Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments
Abstract
:1. Introduction
2. Experimental
2.1. Test Material and Specimen
2.2. Test Apparatus and Condition
3. Results and Analysis
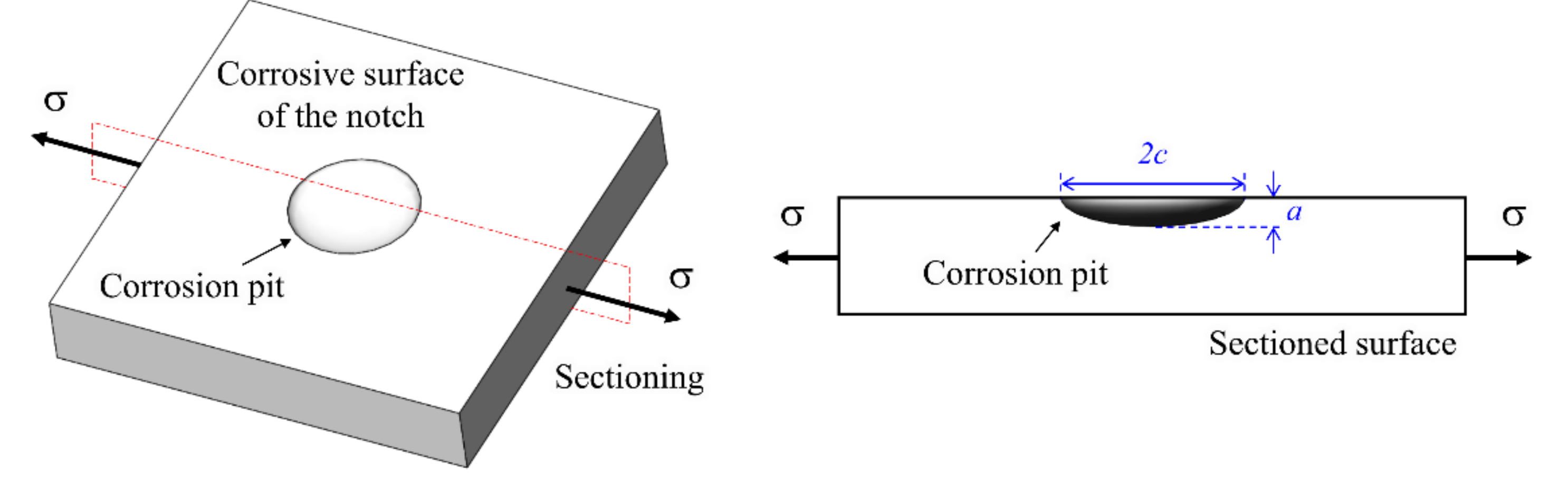
3.1. Observed Corrosion Pit
3.2. Determination of Local Pit Stress Using FE Analysis
4. Corrosion Rate Model
4.1. Existing Corrosion Models
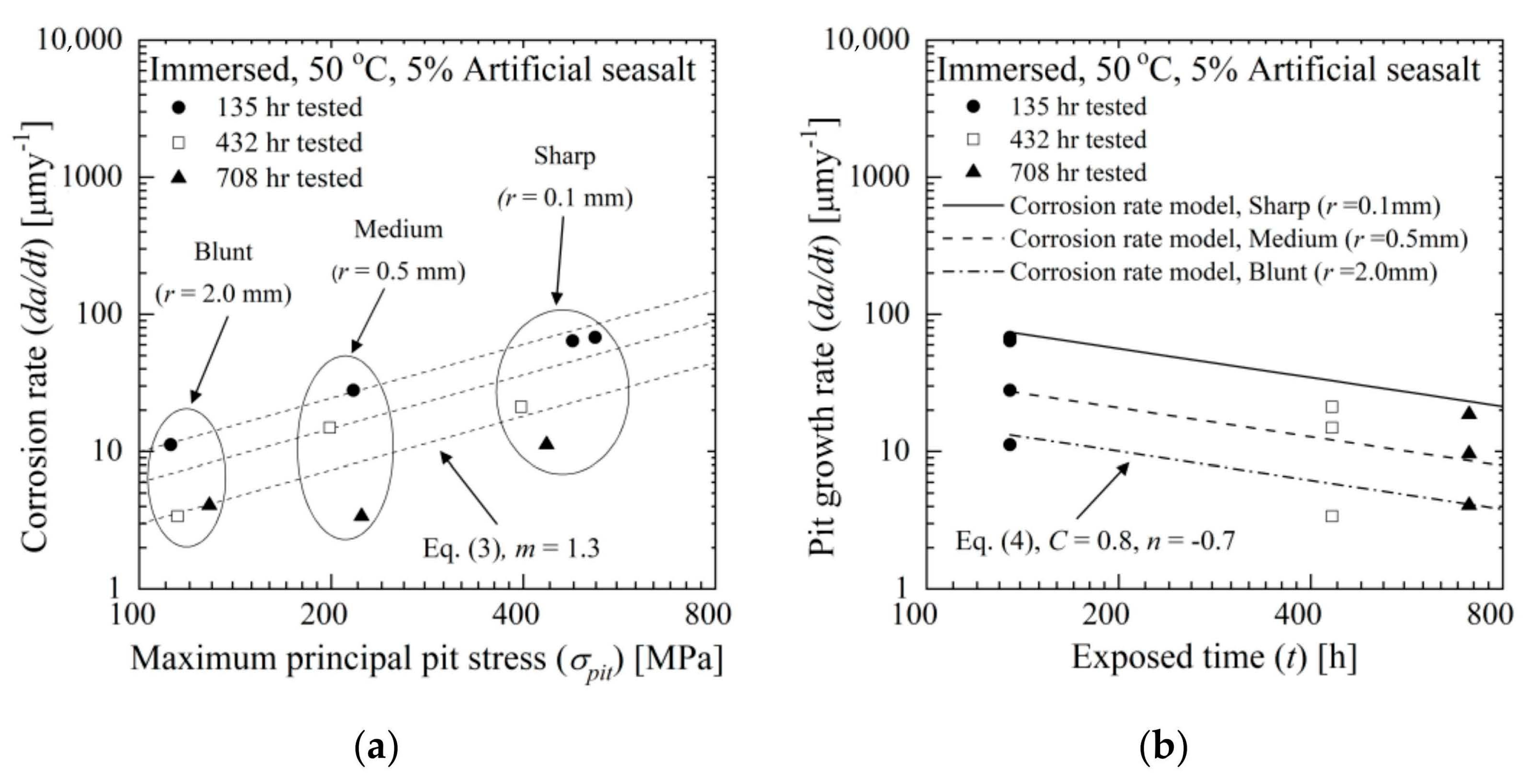
4.2. Stress Effect on Pit Growth Rate
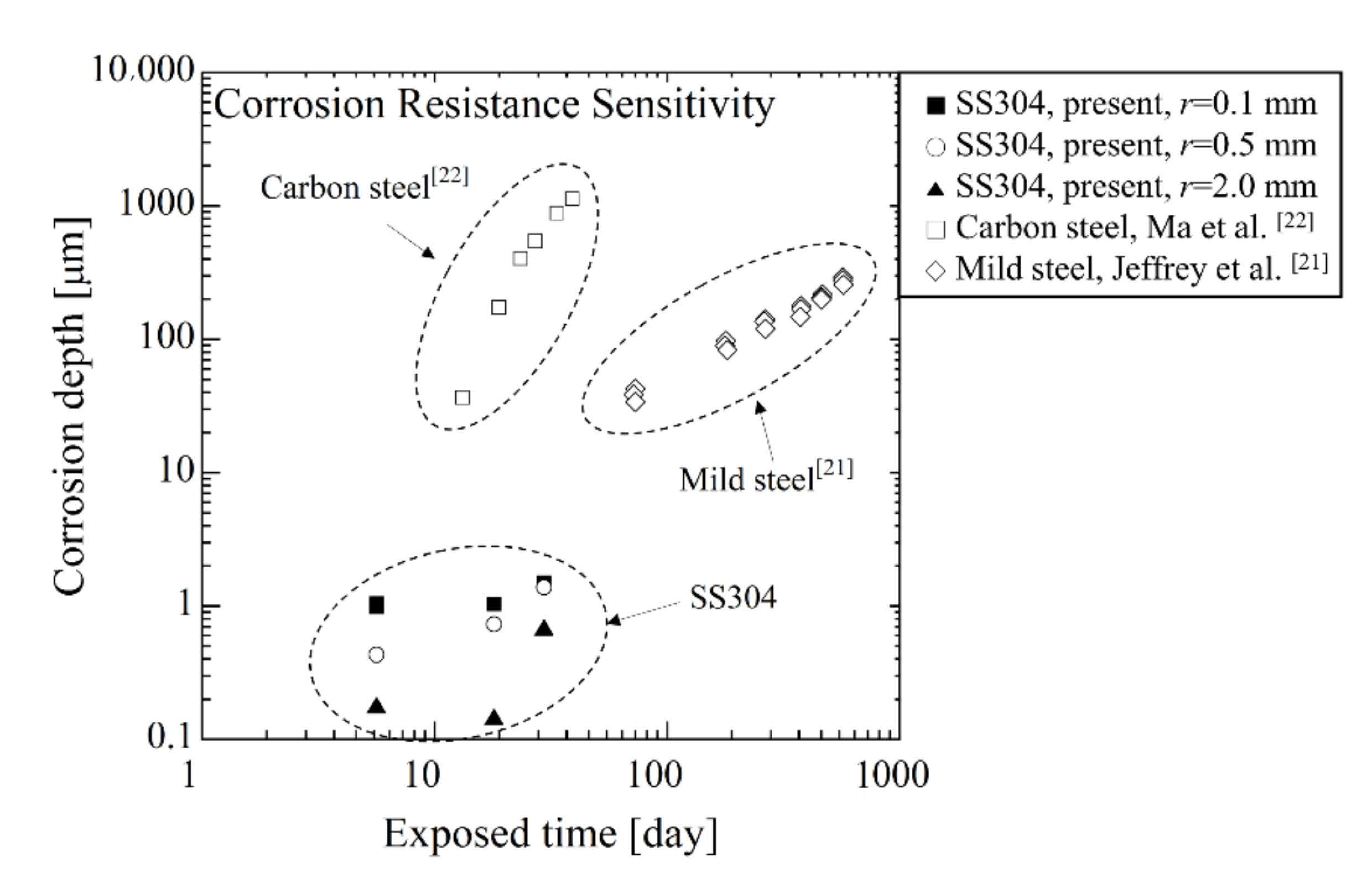
4.3. Comparison with Other Models
5. Discussion
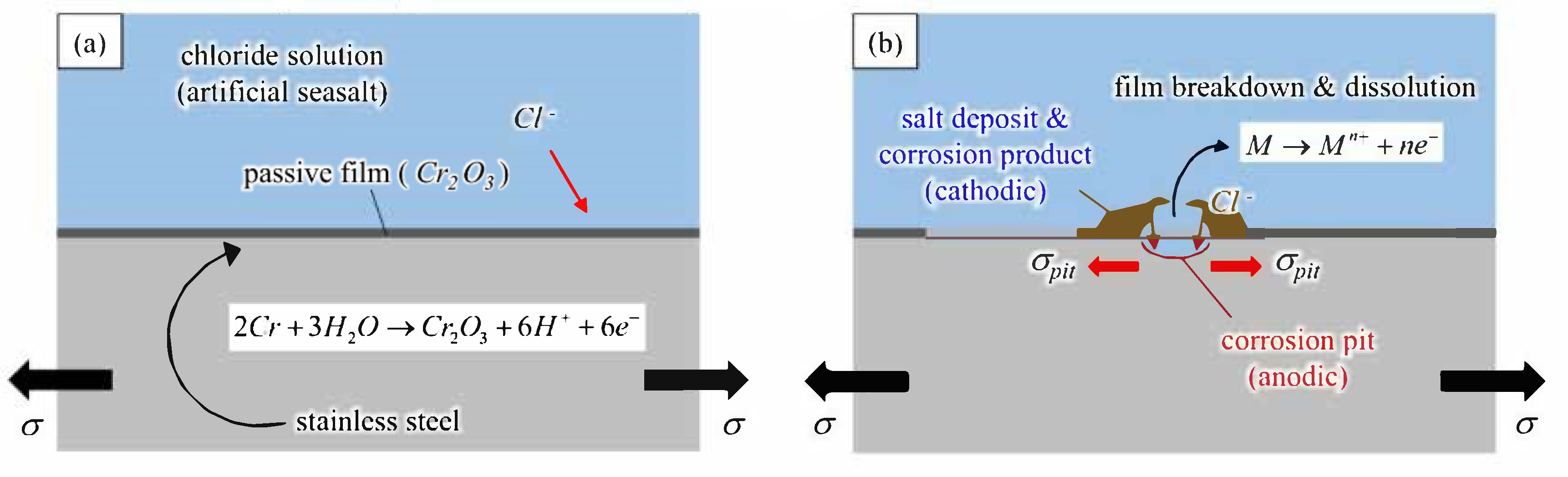
5.1. Surface Morphology
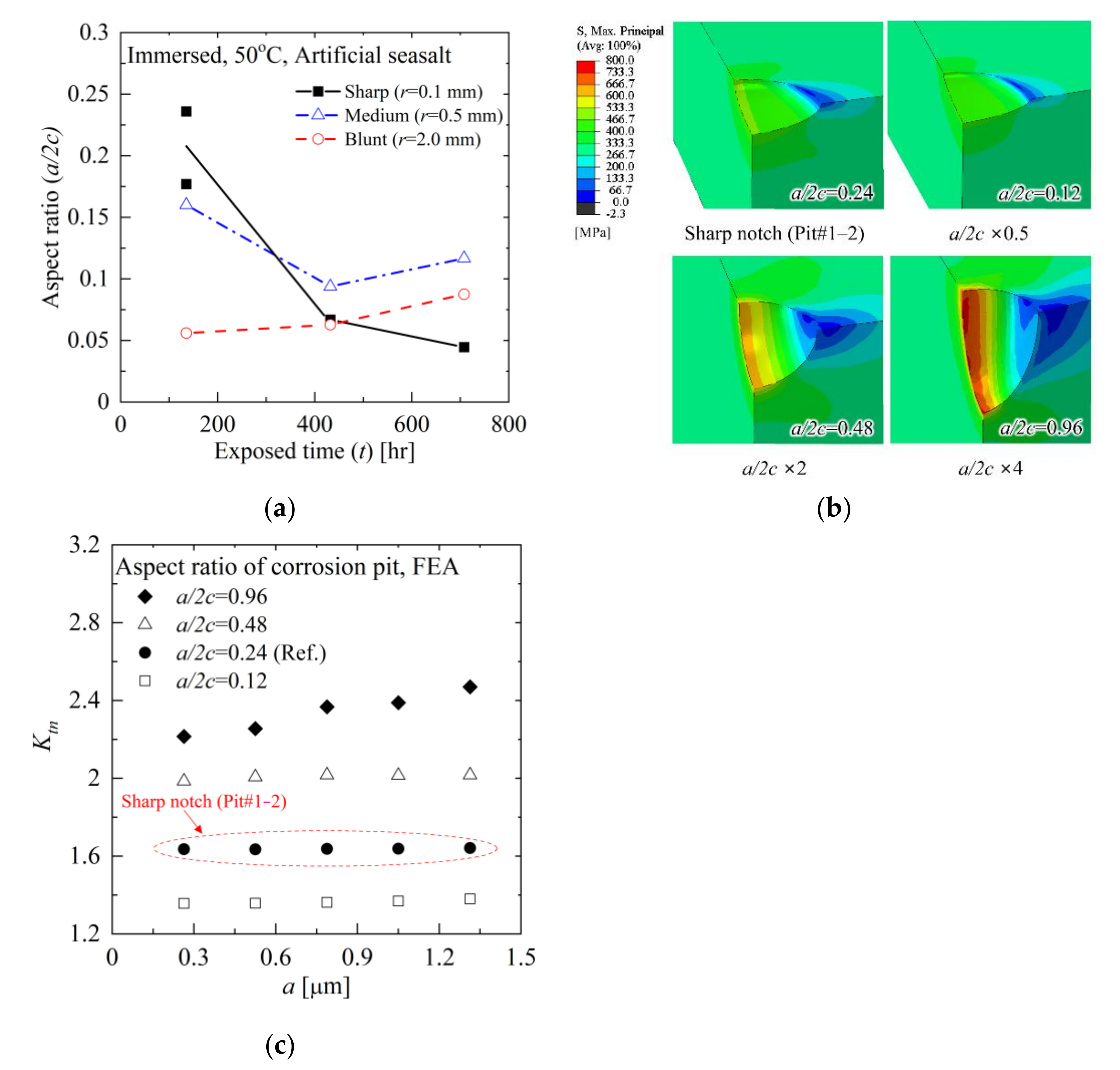
5.2. Aspect Ratio of Corrosion Pit
6. Conclusions
- Based on our experimental findings, the pit growth rate model is suggested in terms of the maximum principal stress on the pit (σpit) and exposure time (t) by fitting the experimental data. The model suggested that the pit growth rate increases exponentially with increasing maximum principal stress and decreasing exposure time.
- SEM analysis of the corrosion pits verified that the electrochemical dissolution facilitated the corrosion pit growth toward the width direction.
- The comparison of the corrosion rate obtained in the present work with those reported in literature showed that the corrosion performance of 304 stainless steel in marine environments is better than those of carbon or mild steel.
- The FE analysis results show that the stress concentration factor (Ktn) is not dependent on a for small a/2c. For large a/2c, Ktn increases linearly with a, which suggests that the stress can be intensified as increasing the corrosion pit depth.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, E.L.; Briant, C.L. Chromium depletion in the vicinity of carbides in sensitized austenitic stainless steels. Met. Mater. Trans. A 1984, 15, 793–811. [Google Scholar] [CrossRef]
- Enos, D.; Bryan, C.R. Residual Stress Measurements from the SNL Mockup Container; Sandia National Lab.(SNL-NM): Albuquerque, NM, USA, 2015. [Google Scholar]
- Wu, X.; Yu, Z.; Gordon, S.; Olson, D.; Liu, S.; Bryan, C.; Schindelholz, E.; Enos, D.; Shayer, Z. Finite Element Analysis of Weld Residual Stresses in Austenitic Stainless Steel Canisters in Dry Storage of Spent Fuel; Sandia National Lab.(SNL-NM): Albuquerque, NM, USA, 2017. [Google Scholar]
- Bryan, C.R.; Enos, D.G. Analysis of dust samples collected from spent nuclear fuel interim storage containers at Hope Creek, Delaware, and Diablo Canyon, California; Sandia National Lab.(SNL-NM): Albuquerque, NM, USA, 2014. [Google Scholar]
- Tani, J.-I.; Mayuzumi, M.; Hara, N. Initiation and Propagation of Stress Corrosion Cracking of Stainless Steel Canister for Concrete Cask Storage of Spent Nuclear Fuel. Corrosion 2009, 65, 187–194. [Google Scholar] [CrossRef]
- Duncan, A.J.; Lam, P.S.; Sindelar, R.L.; Metzger, K.E. Crack Growth Rate Testing of Bolt-Load Compact Tension Specimens Under Chloride-Induced Stress Corrosion Cracking Conditions in Spent Nuclear Fuel Canisters. In Pressure Vessels and Piping Conference; American Society of Mechanical Engineers: New York City, NY, USA, 2018; p. V06BT06A068. [Google Scholar]
- Jeong, J.-Y.; Lee, M.-W.; Kim, Y.-J.; Lam, P.-S.; Duncan, A.J. Chloride-Induced Stress Corrosion Cracking Tester for Austenitic Stainless Steel. J. Test. Evaluation 2020, 49, 2. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. Trans. R. Soc. London. Ser. A Phys. Eng. Sci. 1992, 341, 531–559. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rajeswari, S. Investigation of failures in stainless steel orthopaedic implant devices: Pit-induced stress corrosion cracking. J. Mater. Sci. Lett. 1992, 11, 1039–1042. [Google Scholar] [CrossRef]
- Tokuda, S.; Muto, I.; Sugawara, Y.; Hara, N. Pit initiation on sensitized Type 304 stainless steel under applied stress: Correlation of stress, Cr-depletion, and inclusion dissolution. Corros. Sci. 2020, 167, 108506. [Google Scholar] [CrossRef]
- Khobragade, N.N.; Khan, M.I.; Patil, A.P. Corrosion Behaviour of Chrome–Manganese Austenitic Stainless Steels and AISI 304 Stainless Steel in Chloride Environment. Trans. Indian Inst. Met. 2013, 67, 263–273. [Google Scholar] [CrossRef]
- Pal, S.; Bhadauria, S.S.; Kumar, P. Pitting Corrosion Behavior of F304 Stainless Steel Under the Exposure of Ferric Chloride Solution. J. Bio- Tribo-Corrosion 2019, 5, 91. [Google Scholar] [CrossRef]
- Srinivasan, J.; Weirich, T.D.; Marino, G.; Annerino, A.; Taylor, J.M.; Noell, P.; Griego, J.J.M.; Schaller, R.F.; Bryan, C.; Locke, J.; et al. Long-Term Effects of Humidity on Stainless Steel Pitting in Sea Salt Exposures. J. Electrochem. Soc. 2021, 168, 021501. [Google Scholar] [CrossRef]
- ASTM International. A240/A240M-19. Standard Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM International. E527-16. Standard Practice for Numbering Metals and Alloys in the Unified Numbering System (UNS); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Pilkey, W.D.; Pilkey, D.F.; Bi, Z. Peterson’s Stress Concentration Factors; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Cerit, M. Numerical investigation on torsional stress concentration factor at the semi elliptical corrosion pit. Corros. Sci. 2013, 67, 225–232. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yan, Y.; Qiao, L.J.; Volinsky, A. Stainless steel pitting and early-stage stress corrosion cracking under ultra-low elastic load. Corros. Sci. 2013, 77, 360–368. [Google Scholar] [CrossRef]
- Paik, J.K.; Thayamballi, A.K. Ultimate strength of ageing ships. Proc. Inst. Mech. Eng. Part M: J. Eng. Marit. Environ. 2002, 216, 57–77. [Google Scholar] [CrossRef]
- Melchers, R.E. Progress in developing realistic corrosion models. Struct. Infrastruct. Eng. 2018, 14, 843–853. [Google Scholar] [CrossRef]
- Melchers, R. Modeling of Marine Immersion Corrosion for Mild and Low-Alloy Steels—Part 1: Phenomenological Model. Corrosion 2003, 59, 319–334. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Schumacher, M.M. Seawater Corrosion Handbook; Noyes Data Corporation: Park Ridge, NJ, USA, 1979. [Google Scholar]
- Mohammadi, F.; Nickchi, T.; Attar, M.; Alfantazi, A. EIS study of potentiostatically formed passive film on 304 stainless steel. Electrochimica Acta 2011, 56, 8727–8733. [Google Scholar] [CrossRef]
- Guo, L.; Mi, N.; Mohammed-Ali, H.; Ghahari, M.; Du Plessis, A.; Cook, A.; Street, S.; Reinhard, C.; Atwood, R.C.; Rayment, T.; et al. Effect of Mixed Salts on Atmospheric Corrosion of 304 Stainless Steel. J. Electrochem. Soc. 2019, 166, C3010–C3014. [Google Scholar] [CrossRef]
- Prošek, T.; Iversen, A.; Taxen, C.; Thierry, D. Low-Temperature Stress Corrosion Cracking of Stainless Steels in the Atmosphere in the Presence of Chloride Deposits. Corrosion 2009, 65, 105–117. [Google Scholar] [CrossRef]
- Ghahari, M.; Krouse, D.; Laycock, N.; Rayment, T.; Padovani, C.; Stampanoni, M.; Marone, F.; Mokso, R.; Davenport, A.J. Synchrotron X-ray radiography studies of pitting corrosion of stainless steel: Extraction of pit propagation parameters. Corros. Sci. 2015, 100, 23–35. [Google Scholar] [CrossRef] [Green Version]

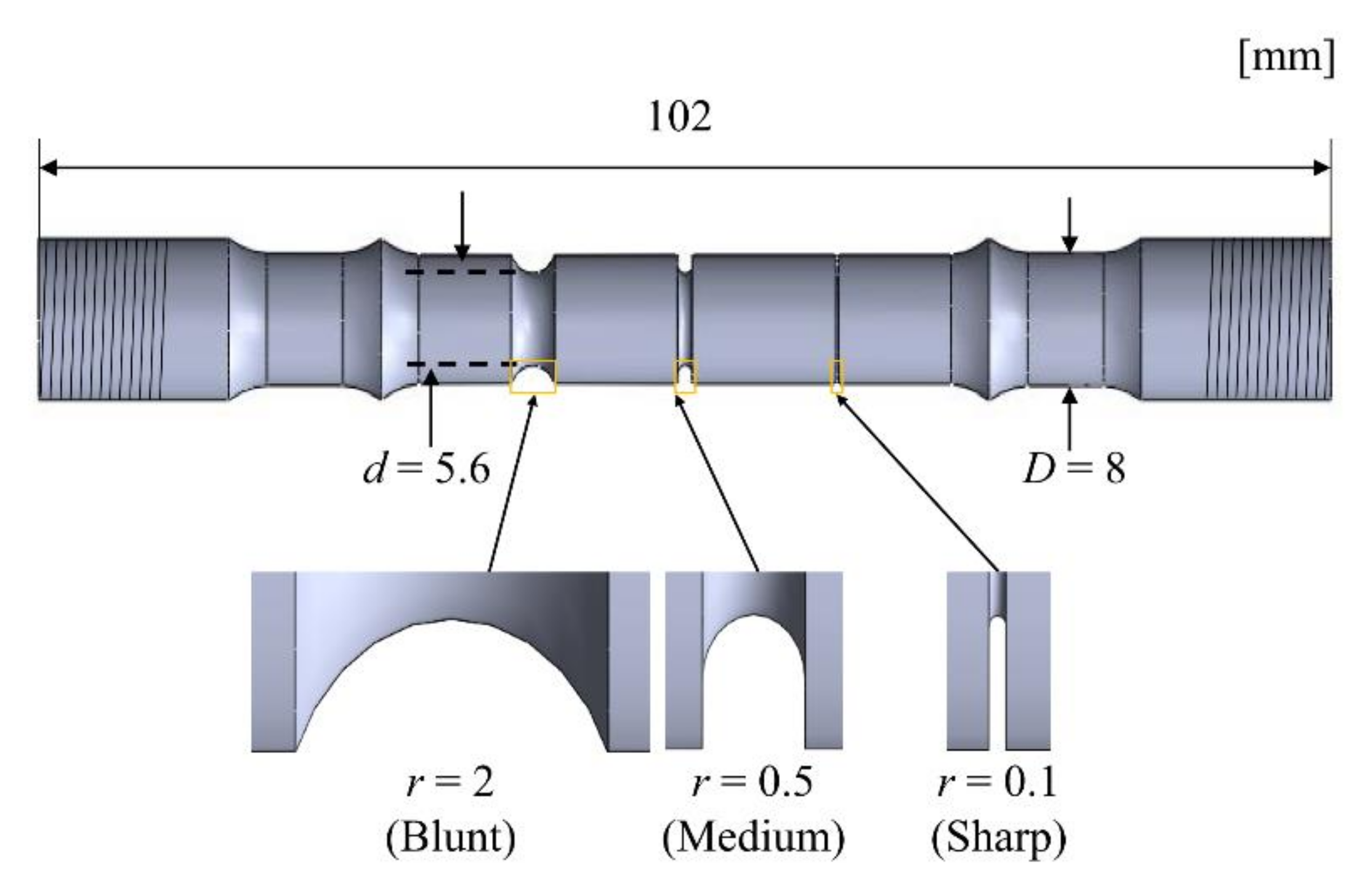
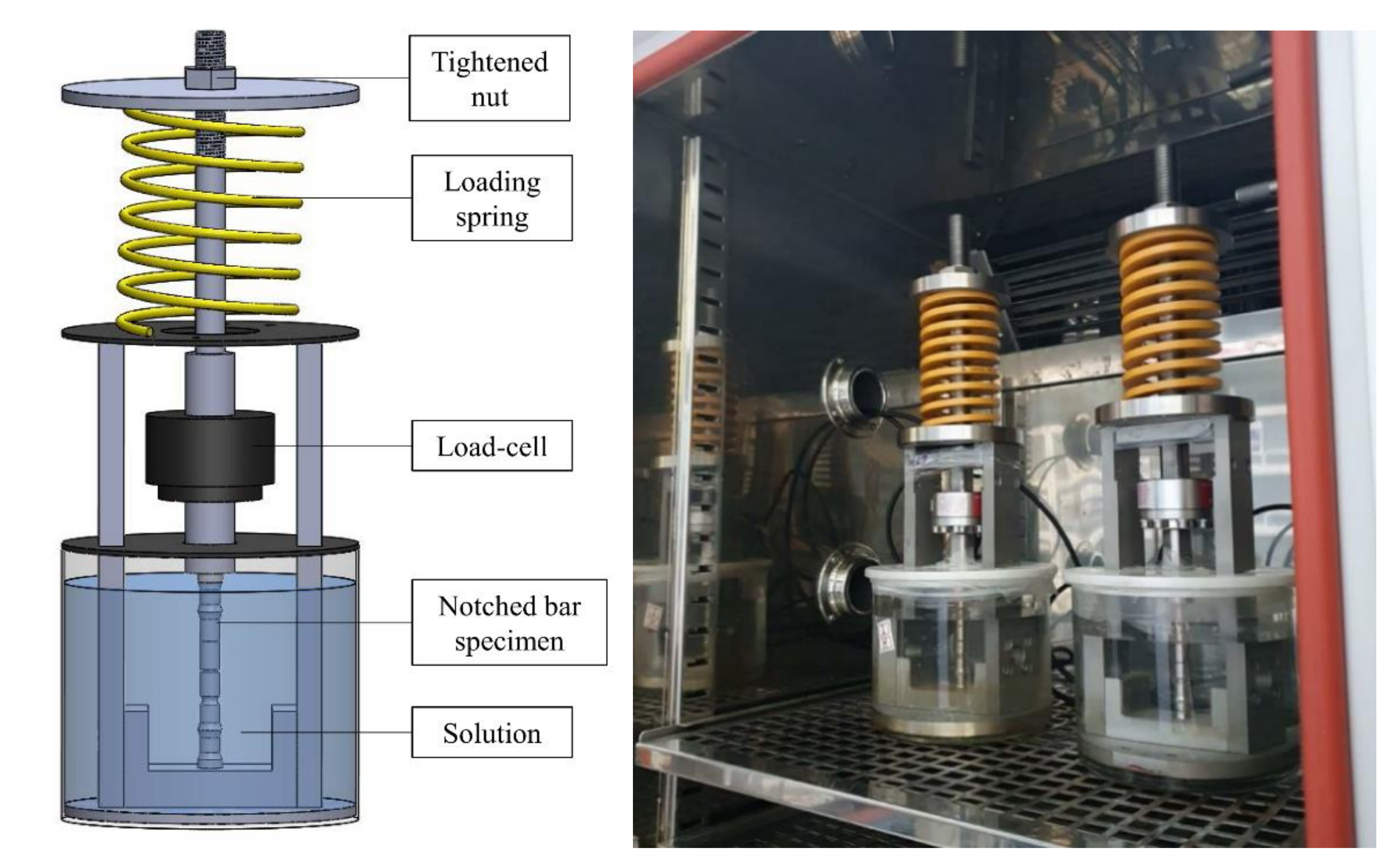
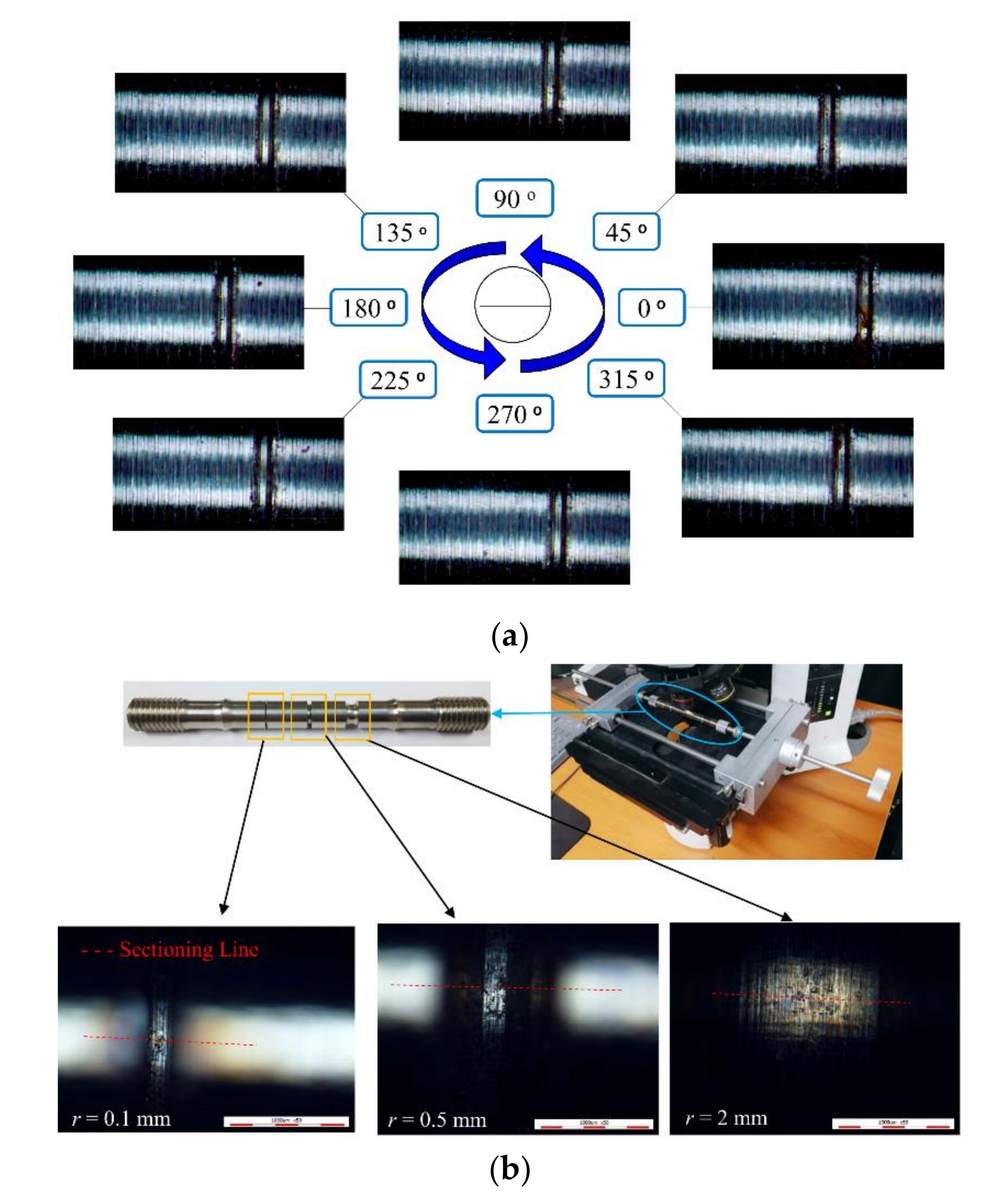
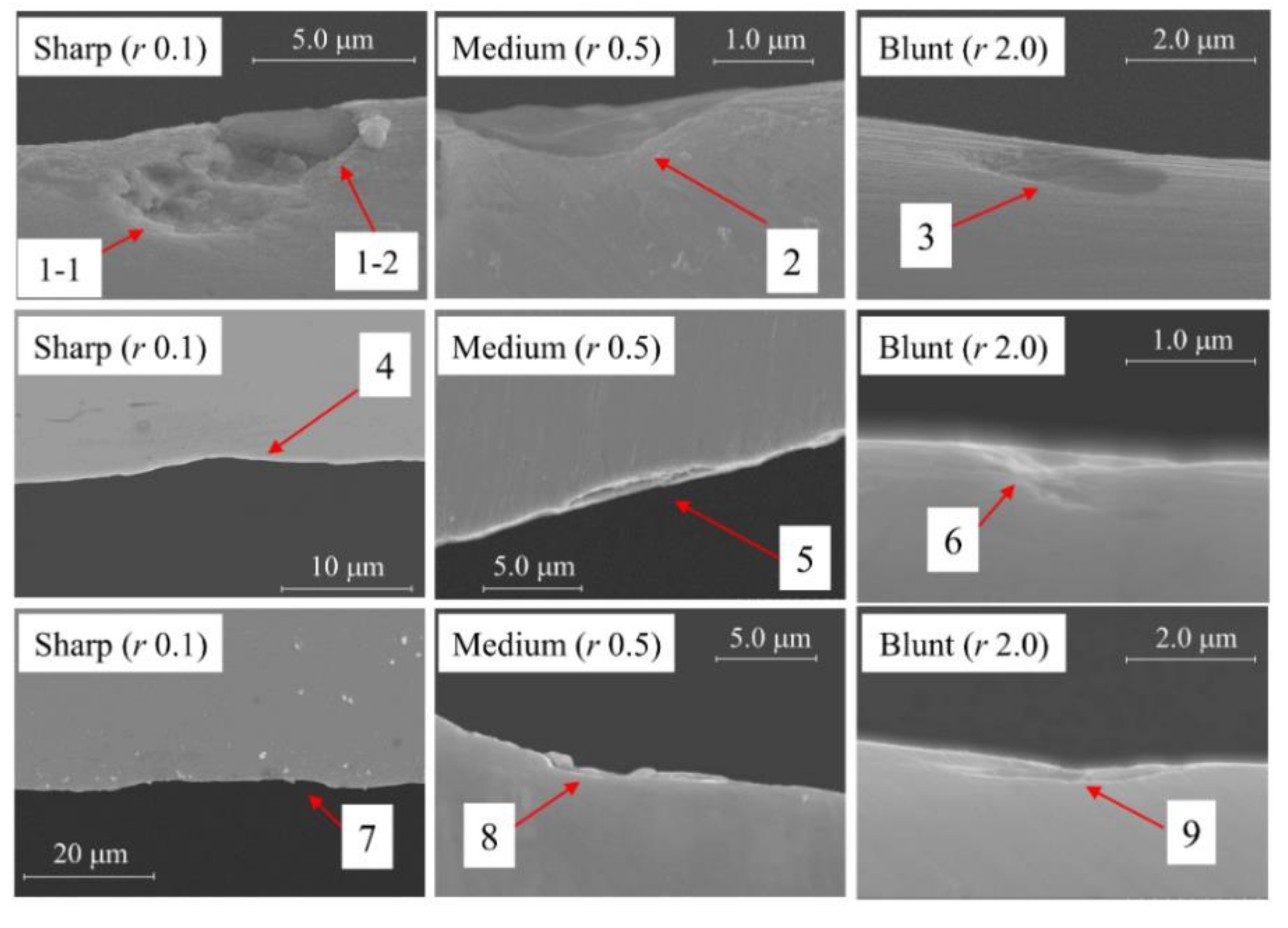


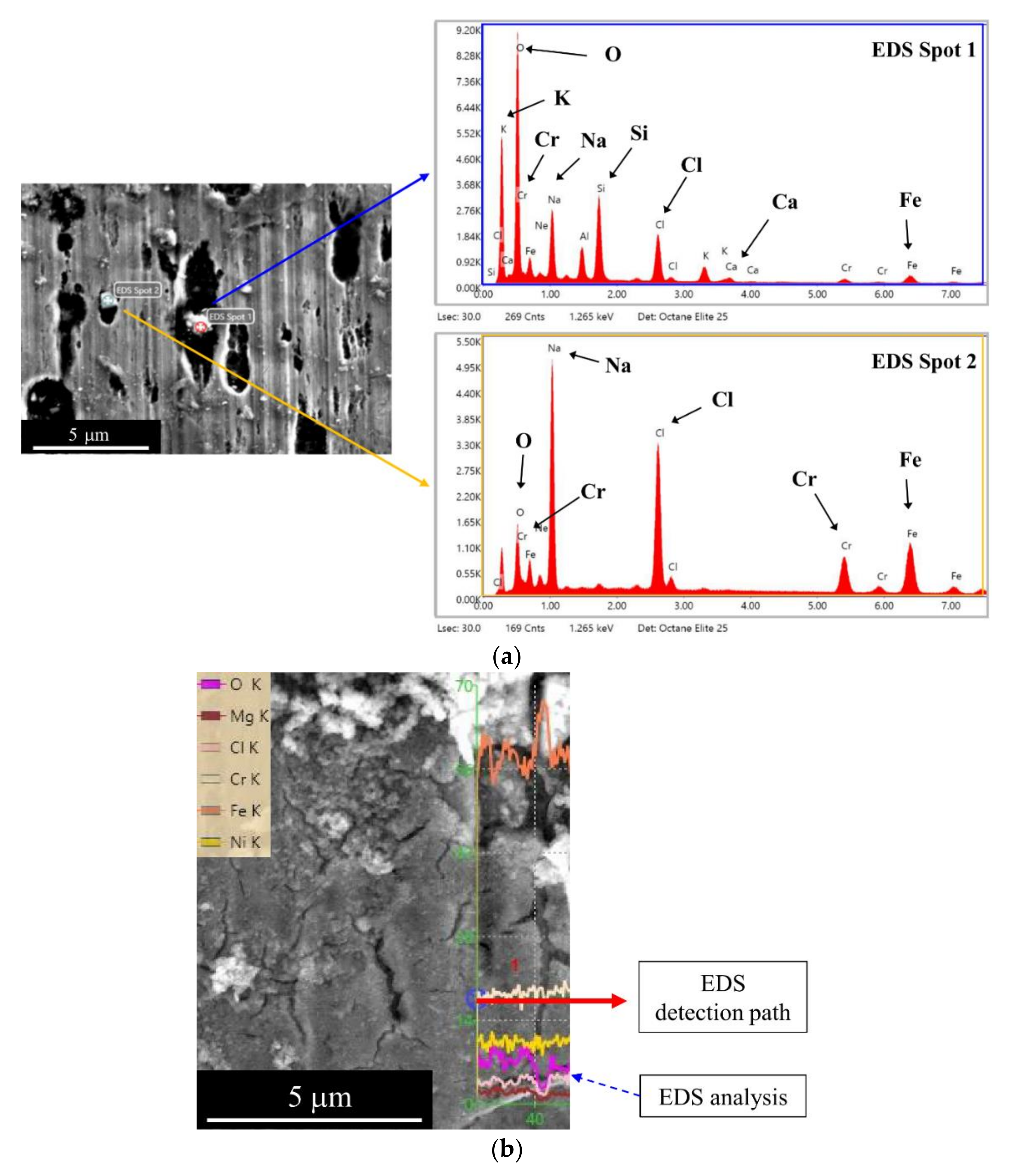
| Chemical Composition | C | Si | Mn | P | S | Ni | Cr |
|---|---|---|---|---|---|---|---|
| Portion [%] | 0.05 | 0.62 | 1.01 | 0.029 | 0.004 | 8.07 | 18.22 |
| Specimen Number | Notch Number | r 1 [mm] | Load [N] | σnet 2 [MPa] | Ktn 3 | σnotch 4 [Mpa] | Test Period [Hour] |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.1 | 1443.1 | 58.6 | 5.4 | 316.4 | 135 |
| 2 | 0.5 | 2.6 | 152.3 | ||||
| 3 | 2.0 | 1.6 | 93.7 | ||||
| 2 | 4 | 0.1 | 1456.2 | 59.1 | 5.4 | 319.3 | 432 |
| 5 | 0.5 | 2.6 | 153.7 | ||||
| 6 | 2.0 | 1.6 | 94.6 | ||||
| 3 | 7 | 0.1 | 1531.5 | 62.2 | 5.4 | 335.8 | 708 |
| 8 | 0.5 | 2.6 | 161.7 | ||||
| 9 | 2.0 | 1.6 | 99.5 |
| Specimen Number | Pit Number | a 1 [μm] | 2c 2 [μm] | a/2c | da/dt 3 [μm/Year] | σpit 4 [MPa] | σpit/σnotch |
|---|---|---|---|---|---|---|---|
| 1 | 1-1 | 0.99 | 5.58 | 0.18 | 64.11 | 477.8 | 1.51 |
| 1-2 | 1.05 | 4.44 | 0.24 | 68.00 | 518.4 | 1.64 | |
| 2 | 0.43 | 2.69 | 0.16 | 27.97 | 216.6 | 1.42 | |
| 3 | 0.17 | 3.09 | 0.06 | 11.23 | 112.0 | 1.20 | |
| 2 | 4 | 1.04 | 15.6 | 0.07 | 21.09 | 396.8 | 1.24 |
| 5 | 0.74 | 7.83 | 0.09 | 14.90 | 199.0 | 1.29 | |
| 6 | 0.17 | 1.49 | 0.06 | 3.386 | 114.9 | 1.21 | |
| 3 | 7 | 1.51 | 33.8 | 0.04 | 18.66 | 435.1 | 1.30 |
| 8 | 0.78 | 9.63 | 0.12 | 9.626 | 223.0 | 1.38 | |
| 9 | 0.33 | 4.48 | 0.09 | 4.083 | 128.8 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-Y.; Jeong, C.; Kim, Y.-J.; Jang, C. Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments. Metals 2021, 11, 1415. https://doi.org/10.3390/met11091415
Jeong J-Y, Jeong C, Kim Y-J, Jang C. Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments. Metals. 2021; 11(9):1415. https://doi.org/10.3390/met11091415
Chicago/Turabian StyleJeong, Jae-Yoon, Chaewon Jeong, Yun-Jae Kim, and Changheui Jang. 2021. "Effect of Stress Magnitude on Pit Growth Rate of 304 Austenitic Stainless Steel in Chloride Environments" Metals 11, no. 9: 1415. https://doi.org/10.3390/met11091415






