based Treatment
3.1.1. Liposomes
3.1.2. Niosomes
3.1.3. Micelles
3.1.4. Nanoemulsion
3.1.5. Polymeric Nanoparticles
3.1.6. Solid Lipid Nanoparticles
3.1.7. Dendrimers
3.1.8. Carbon Nanotubes
3.1.9. Contribution of Authors to the Nano-Based Diabetes Therapy
3.2. Insulin Pump
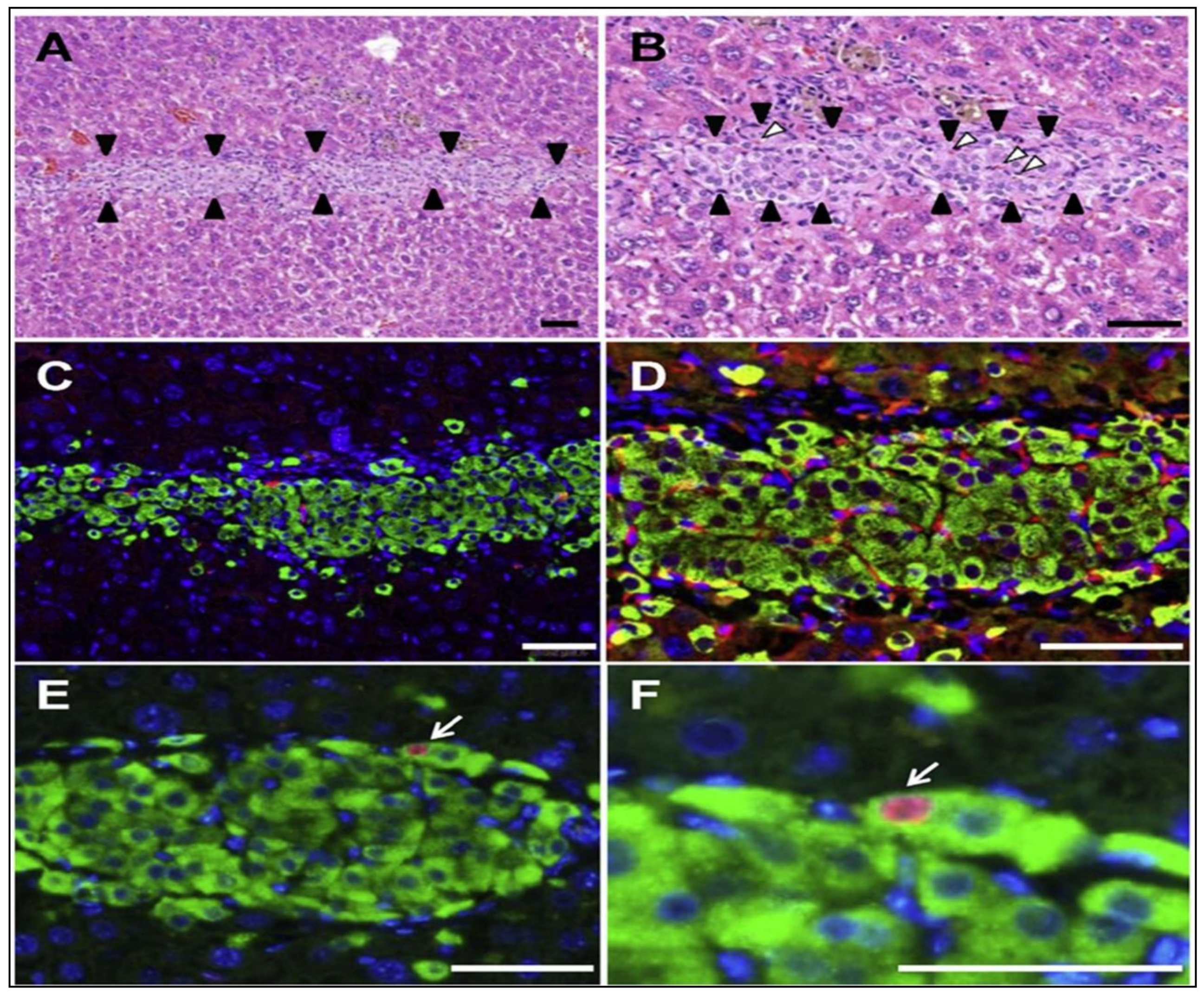
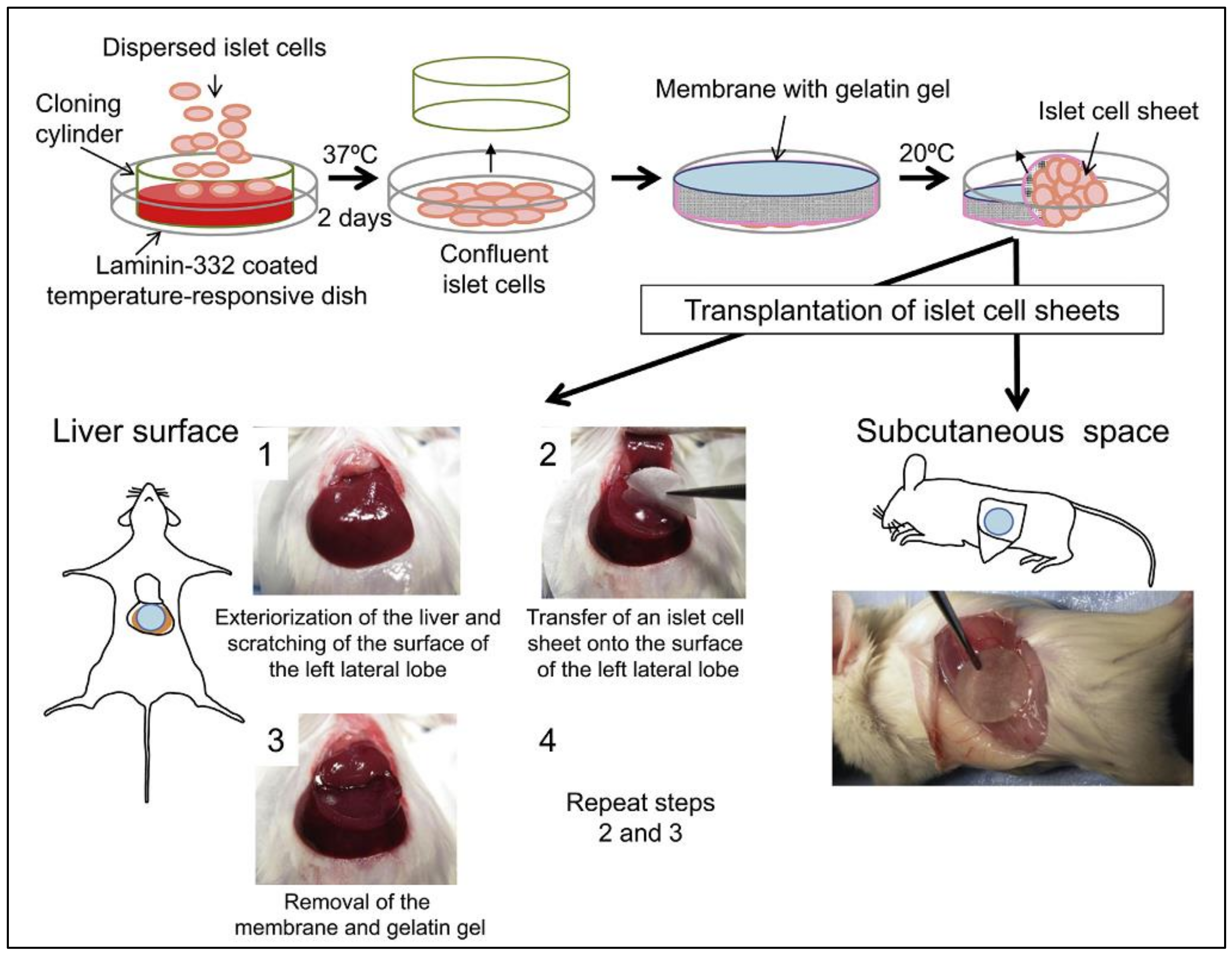
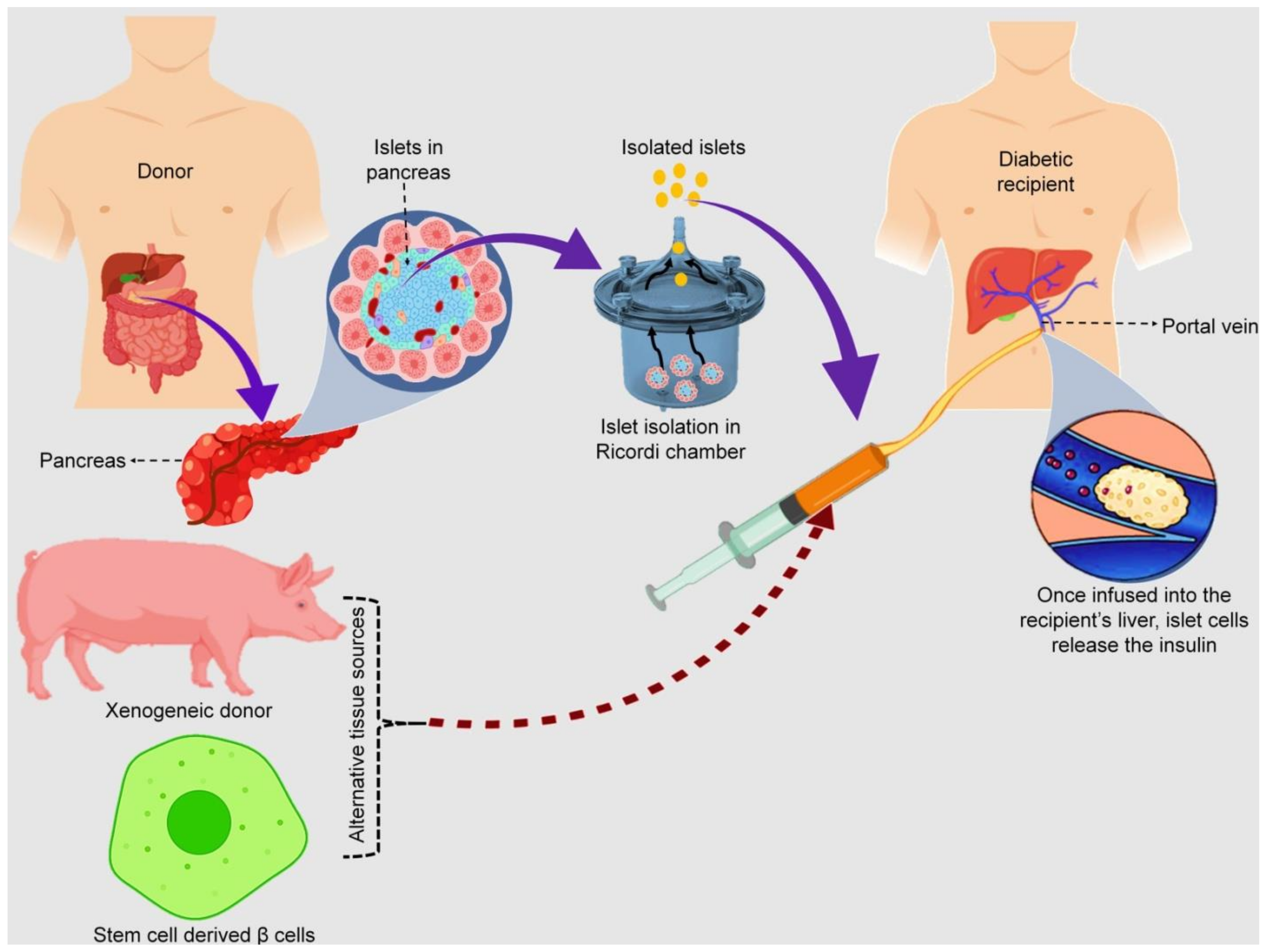
3.3. Pancreatic Islet Cell Transplantation
3.4. Artificial Pancreas
3.5. Tissue Engineering
3.6. Gene Therapy
3.7. Stem Cells Therapy
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Mishra, V.; Hemani, V.; Verma, A.; Jain, A.; Jain, S.; Charyulu, R.N. Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends Food Sci. Technol. 2021, 110, 594–610. [Google Scholar] [CrossRef]
- Global Fact Sheet, 9th ed.; IDF Diabetes Atlas. 2019. Available online: https://diabetesatlas.org/upload/resources/material/20201028_180116_Global-factsheet-final.pdf (accessed on 13 July 2021).
- Demographic and Geographic Outline, 9th ed.; IDF Diabetes Atlas. 2019. Available online: https://diabetesatlas.org/en/sections/demographic-and-geographic-outline.html (accessed on 13 July 2021).
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and Insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009, 137, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Cheng, P.C.; Tu, S.T.; Hsu, S.R.; Cheng, Y.C.; Liu, Y.H. Effect of Metformin monotherapy on serum lipid profile in statin-naïve individuals with newly diagnosed type 2 diabetes mellitus: A cohort study. PeerJ 2018, 6, e4578. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging nanoparticulate drug delivery systems of metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002, 51 (Suppl. 3), S368–S376. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Roumie, C.L.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Liu, X.; Murff, H.J.; Elasy, T.A.; Griffin, M.R. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2012, 157, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Eldor, R.; DeFronzo, R.A.; Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: Glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013, 36 (Suppl. 2), S162–S174. [Google Scholar] [CrossRef]
- Defronzo, R.A.; Tripathy, D.; Schwenke, D.C.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.C.; Gastaldelli, A.; Henry, R.R.; Kitabchi, A.E.; et al. Prevention of diabetes with pioglitazone in act now: Physiologic correlates. Diabetes 2013, 62, 3920–3926. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; Mari, A.; DeFronzo, R.A. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E871–E883. [Google Scholar] [CrossRef] [PubMed]
- Jearath, V.; Vashisht, R.; Rustagi, V.; Raina, S.; Sharma, R. Pioglitazone-induced congestive heart failure and pulmonary edema in a patient with preserved ejection fraction. J. Pharmacol. Pharmacother. 2016, 7, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Yadav, T.P.; Pandey, B.; Gupta, V.; Singh, S.P. Engineering nanomaterials for smart drug release: Recent advances and challenges. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S., Ranjan, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 411–419, Chapter 15. [Google Scholar]
- Singh, A.K. Dipeptidyl peptidase-4 inhibitors: Novel mechanism of actions. Indian J. Endocrinol. Metab. 2014, 18, 753–759. [Google Scholar] [CrossRef]
- Pathak, R.; Bridgeman, M.B. Dipeptidyl Peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharm. Ther. 2010, 35, 509–513. [Google Scholar]
- Brunton, S. GLP-1 Receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: Is one approach more successful or preferable than the other? Int. J. Clin. Pract. 2014, 68, 557–567. [Google Scholar] [CrossRef]
- Bunck, M.C.; Cornér, A.; Eliasson, B.; Heine, R.J.; Shaginian, R.M.; Taskinen, M.-R.; Smith, U.; Yki-Järvinen, H.; Diamant, M. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011, 34, 2041–2047. [Google Scholar] [CrossRef]
- Stonehouse, A.H.; Darsow, T.; Maggs, D.G. Incretin-based therapies. J. Diabetes 2012, 4, 55–67. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Bain, S.; Kanamarlapudi, V. Recent advances in understanding the role of glucagon-like peptide 1. F1000Research 2020, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: A review of their basic and clinical pharmacology. Diabetes Ther. 2014, 5, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Agrawal, A.K.; Jain, S.; Yadav, N.P. Novel drug delivery system: An immense hope for diabetics. Drug Deliv. 2016, 23, 2371–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Hu, S.Q.; Mao, W.W.; Xiang, J.J.; Zhou, Z.X.; Liu, X.R.; Tang, J.; Shen, Y. Assemblies of peptide-cytotoxin conjugates for tumor-homing chemotherapy. Adv. Funct. Mat. 2019, 29, 1807446. [Google Scholar] [CrossRef]
- Zhao, R.; Lu, Z.; Yang, J.; Zhang, L.; Li, Y.; Zhang, X. Drug delivery system in the treatment of diabetes mellitus. Front. Bioeng. Biotechnol. 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Naser, M.; Nasr, M.M.; Shehata, L.H. Nanotechnology in diagnosis and treatment of diabetes mellitus. Int. J. Prog. Sci. Tech. 2021, 24, 586–596. [Google Scholar]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral delivery for protein and peptide drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef]
- Gedawy, A.; Martinez, J.; Al-Salami, H.; Dass, C.R. Oral insulin delivery: Existing barriers and current counter-strategies. J. Pharm. Pharmacol. 2018, 70, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of Insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- Gupta, D.R.; Odayakumar, M.P. Development and characterization of metformin nanoparticles for the effective treatment of diabetes mellitus. Int. J. Life Sci. Pharma Res. 2021, 11, P204–P217. [Google Scholar] [CrossRef]
- Abdel Maksoud, H.A.; Abou Zaid, O.A.R.; Elharrif, M.G.; Omnia, M.A.; Alaa, E.A. Selenium Cleome Droserifolia Nanoparticles (Se-CNPs) and It’s ameliorative effects in experimentally induced diabetes mellitus. Clin. Nutr. ESPEN 2020, 40, 383–391. [Google Scholar] [CrossRef]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B 2019, 197, 111541. [Google Scholar] [CrossRef]
- Nowacka, O.; Milowska, K.; Belica-Pacha, S.; Palecz, B.; Šipošová, K.; Gazova, Z.; Bryszewska, M. Generation-dependent effect of PAMAM dendrimers on human insulin fibrillation and thermal stability. Int. J. Biol. Macromol. 2016, 82, 54–60. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Gowayed, M.A.; Seiffein, N.L.; Abdel-Moneim, R.A.; Kamel, M.A.; Labib, G.S. Valsartan solid lipid nanoparticles integrated hydrogel: A challenging repurposed use in the treatment of diabetic foot ulcer, in-vitro/in-vivo experimental study. Int. J. Pharm. 2021, 592, 120091. [Google Scholar] [CrossRef]
- Radwan, S.E.-S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Al-Trad, B.; Al Gazo, L.; Alomari, G.; Al Zoubi, M.; Alshaer, W.; Al-Batayneh, K.; Kanan, B.; Pal, K.; Tambuwala, M.M. Gold nanoparticles ameliorate diabetic cardiomyopathy in streptozotocin-induced diabetic rats. J. Mol. Struct. 2021, 1231, 130009. [Google Scholar] [CrossRef]
- Mostafa, F.; Abdel-Moneim, A.; Abdul-Hamid, M.; Galaly, S.R.; Mohamed, H.M. Polydatin and polydatin-loaded chitosan nanoparticles attenuate diabetic cardiomyopathy in rats. J. Mol. Histol. 2021, 52, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.Z.; Ma, W.C.; Xu, J.L.; Wang, J.L.; Chen, M.J.; Yu, L.; Chen, Y.N. Therapeutic effect of combined use of FGF1-loaded nano-liposomes and ultrasound-targeted microbubble destruction technique on treating rats with experimental diabetic cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 2017, 45, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xue, L.-F.; Hu, B.; Liu, H.-H.; Huang, S.-B.; Khan, S.; Meng, Y. Calycosin-loaded nanoliposomes as potential nanoplatforms for treatment of diabetic nephropathy through regulation of mitochondrial respiratory function. J. Nanobiotechnol. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Design and optimization of crocetin loaded PLGA nanoparticles against diabetic nephropathy via suppression of inflammatory biomarkers: A formulation approach to preclinical study. Drug Deliv. 2019, 26, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nanocarriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Sharmila, S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today Proc. 2020, 22, 432–438. [Google Scholar] [CrossRef]
- Ramkanth, S.; Anitha, P.; Gayathri, R.; Mohan, S.; Babu, D. Formulation and design optimization of nano-transferosomes using Pioglitazone and Eprosartan mesylate for concomitant therapy against diabetes and hypertension. Eur. J. Pharm. Sci. 2021, 162, 105811. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, M.; Yin, W.; Liang, B.; Li, A.; Li, J.; Li, X.; Zhao, S.; Liu, F. Development of a novel RNAi therapy: Engineered MiR-31 exosomes promoted the healing of diabetic wounds. Bioact. Mater. 2021, 6, 2841–2853. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Yan-yu, X.; Yun-mei, S.; Zhi-peng, C.; Qi-neng, P. Preparation of silymarin proliposome: A new way to increase oral bioavailability of silymarin in beagle dogs. Int. J. Pharm. 2006, 319, 162–168. [Google Scholar] [CrossRef]
- Bergot, A.-S.; Buckle, I.; Cikaluru, S.; Naranjo, J.L.; Wright, C.M.; Zheng, G.; Talekar, M.; Hamilton-Williams, E.E.; Thomas, R. Regulatory T Cells induced by single-peptide liposome immunotherapy suppress islet-specific t cell responses to multiple antigens and protect from autoimmune diabetes. J. Immunol. 2020, 204, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Villalba, A.; Rodriguez-Fernandez, S.; Ampudia, R.-M.; Cano-Sarabia, M.; Perna-Barrull, D.; Bertran-Cobo, C.; Ehrenberg, C.; Maspoch, D.; Vives-Pi, M. Preclinical evaluation of antigen-specific nanotherapy based on phosphatidylserine-liposomes for type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 77–83. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Boarescu, P.M.; Bolboacă, S.D.; Blidaru, M.; Feștilă, D.; Dogaru, G.; Nicula, C.A. Comparative effect of curcumin versus liposomal curcumin on systemic pro-inflammatory cytokines profile, MCP-1 And RANTES in experimental diabetes mellitus. Int. J. Nanomed. 2019, 14, 8961–8972. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Hasan, A.A.; Madkor, H.; Wageh, S. Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug Deliv. 2013, 20, 120–126. [Google Scholar] [CrossRef]
- Mohsen, A.M.; AbouSamra, M.M.; ElShebiney, S.A. Enhanced oral bioavailability and sustained delivery of glimepiride via niosomal encapsulation: In-vitro characterization and in-vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1254–1264. [Google Scholar] [CrossRef]
- Singhal, T.; Mujeeb, M.; Ahad, A.; Aqil, M.; Rahman, S.O.; Najmi, A.K.; Siddiqui, W.A. Preparation, optimization and biological evaluation of gymnemic acid loaded niosomes against streptozotocin-nicotinamide induced diabetic-nephropathy in Wistar rats. J. Drug Deliv. Sci. Technol. 2019, 54, 101328. [Google Scholar] [CrossRef]
- Alam, M.S.; Ahad, A.; Abidin, L.; Aqil, M.; Mir, S.R.; Mujeeb, M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in wistar rats. Biomed. Pharmacother. 2018, 97, 1514–1520. [Google Scholar] [CrossRef]
- Samed, N.; Sharma, V.; Sundaramurthy, A. Hydrogen bonded niosomes for encapsulation and release of hydrophilic and hydrophobic anti-diabetic drugs: An efficient system for oral anti-diabetic formulation. Appl. Surf. Sci. 2018, 449, 567–573. [Google Scholar] [CrossRef]
- Lundqvist, T.; Bredeberg, S. Pharmaceutical development. In Drug Discovery and Development—Technology in Transition; Churchull Livingstone/Elsevier: Edinburgh, UK, 2012; pp. 227–238. [Google Scholar]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abd El-Alim, S.H.; Basha, M.; Salama, A. Phospholipid complex enriched micelles: A novel drug delivery approach for promoting the antidiabetic effect of Repaglinide. Eur. J. Pharm. Sci. 2017, 99, 75–84. [Google Scholar] [CrossRef]
- Uppal, S.; Italiya, K.S.; Chitkara, D.; Mittal, A. Nanoparticulate-based drug delivery systems for small molecule anti-diabetic drugs: An emerging paradigm for effective therapy. Acta Biomater. 2018, 81, 20–42. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Rawat, B.S.; Singh, D.; Rawat, M.S.M. Pharmacosomes: The lipid-based new drug delivery system. Expert Opin. Drug Deliv. 2009, 6, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Lv, J.; Huang, F.; An, Y.; Shi, L.; Ma, R. Glucose and H2O2 dual-responsive polymeric micelles for the self-regulated release of insulin. ACS Appl. Bio Mater. 2020, 3, 1598–1606. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Choudhry, I.; Namdev, A.; Mishra, S.; Soni, S.; Hurkat, P.; Jain, A.; Jain, D. Oral insulin: Myth or reality. Curr. Diabetes Rev. 2018, 14, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.; Filipe, P.; Thomé, N.G.; Vieira, J.; Oliveira, C.; Teodósio, C.; Ferreira, R.; Roque, L.; Fonte, P. A brief overview of the oral delivery of insulin as an alternative to the parenteral delivery. Curr. Mol. Med. 2020, 20, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Taurin, S.; Altayeb, D.; Taha, S.; Bakhiet, M.; Greish, K. Oral insulin delivery using poly (styrene co-maleic acid) micelles in a diabetic mouse model. Pharmaceutics 2020, 12, 1026. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Liu, C.-S.; Huang, C.-L.; Chen, L.; Zheng, Y.-R.; Huang, S.-H.; Long, X.-Y. Nanoemulsion improves hypoglycemic efficacy of berberine by overcoming its gastrointestinal challenge. Colloids Surf. B Biointerfaces 2019, 181, 927–934. [Google Scholar] [CrossRef]
- Santalices, I.; Vázquez-Vázquez, C.; Santander-Ortega, M.J.; Lozano, V.; Araújo, F.; Sarmento, B.; Shrestha, N.; Préat, V.; Chenlo, M.; Alvarez, C.V.; et al. A nanoemulsion/micelles mixed nanosystem for the oral administration of hydrophobically modified insulin. Drug Deliv. Transl. Res. 2021, 11, 524–545. [Google Scholar] [CrossRef]
- Djamil, R.; Zaidan, S.; Rahmat, D.; Pratami, D.K.; Hakim, F. Nanoemulsion of okra fruit extract as antidiabetic treatment. Int. J. Appl. Pharm. 2020, 7, 138–142. [Google Scholar] [CrossRef]
- Gundogdu, G.; Nalci, K.A.; Ugur Kaplan, A.B.; Gundogdu, K.; Demirci, T.; Demirkaya Miloglu, F.; Hacımuftuoglu, A.; Cetin, M. The evaluation of the effects of nanoemulsion formulations containing boron and/or zinc on the wound healing in diabetic rats. Int. J. Low. Extrem. Wounds 2020, 1534734620961892, Epub ahead of print. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Lari, A.S.; Zahedi, P.; Ghourchian, H.; Khatibi, A. Microfluidic-based synthesized carboxymethyl chitosan nanoparticles containing metformin for diabetes therapy: In vitro and in vivo assessments. Carbohydr. Polym. 2021, 261, 117889. [Google Scholar] [CrossRef] [PubMed]
- Hadiya, S.; Radwan, R.; Zakaria, M.; El-Sherif, T.; Hamad, M.A.; Elsabahy, M. Nanoparticles integrating natural and synthetic polymers for in vivo insulin delivery. Pharm. Dev. Technol. 2021, 26, 30–40. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Correa, V.L.R.; Silva, F.K.L.; da Casas, A.A.; Chagas, A.D.L.D.; de Oliveira, L.P.; Miguel, M.P.; Diniz, D.G.A.; Amaral, A.C.; de Menezes, L.B. Wound healing treatment using insulin within polymeric nanoparticles in the diabetes animal model. Eur. J. Pharm. Sci. 2020, 150, 105330. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Anchan, R.B.; Koland, M. Oral insulin delivery by chitosan coated solid lipid nanoparticles: Ex vivo and in vivo studies. J. Young Pharm. 2021, 13, 43–48. [Google Scholar] [CrossRef]
- Muntoni, E.; Anfossi, L.; Milla, P.; Marini, E.; Ferraris, C.; Capucchio, M.T.; Colombino, E.; Segale, L.; Porta, M.; Battaglia, L. Glargine insulin loaded lipid nanoparticles: Oral delivery of liquid and solid oral dosage forms. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Oroojan, A.A.; Ahangarpour, A.; Paknejad, B.; Zareian, P.; Hami, Z.; Abtahi, S.R. Effects of Myricitrin and solid lipid nanoparticle-containing Myricitrin on reproductive system disorders induced by diabetes in male mouse. World J. Mens. Health 2021, 39, 147–157. [Google Scholar] [CrossRef]
- Mishra, V.; Yadav, N.; Saraogi, G.K.; Tambuwala, M.M.; Giri, N. Dendrimer based nanoarchitectures in diabetes management: An overview. Curr. Pharm. Des. 2019, 25, 2569–2583. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Q. Encapsulation of Astragaloside with matrix metalloproteinase-2-responsive hyaluronic acid end-conjugated polyamidoamine dendrimers improves wound healing in diabetes. J. Biomed. Nanotechnol. 2020, 16, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Chandrasekhar, B.; Yousif, M.H.; Renno, W.; Benter, I.F.; El-Hashim, A.Z. Chronic administration of nano-sized PAMAM Dendrimers in vivo inhibits EGFR-ERK1/2-ROCK signaling pathway and attenuates diabetes-induced vascular remodeling and dysfunction. Nanomedicine 2019, 18, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; An, S.; Choi, S.; Nam, K.; Jung, H.S.; Yoon, C.S.; Ko, J.H.; Jun, H.J.; Kim, T.K.; Jung, S.J.; et al. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J. Gene Med. 2012, 14, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Labieniec-Watala, M.; Przygodzki, T.; Sebekova, K.; Watala, C. Can metabolic impairments in experimental diabetes be cured with poly (amido) amine (PAMAM) G4 dendrimers?–In the search for minimizing of the adverse effects of PAMAM administration. Int. J. Pharm. 2014, 464, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Hussain, S.; Butt, F.K.; Jianguo, X.; Zhu, C. Functionalization of carbon nanotubes by a facile chemical method and its application in anti-diabetic activity. J. Nanosci. Nanotechnol. 2017, 17, 8557–8561. [Google Scholar] [CrossRef]
- Awan, T.; Naikoo, G.A.; Salim, H.; Arshad, F.; Hassan, I.U.; Pedram, M.Z.; Ahmed, W.; Faruck, H.L.; Aljabali, A.A.; Mishra, V.; et al. Fourth generation glucose sensors composed of copper nanostructures for diabetes management: A critical review. Bioeng. Transl. Med. 2021, 6, e10248. [Google Scholar]
- Alomari, G.; Al-Trad, B.; Hamdan, S.; Aljabali, A.A.; Al Zoubi, M.S.; Al-Batanyeh, K.; Qar, J.; Eaton, G.J.; Alkaraki, A.K.; Alshaer, W.; et al. Alleviation of diabetic nephropathy by zinc oxide nanoparticles in streptozotocin-induced type 1 diabetes in rats. IET Nanobiotechnol. 2021, 15, 473–483. [Google Scholar] [CrossRef]
- Alomari, G.; Al-Trad, B.; Hamdan, S.; Aljabali, A.; Al-Zoubi, M.; Bataineh, N.; Qar, J.; Tambuwala, M.M. Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Deliv. Transl. Res. 2020, 10, 216–226. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; Tambuwala, M.M.; Mitchell, C.A.; Osman, M.A.; El-Gizawy, S.A.; Faheem, A.M.; El-Tanani, M.; McCarron, P.A. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly (vinyl alcohol)-borate hydrogels. Drug Deliv. Transl. Res. 2018, 8, 1053–1065. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Faheem, A.M.; Tambuwala, M.M.; Osman, M.A.; El-Gizawy, S.A.; O’Hagan, B.; Irwin, N.; McCarron, P.A. Effect of poly(ethylene glycol) content and formulation parameters on particulate properties and intraperitoneal delivery of insulin from PLGA nanoparticles prepared using the double-emulsion evaporation procedure. Pharm. Dev. Technol. 2017, 23, 370–381. [Google Scholar] [CrossRef]
- Bose, S.; Sharma, P.; Mishra, V.; Patial, S.; Saraogi, G.K.; Tambuwala, M.M.; Dua, K. Comparative in vitro evaluation of glimepiride containing nanosuspension drug delivery system developed by different techniques. J. Mol. Struct. 2021, 1231, 129927. [Google Scholar] [CrossRef]
- Peters, C.J.; Hindmarsh, P.C.; Thompson, R.J. Insulin pump therapy. Paediatr Child Health 2017, 27, 160–165. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Shah, K.U.; Khan, T.M.; Rehman, A.U.; Rashid, H.U.; Mahmood, S.; Khan, S.; Farrukh, M.J. Evaluation of current trends and recent development in insulin therapy for management of diabetes mellitus. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), S833–S839. [Google Scholar] [CrossRef]
- Sadrzadeh, N.; Glembourtt, M.J.; Stevenson, C.L. Peptide drug delivery strategies for the treatment of diabetes. J. Pharm. Sci. 2007, 96, 1925–1954. [Google Scholar] [CrossRef] [PubMed]
- Nimri, R.; Nir, J.; Phillip, M. Insulin pump therapy. Am. J. Ther. 2020, 27, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Berget, C.; Lange, S.; Messer, L.; Forlenza, G.P. A clinical review of the t: Slim X2 insulin pump. Expert Opin. Drug Deliv. 2020, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Somali, M.; Paschou, S.A.; Mouslech, Z. Insulin pumps use in Greece: Efficacy and safety data from 140 patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 160, 108026. [Google Scholar] [CrossRef]
- Senstius, J.; Harboe, E.; Westermann, H. The in-vitro stability of insulin aspart is unaffected when stored for up to seven days in user-filled reservoirs of continuous subcutaneous insulin infusion pumps. Diabetes 2005, 54, A101. [Google Scholar]
- Bode, B.W.; Tamborlane, W.V.; Davidson, P.C. Insulin pump therapy in the 21st century. Strategies for successful use in adults, adolescents, and children with diabetes. Postgrad. Med. 2002, 111, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, H.E.; Pretorius, S.G.; Wessels, D.H.; Becker, R.H.A. Pharmacokinetic and Glucodynamic variability: Assessment of Insulin Glargine, NPH Insulin and Insulin ultralente in healthy volunteers using a Euglycaemic Clamp Technique. Diabetologia 2005, 48, 1988–1995. [Google Scholar] [CrossRef]
- DeVries, J.H.; Heine, R.J. Insulin pump therapy: A meta-analysis: Response to Weissberg-Benchell et al. Diabetes Care 2003, 26, 2485. [Google Scholar] [CrossRef]
- Raskin, P.; Bode, B.W.; Marks, J.B.; Hirsch, I.B.; Weinstein, R.L.; McGill, J.B.; Peterson, G.E.; Mudaliar, S.R.; Reinhardt, R.R. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: A randomized, parallel-group, 24-week study. Diabetes Care 2003, 26, 2598–2603. [Google Scholar] [CrossRef]
- Testa, M.; Hayes, J.; Turner, R.; Simonson, D. Patient acceptance and satisfaction with intensive insulin therapy in type 2 diabetes: A randomized trial of the insulin pen vs. pump. Diabetes 2001, 50, A428. [Google Scholar]
- Buchwald, H.; Rohde, T.D.; Dorman, F.D.; Skakoon, J.G.; Wigness, B.D.; Prosl, F.R.; Tucker, E.M.; Rublein, T.G.; Blackshear, P.J.; Varco, R.L. A Totally implantable drug infusion defice: Laboratory and clinical experience using a model with single flow rate and new design for modulated insulin infusion. Diabetes Care 1980, 3, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Irsigler, K.; Kritz, H.; Hagmüller, G.; Franetzki, M.; Prestele, K.; Thurow, H.; Geisen, K. Long-term continuous intraperitoneal insulin infusion with an implanted remote-controlled insulin infusion device. Diabetes 1981, 30, 1072–1075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gin, H.; Renard, E.; Melki, V.; Boivin, S.; Schaepelynck-Bélicar, P.; Guerci, B.; Selam, J.L.; Brun, J.M.; Riveline, J.P.; Estour, B.; et al. Combined improvements in implantable pump technology and insulin stability allow safe and effective long term intraperitoneal insulin delivery in type 1 diabetic patients: The EVADIAC experience. Diabetes Metab. 2003, 29, 602–607. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Saudek, C.D.; Henry, R.R. Why intraperitoneal delivery of insulin with implantable pumps in NIDDM? Diabetes 1992, 41, 657–661. [Google Scholar] [CrossRef]
- Zoltobrocki, M. Insulin delivery by implantable pumps. Horm. Metab. Res. Suppl. Ser. 1992, 26, 140–145. [Google Scholar]
- Miller, K.M.; Foster, N.C.; Beck, R.W.; Bergenstal, R.M.; DuBose, S.N.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V. Current state of type 1 diabetes treatment in the US: Updated data from the T1D Exchange clinic registry. Diabetes Care 2015, 38, 971–978. [Google Scholar] [CrossRef]
- Brouwer, E.; Knol, R.; Vernooij, A.S.N.; van den Akker, T.; Vlasman, P.E.; Klumper, F.J.C.M.; DeKoninck, P.; Polglase, G.R.; Hooper, S.B.; Te Pas, A.B. Physiological-based cord clamping in preterm infants using a new purpose-built resuscitation table: A feasibility study. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F396–F402. [Google Scholar] [CrossRef]
- Blackman, S.M.; Raghinaru, D.; Adi, S.; Simmons, J.H.; Ebner-Lyon, L.; Chase, H.P.; Tamborlane, W.V.; Schatz, D.A.; Block, J.M.; Litton, J.C.; et al. Insulin pump use in young children in the T1D exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr. Diabetes 2014, 15, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Willi, S.M.; Miller, K.M.; DiMeglio, L.A.; Klingensmith, G.J.; Simmons, J.H.; Tamborlane, W.V.; Nadeau, K.J.; Kittelsrud, J.M.; Huckfeldt, P.; Beck, R.W.; et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015, 135, 424–434. [Google Scholar] [CrossRef]
- O’Connor, M.R.; Carlin, K.; Coker, T.; Zierler, B.; Pihoker, C. Disparities in Insulin pump therapy persist in youth with type 1 diabetes despite rising overall pump use rates. J. Pediatr. Nurs. 2019, 44, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ohashi, K.; Utoh, R.; Shimizu, H.; Ise, K.; Suzuki, H.; Yamato, M.; Okano, T.; Gotoh, M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation 2011, 92, 1231–1236. [Google Scholar] [CrossRef]
- Rajab, A. Islet transplantation: Alternative sites. Curr. Diab. Rep. 2010, 10, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, A.; Molano, R.D.; Ricordi, C.; Zahr, E.; Collins, J.; Valdes, R.; Inverardi, L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 2006, 81, 1318–1324. [Google Scholar] [CrossRef]
- Fujita, I.; Utoh, R.; Yamamoto, M.; Okano, T.; Yamato, M. The liver surface as a favorable site for islet cell sheet transplantation in type 1 diabetes model mice. Regen. Ther. 2018, 8, 65–72. [Google Scholar] [CrossRef]
- Kemp, C.B.; Knight, M.J.; Scharp, D.W.; Ballinger, W.F.; Lacy, P.E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia 1973, 9, 486–491. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Pepper, A.; Shapiro, A.J. Islet cell transplantation. In Principles of Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 987–1007. [Google Scholar] [CrossRef]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.T.; Shapiro, A.M.J. Five-Year Follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Owen, R.J.T.; Ryan, E.A.; O’Kelly, K.; Lakey, J.R.T.; McCarthy, M.C.; Paty, B.W.; Bigam, D.L.; Kneteman, N.M.; Korbutt, G.S.; Rajotte, R.V.; et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: Radiologic aspects. Radiology 2003, 229, 165–170. [Google Scholar] [CrossRef]
- Carlsson, P.-O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Snarski, E.; Milczarczyk, A.; Hałaburda, K.; Torosian, T.; Paluszewska, M.; Urbanowska, E.; Król, M.; Boguradzki, P.; Jedynasty, K.; Franek, E.; et al. Immunoablation and Autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: Long-term observations. Bone Marrow Transplant. 2016, 51, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Luu, Q.F.; Villareal, C.J.; Fritschi, C.; Monson, R.S.; Oberholzer, J.; Danielson, K.K. Concerns and hopes of patients with type 1 diabetes prior to islet cell transplantation: A content analysis. J. Diabetes Complicat. 2018, 32, 677–681. [Google Scholar] [CrossRef] [PubMed]
- The Miracle of an Artificial Pancreas. NIH Medlineplus Megazine. Trusted Health Information from the National Institutes of Health. Available online: https://medlineplus.gov/magazine/issues/spring17/articles/spring17pg15-16.html (accessed on 13 July 2021).
- FDA’s Efforts to Advance Artificial Pancreas Device Systems, The Artificial Pancreas Device System. U.S. Food & Drug Administration. Available online: https://www.fda.gov/medicaldevices/productsandmedicalprocedures/homehealthandconsumer/consumerproducts/artificialpancreas/default.htm (accessed on 13 July 2021).
- Oukes, T.; Blauw, H.; van Bon, A.C.; DeVries, J.H.; von Raesfeld, A.M. Acceptance of the artificial pancreas: Comparing the effect of technology readiness, product characteristics, and social influence between invited and self-selected respondents. J. Diabetes Sci. Technol. 2019, 13, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Douglas, H.; Harrison. Plastic and Reconstructive Surgery: Approaches and Techniques; Farhadieh, R., Bulstrode, N., Cugno, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; p. 1777. [Google Scholar]
- Svendsen, B.; Holst, J.J. Paracrine Regulation of Somatostatin Secretion by Insulin and Glucagon in Mouse Pancreatic Islets. Diabetologia 2021, 64, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Rice, M.A.; Dodson, B.T.; Arthur, J.A.; Anseth, K.S. Cell-based therapies and tissue engineering. Otolaryngol. Clin. N. Am. 2005, 38, 199–214. [Google Scholar] [CrossRef]
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S.; Mozafari, M.; Samadikuchaksaraei, A.; Hajibaki, L.; Gholipour, M. Effect of silver concentration on bioactivity and antibacterial properties of SiO2-CaO-P2O5 sol-gel derived bioactive glass. In Key Engineering Materials; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 2012; pp. 74–79. [Google Scholar] [CrossRef]
- Mobini, S.; Solati-Hashjin, M.; Peirovi, H.; Osman, N.A.; Gholipourmalekabadi, M.; Barati, M.; Samadikuchaksaraei, A. Bioactivity and biocompatibility studies on silk-based scaffold for bone tissue engineering. J. Med. Biol. Eng. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Mozafari, M.; Bandehpour, M.; Salehi, M.; Sameni, M.; Caicedo, H.H.; Mehdipour, A.; Hamidabadi, H.G.; Samadikuchaksaraei, A.; Ghanbarian, H. Optimization of nanofibrous silk fibroin scaffold as a delivery system for bone marrow adherent cells: In vitro and in vivo studies. Biotechnol. Appl. Biochem. 2015, 62, 785–794. [Google Scholar] [CrossRef]
- Supartono, B. Tissue Engineering Therapy for Unhealed Diabetic Wound Using Mononuclear Stem Cells, Plasma Rich Platelets and Collagen. Biomed. J. Sci. Tech. Res. 2018, 10, 7838–7841. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Agarwal, D.; Korutla, L.; May, C.L.; Wang, W.; Griffith, N.N.; Hering, B.J.; Kaestner, K.H.; Velazquez, O.C.; Markmann, J.F.; et al. Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat. Metab. 2020, 2, 1013–1020. [Google Scholar] [CrossRef]
- Bell, G.I.; Pilkis, S.J.; Weber, I.T.; Polonsky, K.S. Glucokinase mutations, insulin secretion, and diabetes mellitus. Annu. Rev. Physiol. 1996, 58, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Deng, S.; Huang, X.; Velidedeoglu, E.; Bae, Y.-S.; Liu, C.; Abt, P.; Stephenson, R.; Mohiuddin, M.; Thambipillai, T.; et al. Transplantation for type I diabetes: Comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann. Surg. 2004, 240, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Canelo, R. Liver and Pancreatic Diseases Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Gruessner, A.C.; Gruessner, R.W.G. Pancreas transplant outcomes for united states and non united states cases as reported to the united network for organ sharing and the international pancreas transplant registry as of December 2011. Clin. Transpl. 2012, 23–40. [Google Scholar]
- Najdahmadi, A. Tissue Engineering and Biosensing, Towards Cure and Control of Diabetes; University of California: Irvine, CA, USA, 2018. [Google Scholar]
- Hasse, J.M.; Weseman, R.A.; Marsha Stieber, M.S.; Keithley, J.K.; August, D.A.; Thorn, D. Part VI: Performance outcome indicators 29 solid organ transplantation. In The Science and Practice of Nutrition Support: A Case-Based Core Curriculum; American Society for Parentral and Enteral Nutrition: Silver Spring, MD, USA, 2001. [Google Scholar]
- Hopt, U.T.; Drognitz, O. Pancreas organ transplantation. Langenbeck’s Arch. Surg. 2000, 385, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Paya, C.V. Fungal infections in solid-organ transplantation. Clin. Infect. Dis. 1993, 16, 677–688. [Google Scholar] [CrossRef]
- Couchoud, C.; Cucherat, M.; Haugh, M.; Pouteil-Noble, C. Cytomegalovirus prophylaxis with antiviral agents in solid organ transplantation. Transplantation 1998, 65, 641–647. [Google Scholar] [CrossRef]
- Snydman, D. Infection in solid organ transplantation. Transpl. Infect. Dis. 1999, 1, 21–28. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Åsberg, A.; Chou, S.; Snydman, D.R.; Allen, U.; Humar, A. Transplantation society international CMV consensus group. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010, 89, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hui, H.; Umehara, Y.; LiCalzi, S.; Demetriou, A.A.; Rozga, J.; Perfettit, R. Intrasplenic transplantation of encapsulated genetically engineered mouse insulinoma cells reverses streptozotocin-induced diabetes in rats. Cell Transplant. 2005, 14, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Alismail, H.; Jin, S. Microenvironmental stimuli for proliferation of functional islet β-cells. Cell Biosci. 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Parke, H.G.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. The Role of Glucagon- and Somatostatin-secreting cells in the regulation of insulin release and beta-cell function in heterotypic pseudoislets. Diabetes Metab. Res. Rev. 2010, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.D.; Mahoney, M.J.; Bryant, S.J. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng. Part B Reviews. 2014, 20, 455–467. [Google Scholar] [CrossRef]
- Bernard, A.B.; Lin, C.-C.; Anseth, K.S. A microwell cell culture platform for the aggregation of pancreatic β-cells. Tissue Eng. Part C Methods 2012, 18, 583–592. [Google Scholar] [CrossRef]
- Weber, L.M.; Cheung, C.Y.; Anseth, K.S. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2007, 16, 1049–1057. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Haskins, K.; Anseth, K.S. The Effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 2007, 28, 3004–3011. [Google Scholar] [CrossRef]
- Baidal, D.A.; Ricordi, C.; Berman, D.M.; Alvarez, A.; Padilla, N.; Ciancio, G.; Linetsky, E.; Pileggi, A.; Alejandro, R. Bioengineering of an Intraabdominal Endocrine Pancreas. N. Engl. J. Med. 2017, 376, 1887–1889. [Google Scholar] [CrossRef]
- Berman, D.M.; Molano, R.D.; Fotino, C.; Ulissi, U.; Gimeno, J.; Mendez, A.J.; Kenyon, N.M.; Kenyon, N.S.; Andrews, D.M.; Ricordi, C.; et al. Bioengineering the Endocrine Pancreas: Intraomental Islet Transplantation within a Biologic Resorbable Scaffold. Diabetes 2016, 65, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. Cell–cell communication mimicry with poly (ethylene glycol) hydrogels for enhancing β-cell function. Proc. Natl. Acad. Sci. USA 2011, 108, 6380–6385. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J. The diabetic foot. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Tudurí, E.; Bruin, J.E.; Kieffer, T.J. Restoring insulin production for type 1 diabetes. J. Diabetes 2012, 4, 319–331. [Google Scholar] [CrossRef]
- Jaén, M.L.; Vilà, L.; Elias, I.; Jimenez, V.; Rodó, J.; Maggioni, L.; Ruiz-de Gopegui, R.; Garcia, M.; Muñoz, S.; Callejas, D.; et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol. Ther. Methods Clin. Dev. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Romer, A.I.; Sussel, L. Pancreatic islet cell development and regeneration. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 255–264. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Lam, K.S.L.; Tam, S.; Xiao, W.; Xu, R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J. Biomed. Sci. 2008, 15, 487–497. [Google Scholar] [CrossRef]
- Schwitzgebel, V.M.; Scheel, D.W.; Conners, J.R.; Kalamaras, J.; Lee, J.E.; Anderson, D.J.; Sussel, L.; Johnson, J.D.; German, M.S. Expression of Neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000, 127, 3533–3542. [Google Scholar] [CrossRef]
- Abed, A.; Critchlow, C.; Flatt, P.R.; McClenaghan, N.H.; Kelly, C. Directed differentiation of progenitor cells towards an islet-cell phenotype. Am. J. Stem Cells 2012, 1, 196–204. [Google Scholar]
- Zhao, M.; Amiel, S.A.; Ajami, S.; Jiang, J.; Rela, M.; Heaton, N.; Huang, G.C. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE 2008, 3, e2666. [Google Scholar] [CrossRef]
- Dassaye, R.; Naidoo, S.; Cerf, M.E. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets 2016, 8, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.L.; Anderson, K.R.; Papizan, J.B.; Sussel, L. Regulation of Neurod1 Contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas. PLoS Genet. 2013, 9, e1003278. [Google Scholar] [CrossRef]
- Handorf, A.M.; Sollinger, H.W.; Alam, T. Genetic engineering of surrogate β cells for treatment of type 1 diabetes mellitus. J. Diabetes Mellit. 2015, 5, 295–312. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Jun, H.-S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol. Med. 2002, 8, 62–68. [Google Scholar] [CrossRef]
- Chen, N.K.F.; Wong, J.S.; Kee, I.H.C.; Lai, S.H.; Thng, C.H.; Ng, W.H.; Ng, R.T.H.; Tan, S.Y.; Lee, S.Y.; Tan, M.E.H.; et al. Nonvirally modified autologous primary hepatocytes correct diabetes and prevent target organ injury in a large preclinical model. PLoS ONE 2008, 3, e1734. [Google Scholar] [CrossRef] [PubMed]
- Calne, R.Y.; Gan, S.U.; Lee, K.O. Stem cell and gene therapies for diabetes mellitus. Nat. Rev. Endocrinol. 2010, 6, 173–177. [Google Scholar] [CrossRef]
- Guney, M.A.; Gannon, M. Pancreas cell fate. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 232–248. [Google Scholar] [CrossRef]
- Medvedev, S.P.; Shevchenko, A.I.; Zakian, S.M. Induced pluripotent stem cells: Problems and advantages when applying them in regenerative medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, Y. Genetic and epigenetic variations in IPSCs: Potential causes and implications for application. Cell Stem Cell 2013, 13, 149–159. [Google Scholar] [CrossRef]
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int. 2012, 2012, 738910. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cohrs, C.M.; Stertmann, J.; Bozsak, R.; Speier, S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metab. 2017, 6, 943–957. [Google Scholar] [CrossRef]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gürtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef]
- Youssef, A.A.; Ross, E.G.; Bolli, R.; Pepine, C.J.; Leeper, N.J.; Yang, P.C. The promise and challenge of induced pluripotent stem cells for cardiovascular applications. JACC Basic Transl. Sci. 2016, 1, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, M.-K. β-Cell Regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas. J. Diabetes Investig. 2016, 7, 286–296. [Google Scholar] [CrossRef]
- Xiao, X.; Gittes, G.K. Concise Review: New insights into the role of macrophages in β-cell proliferation. Stem Cells Transl. Med. 2015, 4, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.M.; Sims, E.K.; Moss, D.R.; Yamamoto, W.; Ahn, G.; Diamond, J.; Tong, X.; Day, K.H.; Territo, P.R.; Hanenberg, H.; et al. Human adipose-derived stromal/stem cells protect against Stz-induced hyperglycemia: Analysis of HASC-derived paracrine effectors. Stem Cells 2014, 32, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.S.; Sudhakar, M.; Nair, P.D.; Bhonde, R.R. Reversal of Experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy 2010, 12, 982–991. [Google Scholar] [CrossRef]
- Zang, L.; Hao, H.; Liu, J.; Li, Y.; Han, W.; Mu, Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 36. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemotherapy 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Amer, M.G.; Embaby, A.S.; Karam, R.A.; Amer, M.G. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene 2018, 654, 87–94. [Google Scholar] [CrossRef]
- Hu, J.; Fu, Z.; Chen, Y.; Tang, N.; Wang, L.; Wang, F.; Sun, R.; Yan, S. Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocr. J. 2015, 62, 339–352. [Google Scholar] [CrossRef]
- Nam, J.S.; Kang, H.M.; Kim, J.; Park, S.; Kim, H.; Ahn, C.W.; Park, J.O.; Kim, K.R. Transplantation of insulin-secreting cells differentiated from human adipose tissue-derived stem cells into type 2 diabetes mice. Biochem. Biophys. Res. Commun. 2014, 443, 775–781. [Google Scholar] [CrossRef]
- Xie, Q.-P.; Huang, H.; Xu, B.; Dong, X.; Gao, S.-L.; Zhang, B.; Wu, Y.-L. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation 2009, 77, 483–491. [Google Scholar] [CrossRef]
- Moriscot, C.; de Fraipont, F.; Richard, M.-J.; Marchand, M.; Savatier, P.; Bosco, D.; Favrot, M.; Benhamou, P.-Y. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells 2005, 23, 594–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, L.-F.; Wang, N.-N.; Liu, Y.-S.; Wei, X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Han, Z.; Zhuo, G.; Qu, X.; Li, X.; Wang, X.; Hao, Y.; Yang, S.; Han, Z.C. Transplantation of placenta derived mesenchymal stem cells in type 2 diabetes: A pilot study. Front Med. 2011, 5, 94–100. [Google Scholar] [CrossRef]
- Khurana, V.; Kwatra, D.; Shah, S.; Mandal, A.; Mitra, A.K. Emerging nanotechnology for stem cell therapy. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 85–103. [Google Scholar] [CrossRef]
| Nanocarriers | Composition | Disease | Advantageous Features/Findings | Reference |
|---|---|---|---|---|
| Polymeric NPs | Metformin (MET)-loaded polymeric NPs | Diabetes mellitus |
| [36] |
| Phyto-NPs | Selenium cleome droserifolia NPs | Diabetes mellitus |
| [37] |
| Zinc oxide NPs (ZnO NPs) | Costus igneus-loaded ZnO NPs | Diabetes mellitus |
| [38] |
| Dendrimer | G4 PAMAM | Diabetes mellitus |
| [39] |
| Solid lipid nanoparticles (SLN) | Valsartan (Val)-loaded SLN (Val-SLN) | Diabetic foot ulcer |
| [40] |
| Bovine serum albumin NPs (BSA-NPs) | Apatinib-loaded BSA-NPs coated hyaluronic acid (HA) (Apa-HA-BSA-NPs) | Diabetic retinopathy (DR) |
| [41] |
| Gold NPs (AuNPs) | Streptozotocin (STZ) -loaded AuNPs | Diabetic cardiomyopathy |
| [42] |
| Chitosan NPs (CSNPs) | Polydatin-loaded CSNPs (PD-CSNPs) | Diabetic cardiomyopathy |
| [43] |
| Liposome | Fibroblast growth factor 1 (FGF1) loaded liposome | Diabetic cardiomyopathy |
| [44] |
| Liposome | Calycosin-loaded nanoliposomes | Diabetic nephropathy |
| [45] |
| Poly(lactic-co-glycolic acid) (PLGA) NPs | Crocetin-loaded PLGA-NPs | Diabetic nephropathy |
| [46] |
| Bilosomes | Eprosartan mesylate loaded-bilosomes | Diabetic nephropathy |
| [47] |
| Silver NPs (AgNPs) | Allium cepa extract-loaded AgNPs | Diabetic neuropathy |
| [48] |
| Transferosomes | Pioglitazone and eprosartan mesylate-loaded nano-transferosomes | Coexisted hypertension with Type 2 diabetes |
| [49] |
| Exosome | RNA interference (RNAi)-based exosome | Diabetic chronic wound |
| [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, V.; Nayak, P.; Sharma, M.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A.; Alsowayeh, N.; Tambuwala, M.M. Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update. Pharmaceutics 2021, 13, 1568. https://doi.org/10.3390/pharmaceutics13101568
Mishra V, Nayak P, Sharma M, Albutti A, Alwashmi ASS, Aljasir MA, Alsowayeh N, Tambuwala MM. Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update. Pharmaceutics. 2021; 13(10):1568. https://doi.org/10.3390/pharmaceutics13101568
Chicago/Turabian StyleMishra, Vijay, Pallavi Nayak, Mayank Sharma, Aqel Albutti, Ameen S. S. Alwashmi, Mohammad Abdullah Aljasir, Noorah Alsowayeh, and Murtaza M. Tambuwala. 2021. "Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update" Pharmaceutics 13, no. 10: 1568. https://doi.org/10.3390/pharmaceutics13101568
APA StyleMishra, V., Nayak, P., Sharma, M., Albutti, A., Alwashmi, A. S. S., Aljasir, M. A., Alsowayeh, N., & Tambuwala, M. M. (2021). Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update. Pharmaceutics, 13(10), 1568. https://doi.org/10.3390/pharmaceutics13101568








