Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants
Abstract
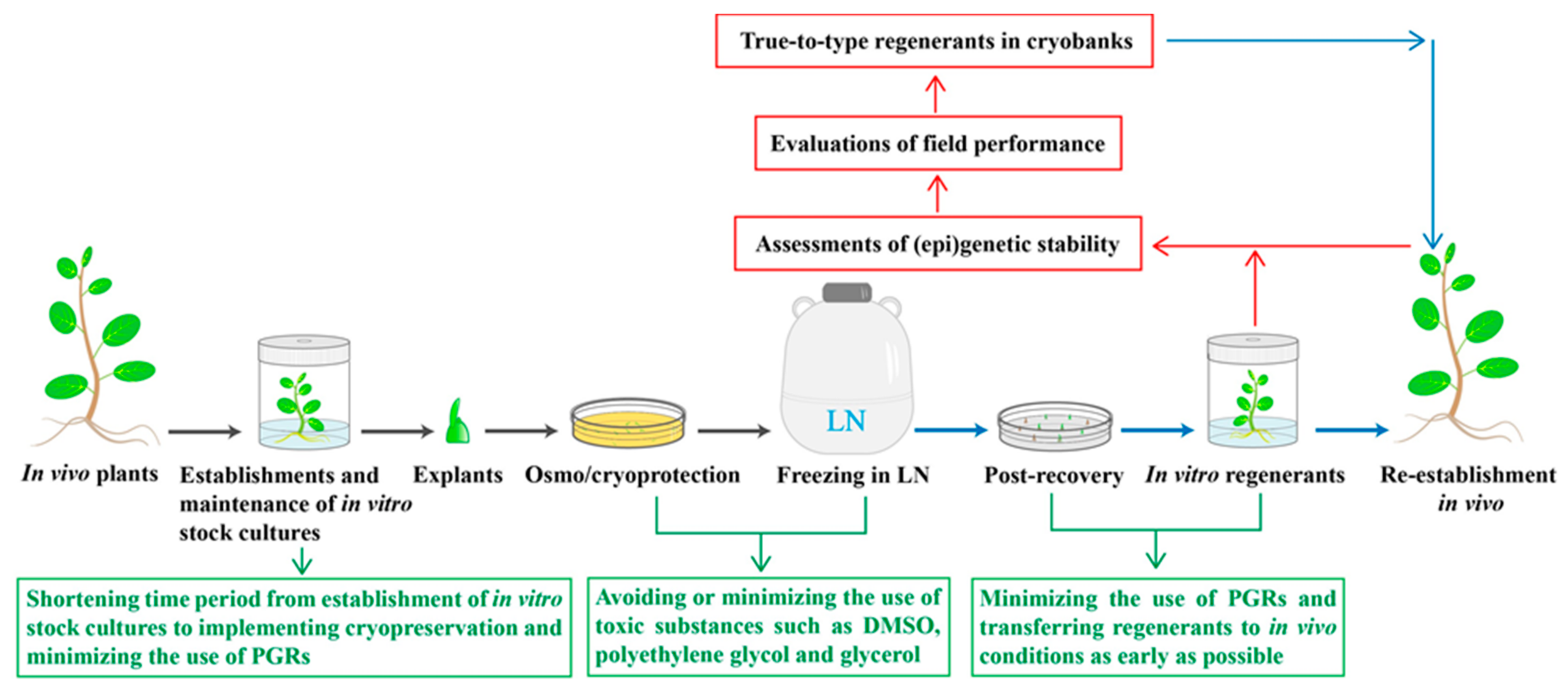
1. Overall Developments and Progresses in Plant Cryopreservation
2. Major Concerns in Recovery of Plants from Cryopreserved Tissues
3. Assessments of Epigenetic and Genetic Integrity
3.1. Epigenetic Integrity
| Plant Species | Explants | Cryopreservation Method * | Molecular Methods ** | DNA Methylation (%) | Causes | Reference |
|---|---|---|---|---|---|---|
| Actinidia chinensis var. deliciosa | Shoot tips | Drop-vitri | MSAP | 1.6 and 12.8 | Cryoprocedures and in vitro cultures | [71] |
| Bactris gasipaes | Somatic embryos | Drop-vitri | The global DNA methylation | 25.2–29.7 | Cryoprocedures | [72] |
| Carica papaya | Shoot tips | Vitri | AMP | 0–0.22 | Genotypes and cryoprocedures | [65] |
| Gentiana | Shoot tips | Encap-dehy | MSAP | 16.61–16.88 | in vitro culture | [75] |
| Mentha × piperita | Shoot tips | Encap-dehy | MSAP | 17.1–32 | Cryoprocedures | [70] |
| Quercus robur | Seed plumules | Desiccation | The global DNA methylation | 8.7–11 | Cryoprocedures | [73] |
| Solanum tuberosum | Shoot tips | DMSO droplet | MSAP | 0.9 | Cryoprocedures and in vitro cultures | [74] |
| Theobroma cacao | Somatic embryos | Encap-dehy | MSAP | 3.6 | Cryoprocedures | [66] |
| Wasabia japonica | Shoot tips | Vitri | MSAP | 0.12–5.5 | Cryoprocedures | [67] |
3.2. Genetic Integrity
4. Metabolic Stability
5. Field Performance
5.1. Seed Germination and Seedling Growth
5.2. Field Performance of Cryopreserved Plants
5.3. Reintroduction of Cryo-Derived Plants to Nature
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFLP | Amplified fragment length polymorphism |
| DMSO | Dimethyl sulfoxide |
| FCM | Flow cytometry |
| HPLC | High performance liquid chromatography |
| ISSR | Inter-simple sequence repeats |
| LN | Liquid nitrogen |
| MSAP | Methylation sensitive amplified polymorphism |
| RAPD | Random amplified polymorphic DNA |
| ROS | Reactive oxygen species |
| SSR | Single sequence repeats |
| SRAP | Sequence-related amplified polymorphism |
References
- Sakai, A. Survival of plant tissue of super-low temperature. Contrib. Inst. Temp. Sci. Haikkaido Univ. Ser. B 1956, 14, 17. [Google Scholar]
- Sakai, A. Survival of the twigs of woody plants at −196 °C. Nature 1960, 185, 392–394. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Org. Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.-C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Tanner, J.D.; Chen, K.Y.; Bonnart, R.M.; Minas, I.S.; Volk, G.M. Considerations for large-scale implementation of dormant budwood cryopreservation. Plant Cell Tissue Org. Cult. 2021, 144, 35–48. [Google Scholar] [CrossRef]
- Wang, M.-R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.-C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant resources. Plant Cell Tissue Org. Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Langis, R.; Schnabel-Preikstas, B.J.; Earle, E.D.; Steponkus, P.L. Cryopreservation of Brassica campestris L. suspensions by vitrification. CryoLetters 1989, 10, 421–428. [Google Scholar]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS-based vitrification and encapsulation-vitrification protocols. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 33–58. [Google Scholar]
- Uragami, M.; Sakai, A.; Nagai, M.; Takahashi, T. Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 1989, 8, 418–421. [Google Scholar] [CrossRef]
- Fabre, J.; Dereuddre, J. Encapsulation-dehydration, a new approach to cryopreservation of Solanum shoot tips. CryoLetters 1990, 11, 413–426. [Google Scholar]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Engelmann, F.; Arnao, M.T.; Wu, Y.; Escobar, R. Development of encapsulation dehydration. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 59–75. [Google Scholar]
- Niino, T.; Yamamoto, S.; Fukui, K.; Martínez, C.R.C.; Arizaga, M.V.; Matsumoto, T.; Engelmann, F. Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. CryoLetters 2013, 34, 549–560. [Google Scholar]
- Yamamoto, S.-I.; Rafque, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminum cryo-plates. CryoLetters 2011, 32, 256–265. [Google Scholar]
- Wang, M.-R.; Chen, L.; Teixeira da Silva, J.A.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Bi, W.-L.; Pan, C.; Hao, X.-Y.; Cui, Z.-H.; Kher, M.M.; Marković, Z.; Wang, Q.-C.; Teixeira da Silva, J.A. Cryopreservation of grapevine (Vitis spp.)—A review. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Bi, W.-L.; Hao, X.-Y.; Cui, Z.-H.; Volk, G.M.; Wang, Q.-C. Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (Vitis spp.). In Vitro Cell. Dev. Biol.-Plant 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Li, J.-W.; Zhang, X.-C.; Wang, M.-R.; Bi, W.-L.; Faisal, M.; Teixeira da Silva, J.A.; Volk, G.M.; Wang, Q.-C. Development, progress and future prospects in cryobiotechnology of Lilium spp. Plant Meth. 2019, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-H.; Yin, Z.-F.; Ma, Y.-L.; Chen, L.; Zhang, Z.-B.; Wang, B.; Li, B.-Q.; Huang, Y.-S.; Wang, Q.-C. Cryopreservation of sweetpotato (Ipomoea batatas) and its pathogen eradication by cryotherapy. Biotechnol. Adv. 2011, 29, 84–93. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Egúsquiza, V.; Espirilla, J.; García, M.; Torres, A.; Rojas, E.; Panta, A.; Barkley, N.; Ellis, D. The potato cryobank at the International Potato Center (CIP): A model for long term conservation of clonal plant genetic resources collections of the future. CryoLetters 2016, 37, 318–329. [Google Scholar]
- Benelli, C.; De Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Yang, X.X.; Popova, E.; Shukla, M.R.; Saxena, P.K. Root cryopreservation to biobank medicinal plants: A case study for Hypericum perforatum L. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 392–402. [Google Scholar] [CrossRef]
- Li, J.-W.; Ozudogru, E.A.; Li, J.; Wang, M.-R.; Bi, W.-L.; Lambardi, M.; Wang, Q.-C. Cryobiotechnology of forest trees: Recent advances and future prospects. Biodivers. Conserv. 2017, 27, 795–814. [Google Scholar] [CrossRef]
- Bi, W.; Saxena, A.; Ayyanath, M.M.; Harpur, C.; Shukla, M.R.; Saxena, P.K. Conservation, propagation, and redistribution (CPR) of Hill’s thistle: Paradigm for plant species at risk. Plant Cell Tissue Org. Cult. 2021, 145, 75–88. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Popova, E.V.; Shukla, M.R.; McIntosh, T.; Saxena, P.K. In vitro and cryobiotechnology approaches to safeguard Lupinus rivularis Douglas ex Lindl., an endangered plant in Canada. Agronomy 2021, 11, 37. [Google Scholar] [CrossRef]
- Streczynski, R.; Clark, H.; Whelehan, L.M.; Ang, S.-T.; Hardstaff, L.K.; Funnekotter, B.; Bunn, E.; Offord, C.A.; Sommerville, K.D.; Mancera, R.L. Current issues in plant cryopreservation and importance for ex situ conservation of threatened Australian native species. Aust. J. Bot. 2019, 67, 1–15. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA National Plant Germplasm System. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Wang, B.; Wang, R.-R.; Cui, Z.-H.; Bi, W.-L.; Li, J.-W.; Li, B.-Q.; Ozudogru, E.A.; Volk, G.M.; Wang, Q.-C. Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol. Adv. 2014, 32, 583–595. [Google Scholar] [CrossRef]
- Salama, A.; Popova, E.; Jones, M.P.; Shukla, M.R.; Fisk, N.S.; Saxena, P.K. Cryopreservation of the critically endangered golden paintbrush (Castilleja levisecta Greenm.): From nature to cryobank to nature. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 69–78. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Yin, Z.; Feng, C.; Wang, Q.C. Novel and potential application of cryopreservation to plant genetic transformation. Biotechnol. Adv. 2012, 30, 604–612. [Google Scholar] [CrossRef]
- Wang, Q.C.; Wang, R.R.; Li, B.Q.; Cui, Z.H. Cryopreservation: A strategy for safe preservation of genetically transformed plant materials. Adv. Genet. Eng. Biotechnol. 2012, 1, 1–2. [Google Scholar] [CrossRef]
- Wang, Q.C.; Panis, B.; Engelmann, F.; Lambardi, M.; Valkonen, J.P.T. Cryotherapy of shoot tips: A technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann. Appl. Biol. 2009, 154, 351–363. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Cryotherapy of shoot tips: Novel pathogen eradication method. Trends Plant Sci. 2009, 14, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Wang, M.-R.; Chen, H.-Y.; Zhao, L.; Cui, Z.-H.; Zhang, Z.; Blystad, D.-R.; Wang, Q.-C. Long-term preservation of potato leafroll virus, potato virus S, and potato spindle tuber viroid in cryopreserved shoot tips. Appl. Microbiol. Biotechnol. 2018, 102, 10743–10754. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-R.; Hamborg, Z.; Ma, X.-Y.; Blystad, D.-R.; Wang, Q.-C. Double-edged effects of cryogenic technique for virus eradication and preservation in shallot shoot tips. Plant Pathol. 2021, accepted. [Google Scholar]
- Wang, M.-R.; Yang, W.; Zhao, L.; Li, J.-W.; Liu, K.; Yu, J.-W.; Wu, Y.-F.; Wang, Q.-C. Cryopreservation of virus: A novel biotechnology for long-term preservation of virus in shoot tips. Plant Meth. 2018, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, M.-R.; Li, J.-W.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology: A double-edged sword for plant obligate pathogens. Plant Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Turner, S.R.; Bunn, E.; Mancera, R.L.; Dixon, K.W. Cryopreservation of threatened native Australian species—What have we learned and where to from here? In Vitro Cell. Dev. Biol.-Plant 2011, 47, 17–25. [Google Scholar] [CrossRef]
- RBG Kew. The State of the World’s Plants Report—2016; Royal Botanic Gardens, Kew: Richmond, UK, 2016; Available online: https://stateoftheworldsplants.org/2016/ (accessed on 30 August 2021).
- Edesi, J.; Tolonen, J.; Ruotsalainen, A.L.; Aspi, J.; Häggman, H. Cryopreservation enables long-term conservation of critically endangered species Rubus humulifolius. Biodivers. Conserv. 2020, 29, 303–314. [Google Scholar] [CrossRef]
- Sharma, N.; Gowthami, R.; Devi, S.V.; Malhotra, E.V.; Pandey, R.; Agrawal, A. Cryopreservation of shoot tips of Gentiana kurroo Royle—A critically endangered medicinal plant of India. Plant Cell Tissue Org. Cult. 2021, 144, 67–72. [Google Scholar] [CrossRef]
- Grout, B.W.W. Cryopreservation of plant cell suspensions. In Cryopreservation and Freeze-Drying Protocols; Methods in Molecular BiologyTM; Day, J.G., Stacey, G.N., Eds.; Human Press: Totowa, NJ, USA, 2007; Volume 368, pp. 153–161. [Google Scholar]
- Kaczmarczyk, A.; Funnekotter, B.; Menon, A.; Phang, P.Y.; Al-Hanbali, A.; Bunn, E.; Mancera, R.L. Current issues in plant cryopreservation. In Current Frontiers in Cryobiology; Katkov, I.I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 417–438. [Google Scholar]
- Kulus, D. Application of cryogenic technologies and somatic embryogensis in the storage and protection of valuable genetic resources of ornamental plants. In Somatic Embryogenesis in Ornanmental and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; pp. 1–26. [Google Scholar]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Org. Cult. 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Miguel, C.; Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 2011, 62, 3713–3725. [Google Scholar] [CrossRef]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tissue Org. Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Wang, N.; Yang, G.; Ying, L. Tissue culture-induced heritable genomic variation in rice, and their phenotypic implications. PLoS ONE 2014, 9, e96879. [Google Scholar]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. CryoLetters 2004, 25, 3–22. [Google Scholar]
- Harding, K.; Johnson, J.W.; Benson, E.E. Exploring the physiological basis of cryopreservation and failure in clonally propagated in vitro crop plant germplasm. Agric. Food Sci. 2009, 18, 103–116. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.J.M.; de Klerk, J.R. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Ito, A.; Yoshida, M. Epigenetics. In Bioprobes: Biochemical Tools for Investigating Cell Functions, 2nd ed.; Osada, H., Ed.; Springer: Tokyo, Japan, 2017; pp. 47–65. [Google Scholar]
- Boyko, A.; Kovalchuk, I. Epigenetic control of plant tress response. Environ. Mol. Mutagen. 2008, 49, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Liu, Q.L.; Deng, X.X. Effect of cryopreservation on apple genetic resources at morphological, chromosomal, and molecular levels. Cryobiology 2001, 43, 46–53. [Google Scholar] [CrossRef]
- Hao, Y.J.; You, C.X.; Deng, X.X. Analysis of ploidy and the patterns of amplified fragment length polymorphism and methylation sensitive amplified polymorphism in strawberry plants recovered from cryopreservation. CryoLetters 2002, 23, 37–46. [Google Scholar]
- Hao, Y.J.; You, C.X.; Deng, X.X. Effects of cryopreservation on developmental competency, cytological and molecular stability of citrus callus. CryoLetters 2002, 23, 27–35. [Google Scholar]
- Peredo, E.L.; Arroyo-García, R.; Reed, B.M.; Revilla, M.A. Genetic stability of in vitro conserved germplasm of Humulu lupulus L. Agric. Food Sci. 2009, 18, 144–151. [Google Scholar] [CrossRef]
- Kaity, A.; Drew, R.A.; Ashmore, S.E. Genetic and epigenetic integrity assessment of acclimatised papaya plants regenerated directly from shoot-tips following short- and long-term cryopreservation. Plant Cell Tissue Org. Cult. 2013, 112, 75–86. [Google Scholar] [CrossRef]
- Adu-Gyamfi, R.; Wetten, A.; Rodriguez, L.C.M. Effect of cryopreservation and post-cryopreservation somatic embryogenesis on the epigenetic fidelity of cocoa (Theobroma cacao L.). PLoS ONE 2016, 11, e0158857. [Google Scholar]
- Maki, S.; Hirai, Y.; Niino, T.; Matsumoto, T. Assessment of molecular genetic stability between long term cryopreservation and tissue cultured wasabi (Wasabia japonica) plants. CryoLetters 2015, 36, 318–324. [Google Scholar]
- Johnston, J.W.; Benson, E.E.; Harding, K. Cryopreservation induces temporal DNA methylation epigenetic changes and differential transcriptional activity in Ribes germplasm. Plant Physiol. Biochem. 2009, 47, 123–131. [Google Scholar] [CrossRef]
- Kaity, A.; Ashmore, S.E.; Drew, R.A. Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep. 2008, 2, 1529–1539. [Google Scholar] [CrossRef]
- Ibáñez, M.A.; Alvarez-Mari, A.; Rodríguez-Sanz, H.; Kremer, C.; González-Benito, M.E.; Martín, C. Genetic and epigenetic stability of recovered mint apices after several steps of a cryopreservation protocol by encapsulation-dehydration. A new approach for epigenetic analysis. Plant Physiol. Biochem. 2019, 143, 299–307. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Bao, W.-W.; Zhang, A.-L.; Pathirana, R.; Wang, Q.-C.; Liu, Z.-D. Cryopreservation of shoot tips, evaluations of vegetative growth, and assessments of genetic and epigenetic changes in cryo-derived plants of Actinidia spp. Cryobiology 2020, 94, 18–25. [Google Scholar] [CrossRef]
- Heringer, A.S.; Steinmacher, D.A.; Fraga, H.P.F.; Vieira, L.N.; Ree, J.F.; Guerra, M.P. Global DNA methylation profiles of somatic embryos of peach palm (Bactris gasipaes Kunth) are influenced by cryoprotectants and droplet-vitrification cryopreservation. Plant Cell Tissue Org. Cult. 2013, 114, 365–372. [Google Scholar] [CrossRef]
- Plitta, B.P.; Michalak, M.; Naskret-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. DNA methylation of Quercus robur L. plumules following cryo-pretreatment and cryopreservation. Plant Cell Tissue Org. Cult. 2014, 117, 31–37. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Houben, A.; Keller, E.R.J.; Mette, M.F. Influence of cryopreservation on the cytosine methylation state of potato genomic DNA. CryoLetters 2010, 31, 380–391. [Google Scholar]
- Mikuła, A.; Tomiczak, K.; Rybczyński, J.J. Cryopreservation enhances embryogenic capacity of Gentiana cruciata (L.) suspension culture and maintains (epi)genetic uniformity of regenerants. Plant Cell Rep. 2011, 30, 565–574. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.J.; Lindpainter, K.; Lincoln, S.E.; Kusumi, K.; Bunker, R.K.; Mao, Y.-P.; Ganten, D.; Dzau, V.J.; Lander, E.S. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell 1991, 67, 213–224. [Google Scholar] [CrossRef]
- Akkaya, M.S.; Bhagwat, A.A.; Cregan, P.B. Lengh polymorphisms of simple sequence repeat DNA in soybean. Genetics 1992, 132, 1131–1139. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.-W.; Zhang, Z.-B.; Wang, R.-R.; Ma, Y.-L.; Blystad, D.-R.; Keller, E.R.J.; Wang, Q.-C. Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J. Biotechnol. 2014, 184, 47–55. [Google Scholar] [CrossRef]
- Kulus, D.; Rewers, M.; Serocka, M. Cryopreservation by encapsulation-dehydration affects the vegetative growth of chrysanthemum but does not disturb its chimeric structure. Plant Cell Tissue Org. Cult. 2019, 138, 153–166. [Google Scholar] [CrossRef]
- Liu, X.-X.; Wen, Y.-B.; Cheng, Z.-H.; Mou, S.-W. Establishment of a garlic cryopreservation protocol for shoot apices from adventitious buds in vitro. Sci. Hortic. 2017, 226, 10–18. [Google Scholar] [CrossRef]
- Wang, R.-R.; Gao, X.-X.; Chen, L.; Huo, L.-Q.; Li, M.-F.; Wang, Q.-C. Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci. Hortic. 2014, 176, 330–339. [Google Scholar] [CrossRef]
- Krajňáková, J.; Sutela, S.; Aronen, T.; Gömöry, D.; Vianello, A.; Häggman, H. Long-term cryopreservation of Greek fir embryogenic cell lines: Recovery, maturation and genetic fidelity. Cryobiology 2011, 63, 17–25. [Google Scholar] [CrossRef]
- Pence, V.C.; Philpott, M.; Culley, T.M.; Plair, B.; Yorke, S.R.; Lindsey, K.; Vanhove, A.C.; Ballesteros, D. Survival and genetic stability of shoot tips of Hedeoma todsenii R.S. Irving after long-term cryostorage. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 28–338. [Google Scholar]
- Martín, C.; Cervera, M.T.; González-Benito, M.E. Genetic stability analysis of chrysanthemum (Chrysanthemum x morifolium Ramat) after different stages of an encapsulation–dehydration cryopreservation protocol. J. Plant Physiol. 2011, 168, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Kremer, C.; González, I.; González-Benito, M.E. Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Org. Cult. 2015, 122, 185–195. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Kremer, C.; Ibáñez, M.A.; Martín, C. Effect of antioxidants on the genetic stability of cryopreserved mint shoot tips by encapsulation–dehydration. Plant Cell Tissue Org. Cult. 2016, 127, 359–368. [Google Scholar] [CrossRef]
- Castillo, N.R.F.; Bassil, N.V.; Wada, S.; Reed, B.M. Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell. Dev. Biol.-Plant 2010, 46, 246–256. [Google Scholar] [CrossRef]
- Aronen, T.S.; Krajnakov, J.; Häggman, H.M.; Ryynänen, L.A. Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Sci. 1999, 142, 163–172. [Google Scholar] [CrossRef]
- Merhy, T.S.M.; Vianna, M.G.; Garcia, R.O.; Pacheco, G.; Mansur, E. Cryopreservation of Passiflora pohlii nodal segments and assessment of gentic stability of regenerated plants. CryoLetters 2014, 35, 204–215. [Google Scholar]
- Li, J.-W.; Chen, H.-Y.; Li, X.-Y.; Zhang, Z.; Blystad, D.-R.; Wang, Q.-C. Cryopreservation and evaluations of vegetative growth, microtuber production and genetic stability in regenerants of purple-fleshed potato. Plant Cell Tissue Org. Cult. 2017, 128, 641–653. [Google Scholar] [CrossRef]
- Galdiano, R.F., Jr.; de Macedo Lemos, E.G.; Vendrame, W.A. Cryopreservation, early seedling development, and genetic stability of Oncidium flexuosum Sims. Plant Cell Tissue Org. Cult. 2013, 114, 139–148. [Google Scholar] [CrossRef]
- Bi, W.-L.; Pan, C.; Liu, J.; Wang, Q.-C. Greenhouse performance, genetic stability and biochemical compounds in Chrysanthemum morifolium ‘Hangju’ plants regenerated from cryopreserved shoot tips. Acta Physiol. Plant. 2016, 38, 268. [Google Scholar] [CrossRef]
- Zhang, Z.; Skjeseth, G.; Elameen, A.; Haugslien, S.; Sivertsen, A.; Wang, Q.-C.; Blystad, D.R. Field performance evaluation and genetic integrity assessment in Argyranthemum maderense plants recovered from cryopreserved shoot tips. In Vitro Cell. Dev. Biol. -Plant 2015, 51, 505–513. [Google Scholar] [CrossRef]
- Li, J.-W.; Li, H.-H.; Wang, R.-R.; Gao, X.-X.; Wang, Q.-C. Cryopreservation for retaining morphology, genetic integrity, and foreign genes in transgenic plants of Torenia fournieri. Acta Physiol. Plant. 2016, 38, 8. [Google Scholar] [CrossRef]
- Cheng, W.; Li, H.; Zhou, F.; Zhu, B.; Yu, J.; Ding, Z. Cryopreservation of Pleione bulbocodioides (Franch.) Rolfe protocorm-like bodies by vitrification. Acta Physiol. Plant. 2020, 42, 82. [Google Scholar] [CrossRef]
- Cejas, I.; Vives, K.; Laudat, T.; González-Olmedo, J.; Engelmann, F.; Martínez-Montero, M.E.; Lorenzo, J.C. Effects of cryopreservation of Phaseolus vulgaris L. seeds on early stages of germination. Plant Cell Rep. 2012, 31, 2065–2073. [Google Scholar] [CrossRef]
- Carmona-Martín, E.; Regalado, J.J.; Perán-Quesada, R. Cryopreservation of rhizome buds of Asparagus officinalis L. (cv. Morado de Huétor) and evaluation of their genetic stability. Plant Cell Tissue Org. Cult. 2018, 133, 395–403. [Google Scholar] [CrossRef]
- Wang, M.-R.; Hamborg, Z.; Slimestad, R.; Elameen, A.; Blystad, D.-R.; Haugslien, S.; Skjeseth, G.; Wang, Q.-C. Assessments of rooting, vegetative growth, bulb production, genetic integrity and biochemical compounds in cryopreserved plants of shallot. Plant Cell Tissue Org. Cult. 2021, 144, 123–131. [Google Scholar] [CrossRef]
- Agrawal, A.; Sanayaima, R.; Singh, R.; Tandon, R.; Verma, S.; Tyagi, R.K. Phenotypic and molecular studies for genetic stability assessment of cryopreserved banana meristems derived from field and in vitro explant sources. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 345–356. [Google Scholar] [CrossRef]
- Li, B.-Q.; Feng, C.-H.; Hu, L.-Y.; Wang, M.-R.; Chen, L.; Wang, Q.-C. Shoot regeneration and cryopreservation of shoot tips of apple (Malus) by encapsulation–dehydration. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 357–368. [Google Scholar] [CrossRef]
- Li, B.-Q.; Feng, C.-H.; Wang, M.-R.; Hu, L.-Y.; Volk, G.M.; Wang, Q.-C. Recovery patterns, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J. Biotechnol. 2015, 214, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Li, Y.-D.; Sun, H.-Y.; Liu, H.-G.; Tang, X.-D.; Wang, Q.-C.; Zhang, Z.-D. An efficient droplet-vitrification cryopreservation for valuable blueberry germplasm. Sci. Hortic. 2017, 219, 60–69. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, J.; Pan, C.; Yu, J.-W.; Wang, Q.-C. In vitro regeneration of adventitious buds from leaf explants and their subsequent cryopreservation in highbush blueberry. Plant Cell Tissue Org. Cult. 2018, 134, 193–204. [Google Scholar] [CrossRef]
- Ai, P.-F.; Lu, L.-P.; Song, J.-J. Cryopreservation of in vitro-grown shoot-tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell Tissue Org. Cult. 2012, 108, 381–387. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, R.; Pandey, R.; Kaushik, N. Genetic and biochemical stability assessment of plants regenerated from cryopreserved shoot tips of a commercially valuable medicinal herb Bacopa monnieri (L.) Wettst. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 346–351. [Google Scholar] [CrossRef]
- Wang, R.-R.; Mou, H.-Q.; Gao, X.-X.; Chen, L.; Li, M.-F.; Wang, Q.-C. Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann. Appl. Biol. 2015, 166, 218–228. [Google Scholar] [CrossRef]
- Tavazza, R.; Lucioli, A.; Benelli, C.; Giorgi, D.; D’Aloisio, E.; Papacchioli, V. Cryopreservation in artichoke: Towards a phytosanitary qualified germplasm collection. Ann. Appl. Biol. 2013, 163, 231–241. [Google Scholar] [CrossRef]
- Li, J.-W.; Chen, H.-Y.; Li, J.; Zhang, Z.; Blystad, D.-R.; Wang, Q.-C. Growth, microtuber production and physiological metabolism in virus-free and virus-infected potato in vitro plantlets grown under NaCl-induced salt stress. Eur. J. Plant Pathol. 2018, 152, 417–432. [Google Scholar] [CrossRef]
- Meijer, E.G.M.; Iren, E.; Schrijnemakers, E.; Hensgens, L.A.M.; van Zijderveld, M.; Schilperoort, R.A. Retention of the capacity to produce plants from protoplasts in cryopreserved cell lines of rice (Oryza sativa L). Plant Cell Rep. 1991, 10, 171–174. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, S.M.; Joo, S.Y.; Yoo, J.S.; Kim, D.I. Cryopreservation of transgenic rice suspension cells producing recombinant hCTLA4Ig. Appl. Microbiol. Biotechnol. 2007, 73, 1470–1476. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P. Continued expression of plant-made vaccines following long-term cryopreservation of antigen-expressing tobacco cell cultures. In Vitro Cell. Dev. Biol.-Plant 2009, 45, 750–757. [Google Scholar] [CrossRef]
- Vendrame, W.A.; Holliday, C.P.; Montello, P.M.; Smith, D.R.; Merkle, S.A. Cryopreservation of yellow-poplar (Liriodendron tulipifera) and sweetgum (Liquidambar spp.) embryogenic cultures. New For. 2001, 21, 283–292. [Google Scholar] [CrossRef]
- Hao, Y.J.; Deng, X.X. GUS gene remains stable in transgenic Citrus callus recovered from cryopreservation. CryoLetters 2003, 24, 375–380. [Google Scholar]
- Ryynänen, L.; Sillanpää, M.; Kontunen-Soppela, S.; Tiimonen, H.; Kangasjärvi, J.; Vapaavuori, E.; Häggman, H. Preservation of transgenic silver birch (Betula pendula Roth) lines by means of cryopreservation. Mol. Breed. 2002, 10, 143–152. [Google Scholar] [CrossRef]
- Jokipii, S.; Ryynänen, L.; Kallio, P.T.; Aronen, T.; Häggman, H. A cryopreservation method maintaining the genetic fidelity of a model forest tree, Populus tremula L. × Populus tremuloides Michx. Plant Sci. 2004, 166, 799–806. [Google Scholar] [CrossRef]
- Schmale, K.; Rademacher, T.H.; Fischer, R.; Hellwig, S. Towards industrial usefulness—Cryocell banking of transgenic BY-2 cell cultures. J. Biotechnol. 2006, 124, 302–311. [Google Scholar] [CrossRef]
- Corredoira, E.; San-Jose, M.C.; Vieitez, A.M.; Ballester, A. Improving genetic transformation of European chestnut and cryopreservation of transgenic lines. Plant Cell Tissue Org. Cult. 2007, 91, 281–288. [Google Scholar] [CrossRef]
- Dolce, N.R.; Faloci, M.M.; Gonzalez, A.M. In vitro plant regeneration and cryopreservation of Arachis glabrata (Fabaceae) using leaflet explants. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 133–144. [Google Scholar] [CrossRef]
- Espasandin, F.D.; Brugnoli, E.A.; Ayala, P.G.; Ayala, L.P.; Ruiz, O.A. Long-term preservation of Lotus tenuis adventitious buds. Plant Cell Tissue Org. Cult. 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Martín, C.; Senula, A.; González, E.; Acosta, A.; Keller, E.R.J.; González-Benito, M.E. Genetic identity of three mint accessions stored by different conservation procedures: Field collection, in vitro and cryopreservation. Genet. Resour. Crop Evol. 2013, 60, 243–249. [Google Scholar] [CrossRef]
- Agrawal, A.; Tyagi, R.K.; Goswami, R. Cryobanking of Banana (Musa sp.) germplasm in India: Evaluation of agronomic and molecular traits of cryopreserved plants. Acta Hortic. 2011, 908, 129–138. [Google Scholar] [CrossRef]
- Cejas, I.; Méndez, R.; Villalobos, A.; Palau, F.; Aragón, C.; Engelmann, F.; Carputo, D.; Aversano, R.; Martínez, M.E.; Lorenzo, J.C. Phenotypic and molecular characterization of Phaseolus vulgaris plants from non-cryopreserved and cryopreserved seeds. Am. J. Plant Sci. 2013, 4, 844–849. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Chmielarz, P.; Michalak, M.; Dering, M.; Bojarczuk, K. Survival and genetic stability of Picea abies embryogenic cultures after cryopreservation using a pregrowth-dehydration method. Plant Cell Tissue Org. Cult. 2013, 113, 303–313. [Google Scholar] [CrossRef]
- Salaj, T.; Matušíková, I.; Fráterová, L.; Piršelová, B.; Salaj, J. Regrowth of embryogenic tissues of Pinus nigra following Cryopreservation. Plant Cell Tissue Org. Cult. 2013, 106, 55–61. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Tilkat, E.; Yildirim, H.; Onay, A.; Çiftçi, Y.O. In vitro conservation and cryopreservation of mature pistachio (Pistacia vera L.) germplasm. J. Plant Biochem. Biotechnol. 2013, 22, 43–51. [Google Scholar] [CrossRef]
- Kaya, E.; Souza, F.V.D. Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 410–417. [Google Scholar] [CrossRef]
- Coelho, N.; González-Benito, M.E.; Martín, C.; Romano, A. Cryopreservation of Thymus lotocephalus shoot tips and assessment of genetic stability. CryoLetters 2014, 35, 119–128. [Google Scholar]
- Solov’eva, A.I.; Dolgikh, Y.I.; Vysotskaya, O.N.; Popov, A.S. Patterns of ISSR and REMAP DNA markers after cryogenic preservation of spring wheat calli by dehydration method. Russ. J. Plant Physiol. 2011, 58, 423–430. [Google Scholar] [CrossRef]
- Hirano, T.; Godo, T.; Miyoshi, K.; Ishikawa, K.; Ishikawa, M.; Mii, M. Cryopreservation and low-temperature storage of seeds of Phaius tankervilleae. Plant Biotechnol. Rep. 2009, 3, 103–109. [Google Scholar] [CrossRef]
- Cejas, I.; Rumlow, A.; Turcios, A.; Engelmann, F.; Martínez, M.E.; Yabor, L.; Papenbrock, J.; Lorenzo, J.C. Exposure of common bean seeds to liquid nitrogen modifies mineral composition of young plantlet leaves. Am. J. Plant Sci. 2016, 7, 1612–1617. [Google Scholar] [CrossRef]
- Zevallos, B.; Cejas, I.; Valle, B.; Yabor, L.; Aragón, C.; Engelmann, F.; Martínez, M.E.; Lorenzo, J.C. Short-term liquid nitrogen storage of wild tomato (Solanum lycopersicum Mill.) seeds modifies the levels of phenolics in 7 day-old seedlings. Sci. Hortic. 2013, 160, 264–267. [Google Scholar] [CrossRef]
- Arguedas, M.; Gómez, D.; Hernández, L.; Engelmann, F.; Garramone, R.; Cejas, I.; Yabor, L.; Martínez-Monter, M.E. Maize seed cryo-storage modifies chlorophyll, carotenoid, protein, aldehyde and phenolics levels during early stages of germination. Acta Physiol. Plants. 2018, 40, 118. [Google Scholar] [CrossRef]
- Arguedas, M.; Perez, A.; Abdelnour, A.; Hernandez, M.; Engelmann, F.; Martínez, M.E.; Yabor, L.; Lorenzo, J.C. Short-term liquid nitrogen storage of maize, common bean and soybean seeds modifies their biochemical composition. Agric. Sci. 2016, 4, 6–12. [Google Scholar] [CrossRef]
- Zevallos, B.; Cejas, I.; Rodríguez, R.C.; Yabor, L.; Aragón, C.; González, J.; Engelmann, F.; Martínez, M.E.; Lorenzo, J.C. Biochemical characterization of Ecuadorian wild Solanum lycopersicum Mill. Plants produced from non-cryopreserved and cryopreserved seeds. CryoLetters 2013, 34, 413–421. [Google Scholar]
- Acosta, Y.; Pérez, L.; Linares, C.; Hernández, L.; Escalante, D.; Pérez, A.; Zevallos, B.E.; Yabor, L.; Martínez-Montero, M.E.; Cejas, I.; et al. Effects of Teramnus labialis (L.f.) Spreng seed cryopreservation on subsequent seed and seedling growth and biochemistry. Acta Physiol. Plant. 2020, 42, 7. [Google Scholar] [CrossRef]
- Moraes, R.M.; Souza, L.B.; Nery, F.C.; Paiva, R.; Barbosa, S. Seed cryopreservation as an alternative for the conservation of H. sabdariffa L. (Malvaceae) germplasm. Acta Hortic. 2018, 1224, 165–174. [Google Scholar] [CrossRef]
| Plant Species | Explants | Cryopreservation Method * | Molecular Markers ** | Polymorphism (%) | Causes | Reference |
|---|---|---|---|---|---|---|
| Abies | Embryogenic cells | Vitri | RAPD | Not specified | Cryoprocedures and in vitro culture | [83] |
| Actinidia chinensis var. deliciosa | Shoot tips | Drop-vitri | AFLP and ISSR | None | [71] | |
| Allium cepa var. aggregatum | Shoot tips | Drop-vitri | AFLP and ISSR | None | [99] | |
| Allium sativum | Shoot tips | Vitri | SSR and FCM | None | [71] | |
| Arachis glabrata | Leaflets | Drop-vitri | RAPD | 0–3.4 | Cryoprocedures | [119] |
| Asparagus officinalis | Rhizome buds | Encap-dehy | EST-SSR and FCM | None | [98] | |
| Bacopa monnieri | Shoot tips | Vitri | RAPD | None | [106] | |
| Carica papaya | Shoot tips | Vitri | RAF | 0–0.7 | Genotypes and cryoprocedures | [66] |
| Chrysanthemum × grandiforum | Shoot tips | Encap-dehy | ISSR | 0–2 | Genotypes and cryoprocedures | [80] |
| RAPD | 0–7.8 | |||||
| FCM | None | |||||
| Chrysanthemum × morifolium | Shoot tips | Encap-dehy | AFLP | 40.1 | Sucrose preculture | [85] |
| RAPD | 5.78 | |||||
| Drop-vitri | SSR | None | [82] | |||
| FCM | None | |||||
| Drop-vitri | ISSR and RAPD | None | [93] | |||
| Cynara scolymus | Shoot tips | Vitri | FCM | None | [108] | |
| Hedeoma todsenii | Shoot tips | Encap-dehy and Encap-vitri | Microsatellite | 5.36–13.04 | Genotypes and cryoprocedures | [84] |
| SRAP | 4.55–20.45 | Genotypes and cryoprocedures | ||||
| Lotus tenuis | Adventitious buds clusters | Vitri | ISSR | 63 | Cryoprocedures | [120] |
| Malus spp. | Shoot tips | Encap-dehy | ISSR | None | [101] | |
| Drop-vitri or Encap-dehy | ISSR and RAPD | None | [102] | |||
| Mentha × piperita | Shoot tips | Drop-vitri | RAPD | 30–40 | Genotypes and cryoprocedures | [121] |
| RAPD | 1–20 | Genotypes and cryoprocedures | [86] | |||
| Encap-dehy | RAPD | 13–76 | Genotypes and cryoprocedures | |||
| AFLP | 0–85.7 | Genotypes, cryoprocedures, and in vitro culture | [87] | |||
| RAPD | 0–62 | |||||
| AFLP | 2.65 | Sucrose preculture and encapsulation | [70] | |||
| RAPD | None | |||||
| Musa spp. | Sucker meristems | Vitri | SSR | None | [122] | |
| Passiflora pohlii | Nodal segments | Encap-vitri | ISSR and RAPD | None | [90] | |
| Vitri | ISSR and RAPD | None | ||||
| Phaseolus vulgaris | Seeds | Direct immersion into LN | SSR | None | [123] | |
| Picea abies | Embryogenic tissues | Vitri | SSR | None | [124] | |
| Pinus nigra | Embryogenic tissues | Slow-freezing | RAPD | None | [125] | |
| Pistacia vera | Shoot tips | Vitri | RAPD | 5.4 | Cryoprotants and post-culture | [126] |
| Pleione bulbocodioides | Protocorm-like bodies | Vitri | ISSR | None | [96] | |
| Rabdosia rubescens | Shoot tips | Encap-dehy | SRAP | 0.01 | Cryoprocedures | [105] |
| FCM | None | |||||
| Saccharum spp. | Shoot tips | Drop-vitri | ISSR | 1.5 | Cryoprotection | [127] |
| Solanum tuberosum | Shoot tips | Vitri | AFLP and ISSR | None | [79] | |
| Drop-vitri | ISSR and RAPD | None | [91] | |||
| Encap-vitri | ||||||
| Thymus lotocephalus | Shoot tips | Drop-vitri | RAPD | 0.06 | Cryoprocedures | [128] |
| Torenia fournieri | Shoot tips | Drop-vitri | ISSRFCM | None | [95] | |
| Triticum aestivum | Calli | Dehy | ISSR | None | [129] | |
| REMAP | 0.3 | Cryoprocedures | ||||
| Vaccinium corymbosum | Shoot tips | Drop-vitri | ISSR and RAPD | None | [103] | |
| Adventitious buds | Drop-vitri | ISSR and RAPD | None | [104] | ||
| Vitis spp. | Shoot tips | Drop-vitri | ISSR and RAPD | None | [17] | |
| Wasabia japonica | Shoot tips | Vitri | AFLP | 0.27–2.2 | Cryoprocedures | [67] |
| Ziziphus jujuba | Shoot tips | Drop-vitri | FCM | None | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants 2021, 10, 1889. https://doi.org/10.3390/plants10091889
Wang M-R, Bi W, Shukla MR, Ren L, Hamborg Z, Blystad D-R, Saxena PK, Wang Q-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants. 2021; 10(9):1889. https://doi.org/10.3390/plants10091889
Chicago/Turabian StyleWang, Min-Rui, Wenlu Bi, Mukund R. Shukla, Li Ren, Zhibo Hamborg, Dag-Ragnar Blystad, Praveen K. Saxena, and Qiao-Chun Wang. 2021. "Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants" Plants 10, no. 9: 1889. https://doi.org/10.3390/plants10091889
APA StyleWang, M.-R., Bi, W., Shukla, M. R., Ren, L., Hamborg, Z., Blystad, D.-R., Saxena, P. K., & Wang, Q.-C. (2021). Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants, 10(9), 1889. https://doi.org/10.3390/plants10091889








