A Pectin-Rich, Baobab Fruit Pulp Powder Exerts Prebiotic Potential on the Human Gut Microbiome In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Products
2.2. Dialysis of Test Product
2.3. Short-Term Colonic Batch Incubations
2.4. Microbial Metabolic Activity
2.5. Quantification of Specific Taxonomic Groups
2.6. Statistics
3. Results
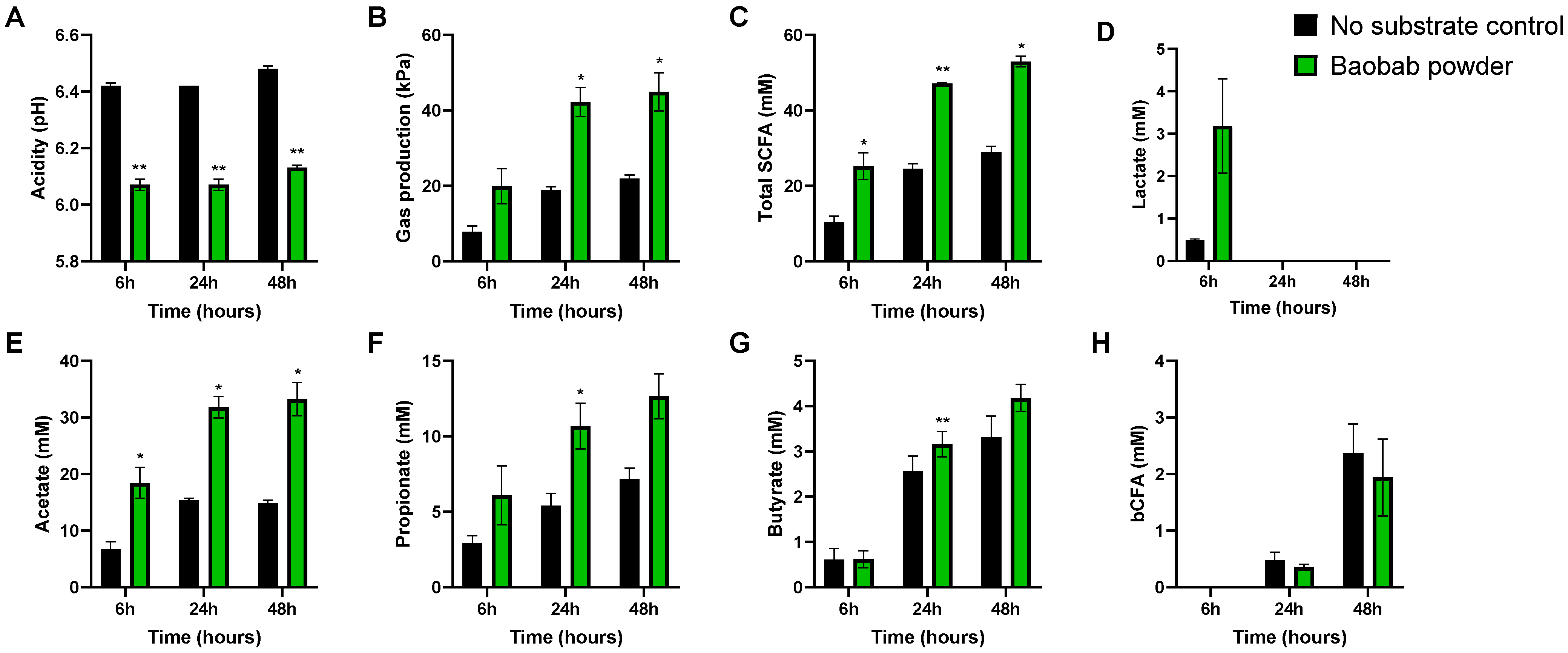
3.1. Baobab Fruit Pulp Powder Stimulated Microbial Metabolic Activity from 0–24 h with Some Interindividual Differences
3.2. Baobab Fruit Pulp Powder Stimulated Acetate, Propionate, Lactate, and Butyrate Production
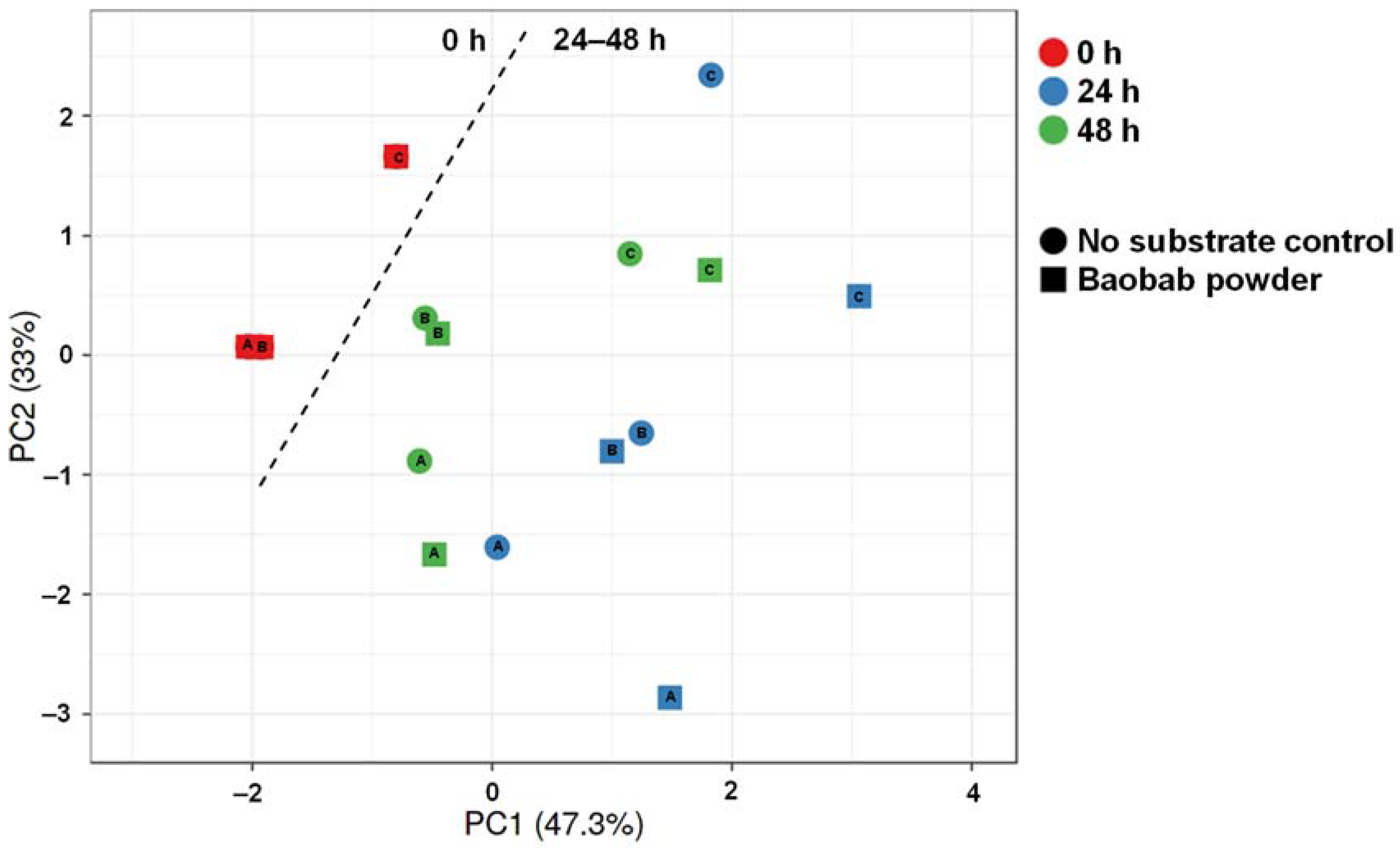
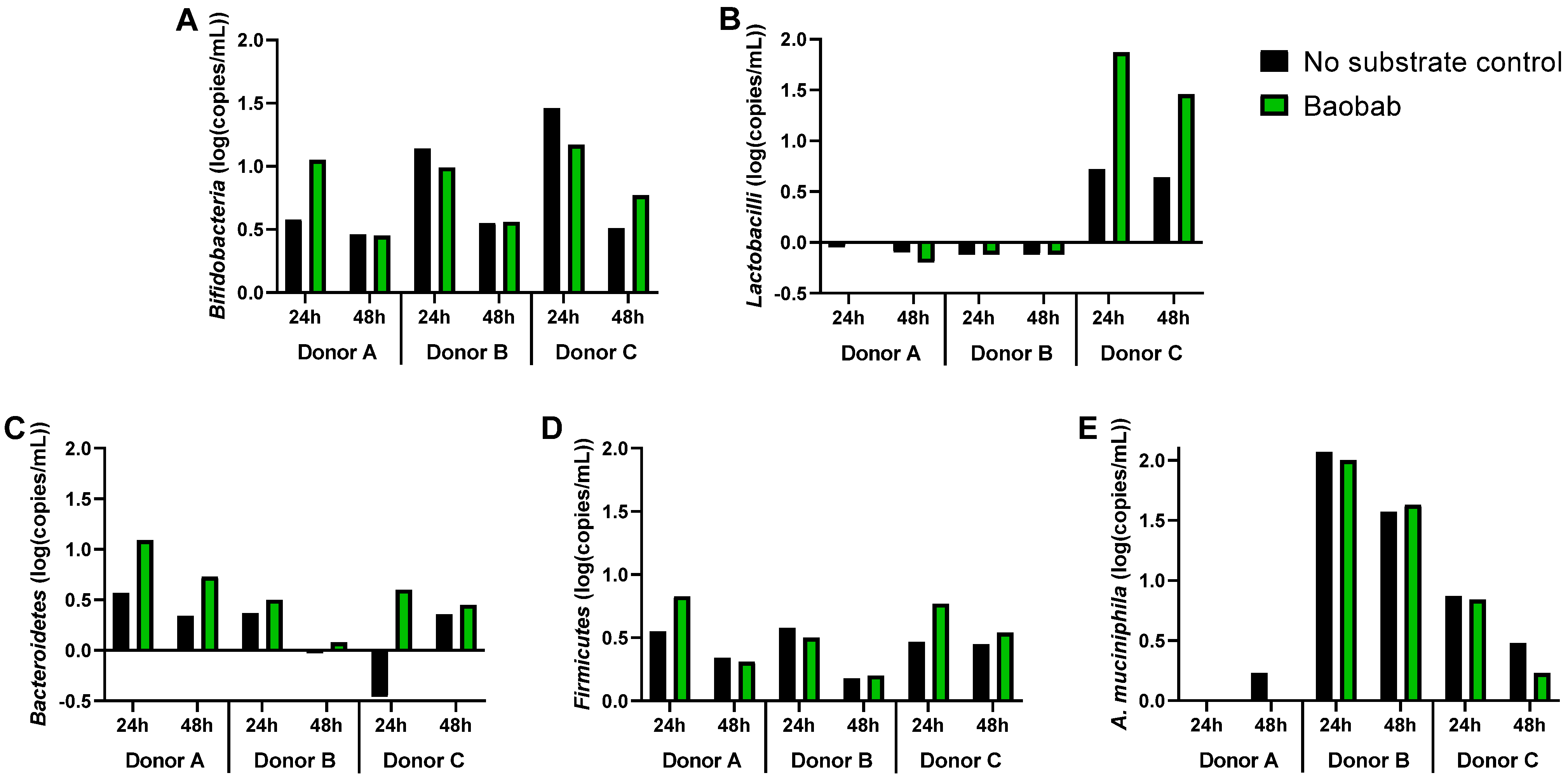
3.3. Baobab Fruit Pulp Powder Altered the Abundance of Specific Members of the Microbial Community in a Donor-Dependent Fashion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robles Alonso, V.; Guarner, F. Linking the Gut Microbiota to Human Health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Xiao, H. Whole Food–Based Approaches to Modulating Gut Microbiota and Associated Diseases. Annu. Rev. Food Sci. Technol. 2020, 11, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual Variability in Gut Microbiota and Host Response to Dietary Interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef] [Green Version]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of Diet and Individual Variation on Intestinal Microbiota Composition and Fermentation Products in Obese Men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host Variables Confound Gut Microbiota Studies of Human Disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Neri-Numa, I.A.; Pastore, G.M. Novel Insights into Prebiotic Properties on Human Health: A Review. Food Res. Int. 2020, 131, 108973. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livesey, G. Tolerance of Low-Digestible Carbohydrates: A General View. Br. J. Nutr. 2001, 85, S7–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marteau, P.; Seksik, P. Tolerance of Probiotics and Prebiotics. J. Clin. Gastroenterol. 2004, 38, S67–S69. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stott, K.; et al. Dietary Pectic Glycans Are Degraded by Coordinated Enzyme Pathways in Human Colonic Bacteroides. Nat. Microbiol. 2018, 3, 210–219. [Google Scholar] [CrossRef]
- Muthai, K.U.; Karori, M.S.; Muchugi, A.; Indieka, A.S.; Dembele, C.; Mng’omba, S.; Jamnadass, R. Nutritional Variation in Baobab (Adansonia digitata L.) Fruit Pulp and Seeds Based on Africa Geographical Regions. Food Sci. Nutr. 2017, 5, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Garvey, R.; Clegg, M.; Coe, S. The Acute Effects of Baobab Fruit (Adansonia digitata) on Satiety in Healthy Adults. Nutr. Health 2017, 23, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Coe, S.A.; Clegg, M.; Armengol, M.; Ryan, L. The Polyphenol-Rich Baobab Fruit (Adansonia digitata L.) Reduces Starch Digestion and Glycemic Response in Humans. Nutr. Res. 2013, 33, 888–896. [Google Scholar] [CrossRef]
- Hernandez-Sanabria, E.; Vázquez-Castellanos, J.F.; Raes, J. In Vitro Ecology: A Discovery Engine for Microbiome Therapies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Duysburgh, C.; Ghyselinck, J.; Goltz, S.; Berezhnaya, Y.; Boileau, T.; De Blaiser, A.; Marzorati, M. Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium Animalis Subsp. Lactis BB-12 in the Human Gut Microbiome In Vitro. Appl. Sci. 2021, 11, 598. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Kamil, A.; Fleige, L.; Chung, Y.; De Chavez, P.; Marzorati, M. Different Oat Ingredients Stimulate Specific Microbial Metabolites in the Gut Microbiome of Three Human Individuals in Vitro. ACS Omega 2018, 3, 12446–12456. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Moens, F.; Pignataro, G.; Schnurr, J.; Ribecco, C.; Gramenzi, A.; Marzorati, M. Yeast-Derived Formulations Are Differentially Fermented by the Canine and Feline Microbiome As Assessed in a Novel In Vitro Colonic Fermentation Model. J. Agric. Food Chem. 2020, 68, 13102–13110. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (CRG-I) Selectively Modulates the Human Gut Microbiota While Promoting Gut Barrier Integrity: An Integrated in Vitro Approach. Nutrients 2020, 12, 1917. [Google Scholar] [CrossRef]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.W.; Boschker, H.T.S.; Verstraete, W.; Van de Wiele, T. Human Faecal Microbiota Display Variable Patterns of Glycerol Metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Furet, J.-P.; Firmesse, O.; Gourmelon, M.; Bridonneau, C.; Tap, J.; Mondot, S.; Doré, J.; Corthier, G. Comparative Assessment of Human and Farm Animal Faecal Microbiota Using Real-Time Quantitative PCR. FEMS Microbiol. Ecol. 2009, 68, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an Extensive Set of 16S RDNA-Targeted Primers for Quantification of Pathogenic and Indigenous Bacteria in Faecal Samples by Real-Time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia Muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a Real-Time PCR Method for Firmicutes and Bacteroidetes in Faeces and Its Application to Quantify Intestinal Population of Obese and Lean Pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Tingirikari, J.M.R. In-Vitro Prebiotic Analysis of Microbiota Accessible Pectic Polysaccharides. Curr. Microbiol. 2019, 76, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Nowak, A.; Libudzisz, Z. Influence of Phenol, p-Cresol and Indole on Growth and Survival of Intestinal Lactic Acid Bacteria. Anaerobe 2006, 12, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Kato, T. Formation of a Mutagenic Diazoquinone by Interaction of Phenol with Nitrite. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1988, 26, 209–214. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, E.; Manuel, G.A.S. Effect of Fruit Pectin on Growth of Lactic Acid Bacteria. J. Probiotics Health 2016, 4. [Google Scholar] [CrossRef]
- Afolabi, O.R.; Popoola, T.O.S. The Effects of Baobab Pulp Powder on the Micro Flora Involved in Tempe Fermentation. Eur. Food Res. Technol. 2005, 220, 187–190. [Google Scholar] [CrossRef]
- Ng, S.K.C.; Hamilton, I.R. Lactate Metabolism by Veillonella Parvula. J. Bacteriol. 1971, 105, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of PH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The Effects of Inulin on Gut Microbial Composition: A Systematic Review of Evidence from Human Studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
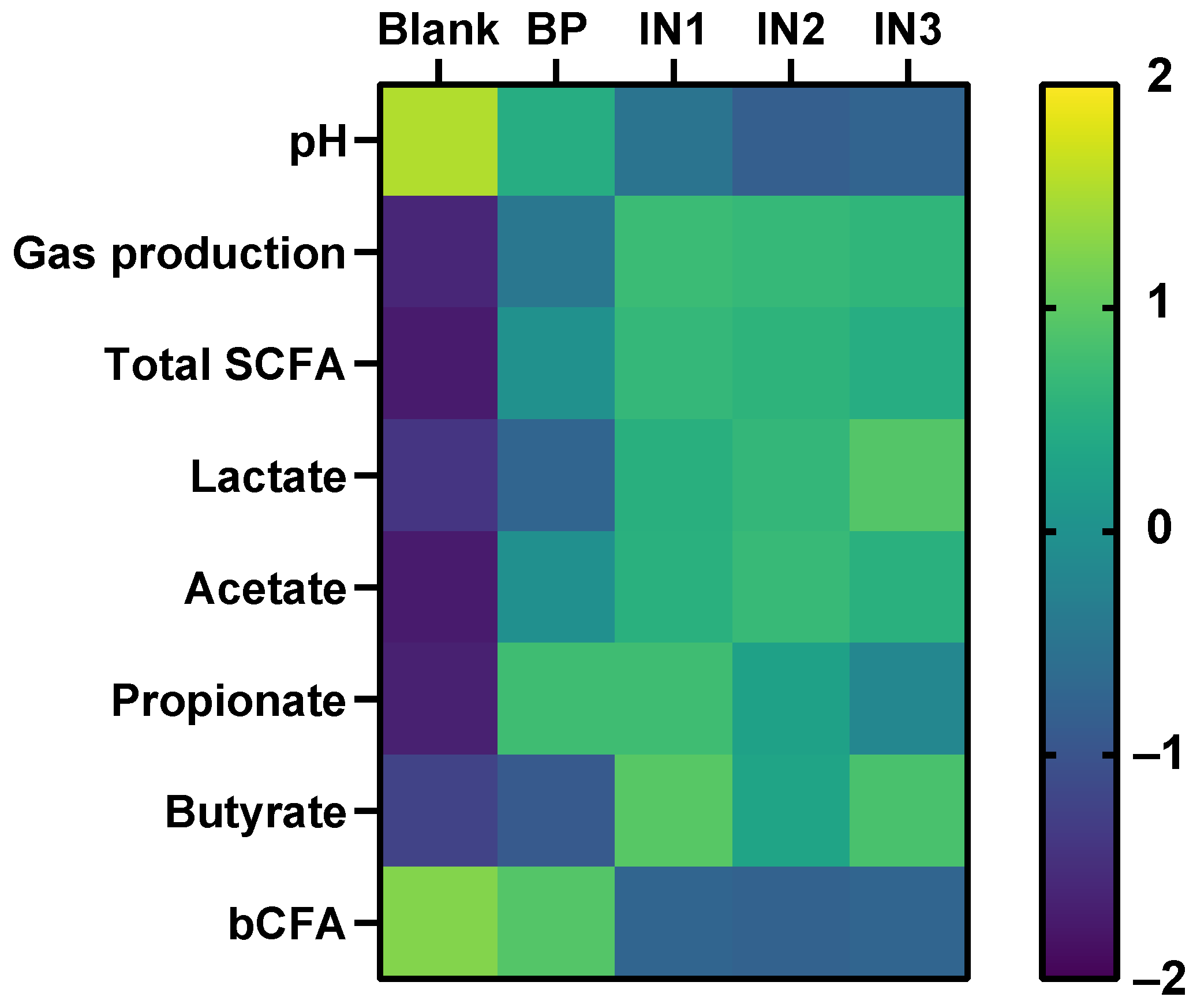
 ) and levels of specific taxonomic groups via qPCR (
) and levels of specific taxonomic groups via qPCR (  ) compared to ‘no substrate control’ incubations for three healthy adult donors. (B) Sampling scheme to evaluate the effect of the dialyzed baobab fruit pulp powder. SCFA = short-chain fatty acid, bCFA = branched-chain fatty acid, qPCR = quantitative polymerase chain reaction.
) compared to ‘no substrate control’ incubations for three healthy adult donors. (B) Sampling scheme to evaluate the effect of the dialyzed baobab fruit pulp powder. SCFA = short-chain fatty acid, bCFA = branched-chain fatty acid, qPCR = quantitative polymerase chain reaction.
 ) and levels of specific taxonomic groups via qPCR (
) and levels of specific taxonomic groups via qPCR (  ) compared to ‘no substrate control’ incubations for three healthy adult donors. (B) Sampling scheme to evaluate the effect of the dialyzed baobab fruit pulp powder. SCFA = short-chain fatty acid, bCFA = branched-chain fatty acid, qPCR = quantitative polymerase chain reaction.
) compared to ‘no substrate control’ incubations for three healthy adult donors. (B) Sampling scheme to evaluate the effect of the dialyzed baobab fruit pulp powder. SCFA = short-chain fatty acid, bCFA = branched-chain fatty acid, qPCR = quantitative polymerase chain reaction.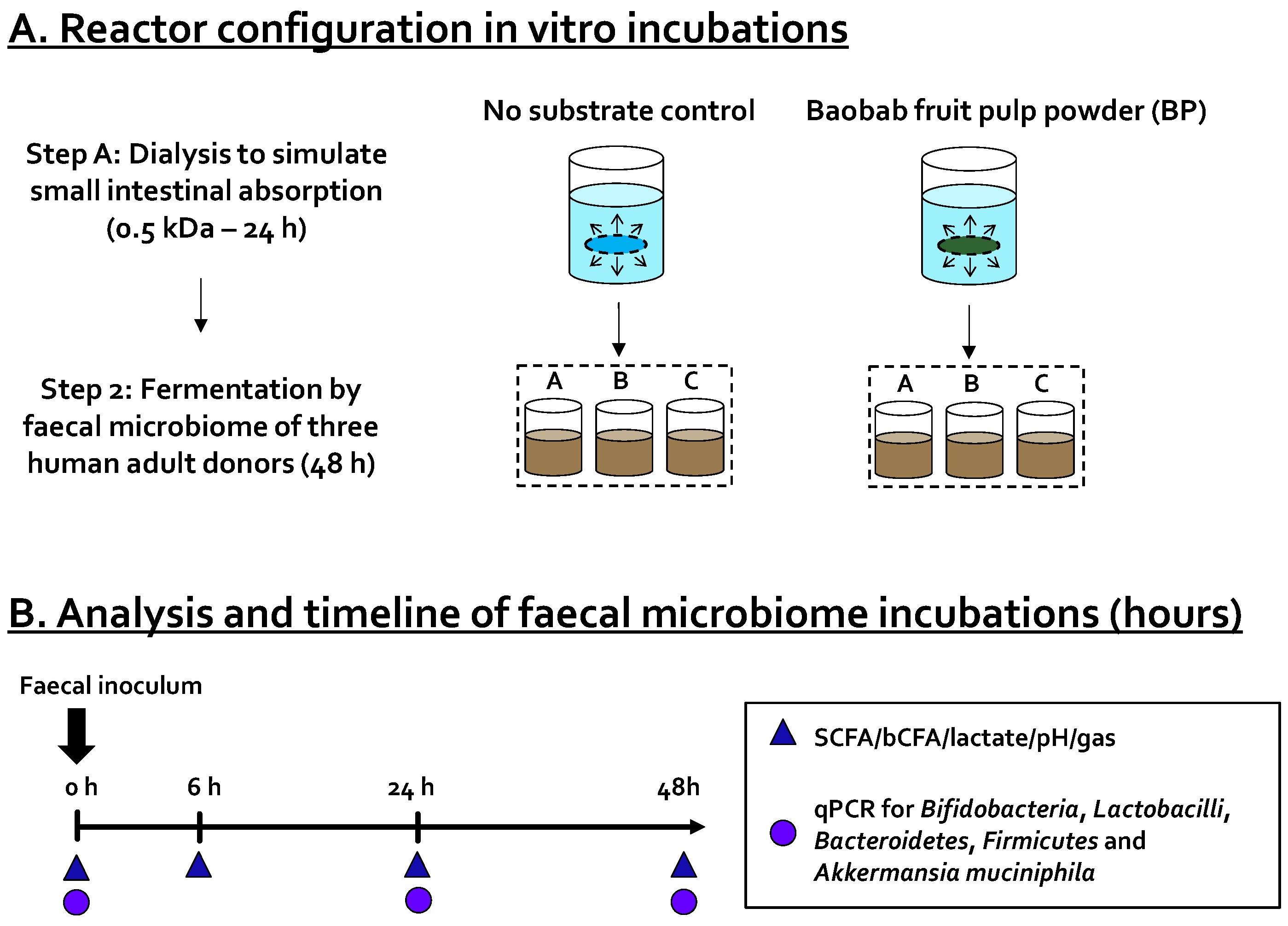


| Taxonomic Group | Primer Sequences 5′–3′ and 3′–5′ | Reference |
|---|---|---|
| Lactobacillus spp. | AGCAGTAGGGAATCTTCCA CGCCACTGGTGTTCYTCCATATA | [30] |
| Bifidobacterium spp. | TCGCGTCYGGTGTGAAAG CCACATCCAGCYTCCAC | [31] |
| Akkermansia muciniphila | CAGCACGTGAAGGTGGGGAC CCTTGCGGTTGGCTTCAGAT | [32] |
| Bacteroidetes | GGAACATGTGGTTTAATTCGATGAT AGCTGACGACAACCATGCAG | [33] |
| Firmicutes | GGAGYATGTGGTTTAATTCGAAGCA AGCTGACGACAACCATGCAC | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foltz, M.; Zahradnik, A.C.; Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M. A Pectin-Rich, Baobab Fruit Pulp Powder Exerts Prebiotic Potential on the Human Gut Microbiome In Vitro. Microorganisms 2021, 9, 1981. https://doi.org/10.3390/microorganisms9091981
Foltz M, Zahradnik AC, Van den Abbeele P, Ghyselinck J, Marzorati M. A Pectin-Rich, Baobab Fruit Pulp Powder Exerts Prebiotic Potential on the Human Gut Microbiome In Vitro. Microorganisms. 2021; 9(9):1981. https://doi.org/10.3390/microorganisms9091981
Chicago/Turabian StyleFoltz, Martin, Alicia Christin Zahradnik, Pieter Van den Abbeele, Jonas Ghyselinck, and Massimo Marzorati. 2021. "A Pectin-Rich, Baobab Fruit Pulp Powder Exerts Prebiotic Potential on the Human Gut Microbiome In Vitro" Microorganisms 9, no. 9: 1981. https://doi.org/10.3390/microorganisms9091981
APA StyleFoltz, M., Zahradnik, A. C., Van den Abbeele, P., Ghyselinck, J., & Marzorati, M. (2021). A Pectin-Rich, Baobab Fruit Pulp Powder Exerts Prebiotic Potential on the Human Gut Microbiome In Vitro. Microorganisms, 9(9), 1981. https://doi.org/10.3390/microorganisms9091981





