Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection
Abstract
1. Introduction
2. Growth Factors in Liver Tumorigenesis and Liver Regeneration
2.1. Hepatocyte Growth Factor (HGF)
2.2. Epidermal Growth Factor (EGF)
2.3. Insulin-Like Growth Factor-1 (IGF-1)
2.4. Hepatocyte Growth Factor, Insulin-Like Growth Factor and Epidermal Growth Factor and Their Link with Vascular Endothelial Growth Factor
3. Pharmacological Approaches Targeting HGF, EGF and IGF-1 Approved for the Treatment of Liver Tumorigenesis
3.1. Clinical Strategies in Liver Tumorigenesis and Liver Regeneration Based on Growth Factor Modulation
3.2. Other Drugs Blocking the HGF/c-Met Axis in Liver Tumorigenesis
3.3. Pharmacological Approaches to Modulate EGF and IGF-1 Pathways in Liver Tumorigenesis
3.4. Role of HGF, EGF and IGF-1 in Liver Hepatectomy: Implications of External Regulation
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcR | Acute rejection |
| Akt | Protein kinase B |
| ALT | Alanine aminotransferase |
| AR | Amphiregulin |
| Bim | Bcl-2-like protein 11 |
| BrdU | Bromodeoxyuridine |
| BUN | Blood urea nitrogen |
| BW | Body weight |
| c-Met | Mesenchymal–epithelial transition factor |
| DBD | Brain-dead donor |
| dHGF | deletion variant of HGF |
| DKK1 | Dickkopf-1 |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ERBB3 | Erb-B2 Receptor Tyrosine Kinase 3 |
| ERK | Extracellular signal-regulated kinase |
| FasL | Fas Ligand |
| FoxO3a | Forkhead box protein O3a |
| GH | Growth hormone |
| GOT | Glutamic oxaloacetic transaminase |
| GPT | Glutamate pyruvate transaminase |
| GR | Growth rate |
| GSD Ib: | Glycogen storage disease type Ib |
| HB-EGF | Heparin-binding epidermal growth factor |
| HCC | Hepatocellular carcinoma |
| HGF | Hepatocyte growth factor |
| hHGF | Human hepatocyte growth facto |
| HMGA2 | High-mobility group protein A2 |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-1 R | Insulin-like growth factor receptor |
| IGFBP-1 | Insulin-like growth factor 1 binding protein |
| IL-1β | Interleukin-1β |
| IL-2 | Interleukin 2 |
| IL-8 | Interleukin 8 |
| IRS | Insulin receptor substrate |
| Jnk | c-Jun N-terminal kinase |
| LD | Liver dysfunction |
| LR | Liver regeneration |
| LT | Liver transplantation |
| LW | Liver weight |
| MAPK | Mitogen-activated protein kinase |
| MELD | Model for end-stage liver disease |
| MET | Mesenchymal–epithelial transition factor |
| miRNA | MicroRNAs |
| mTOR | mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OLT | Orthotropic liver transplantation |
| PCNA | Proliferating cell nuclear antigen |
| PD-L1 | Programmed death-ligand 1 |
| PERK | protein kinase R-like endoplasmic reticulum kinase |
| PH | Partial hepatectomy |
| PI3K | Phosphoinositide 3-kinase |
| PLT | Partial liver transplantation |
| rh-HGF activator | recombinant human HGF activator |
| ROS | Reactive oxygen species |
| RSLT | Reduced size liver transplantation |
| SCr | Serum creatinine |
| SD | Sprague-Dawley |
| SF | Scatter factor |
| SIRT1 | Deacetylase sirtuin1 |
| STAT | Signal transducer and activator of transcription |
| TGF- 1β | Tumor growth factor-1β |
| TGFα | Tumor growth factor alpha |
| TNFα | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Sharma, R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: Evidence from GLOBOCAN 2018. Jpn. J. Clin. Oncol. 2020, 50, 1370–1379. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Bai, X.; Liang, T. Evaluation of Intra-Tumoral Vascularization in Hepatocellular Carcinomas. Front. Med. 2020, 7, 584250. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment strategies for hepatocellular carcinoma—A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Bhaijee, F.; Krige, J.E.J.; Locketz, M.L.; Kew, M.C. Liver resection for non-cirrhotic hepatocellular carcinoma in South African patients. S. Afr. J. Surg. 2011, 49, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tomás, T.; Rueda-Robles, A.; Álvarez-Mercado, A.I. Insights into the Role of Dysbiosis in the Progression of Liver Disease: An Update; Cowell, R.I., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; ISBN 978-1-53618-332-0. [Google Scholar]
- Velasco-Loyden, G.; Pérez-Martínez, L.; Vidrio-Gómez, S.; Pérez-Carreón, J.I.; Chagoya de Sánchez, V. Cancer chemoprevention by an adenosine derivative in a model of cirrhosis-hepatocellular carcinoma induced by diethylnitrosamine in rats. Tumor Biol. 2017, 39, 101042831769119. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; Cantarini, M.C.; Wands, J.R.; Bernardi, M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis 2008, 29, 1299–1305. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Ye, S.L.; Liu, Y.K.; Qin, L.X.; Sun, H.C.; Ye, Q.H.; Wang, L.; Zhou, J.; Qiu, S.J.; Li, Y.; et al. A decade’s studies on metastasis of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 187–196. [Google Scholar] [CrossRef]
- Qing, X.; Xu, W.; Zong, J.; Du, X.; Peng, H.Z.Y. Emerging treatment modalities for systemic therapy in hepatocellular carcinoma. Biomark. Res. 2021, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Delou, J.M.A.; Souza, A.S.O.; Souza, L.C.M.; Borges, H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells 2019, 8, 1013. [Google Scholar] [CrossRef]
- Tadatoshi Takayama Surgical treatment for hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2011, 41, 447–454. [CrossRef]
- Jarnagin, W.; Chapman, W.C.; Curley, S.; D’Angelica, M.; Rosen, C.; Dixon, E.; Nagorney, D. Surgical treatment of hepatocellular carcinoma: Expert consensus statement. HPB 2010, 12, 302–310. [Google Scholar] [CrossRef]
- Firtina Karagonlar, Z.; Koc, D.; Iscan, E.; Erdal, E.; Atabey, N. Elevated hepatocyte growth factor expression as an autocrine c-Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci. 2016, 107, 407–416. [Google Scholar] [CrossRef]
- Fu, R.; Jiang, S.; Li, J.; Chen, H.; Zhang, X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med. Oncol. 2020, 37, 24. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-Term Survival and Pattern of Recurrence After Resection of Small Hepatocellular Carcinoma in Patients With Preserved Liver Function. Ann. Surg. 2002, 235, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, R.; Sato, Y.; Kobayashi, Y. Cholangiocarcinoma prognosis varies over time depending on tumor site and pathology. J. Gastrointest. Liver Dis. 2018, 27, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Oña, L.; Lachmann, M. Signalling architectures can prevent cancer evolution. Sci. Rep. 2020, 10, 674. [Google Scholar] [CrossRef]
- Taylor, P.; Stadler, P.F. The hallmarks of cancer A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar]
- Wang, X.; Ding, J.; Feng, Y.; Weng, L.; Zhao, G.; Xiang, J.; Zhang, M.; Xing, D. Targeting of growth factors in the treatment of hepatocellular carcinoma: The potentials of polysaccharides (Review). Oncol. Lett. 2017, 13, 1509–1517. [Google Scholar] [CrossRef]
- Pinter, M.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 2016, 1, e000042. [Google Scholar] [CrossRef]
- Loyer, P.; Cariou, S.; Glaise, D.; Bilodeau, M.; Baffet, G.; Guguen-Guillouzo, C. Growth Factor Dependence of Progression through G1 and S Phases of Adult Rat Hepatocytes in Vitro. J. Biol. Chem. 1996, 271, 11484–11492. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Nagel, A.J.; Tanabe, K.; Fuchs, J.; Dehlke, K.; Ghamarnejad, O.; Lemekhova, A.; Mehrabi, A. Markers of liver regeneration—The role of growth factors and cytokines: A systematic review. BMC Surg. 2020, 20, 31. [Google Scholar] [CrossRef]
- Breuhahn, K.; Longerich, T.; Schirmacher, P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene 2006, 25, 3787–3800. [Google Scholar] [CrossRef] [PubMed]
- Orcutt, S.T.; Anaya, D.A. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control 2018, 25, 1073274817744621. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Casillas-Ramírez, A.; Peralta, C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: A 2015 update. Clin. Sci. 2015, 129, 345–362. [Google Scholar] [CrossRef]
- Kong, X.; Wu, W.; Yuan, Y.; Pandey, V.; Wu, Z.; Lu, X.; Zhang, W.; Chen, Y.; Wu, M.; Zhang, M.; et al. Human growth hormone and human prolactin function as autocrine/paracrine promoters of progression of hepatocellular carcinoma. Oncotarget 2016, 7, 29465–29479. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Bujaldon, E.; Gracia-Sancho, J.; Peralta, C. The role of adipokines in surgical procedures requiring both liver regeneration and vascular occlusion. Int. J. Mol. Sci. 2018, 19, 3395. [Google Scholar] [CrossRef]
- Shi, J.H.; Line, P.D. Effect of liver regeneration on malignant hepatic tumors. World J. Gastroenterol. 2014, 20, 16167–16177. [Google Scholar] [CrossRef] [PubMed]
- Shiota, G.; Wang, T.C.; Nakamura, T.; Schmidt, E.V. Hepatocyte growth factor in transgenic mice: Effects on hepatocyte growth, liver regeneration and gene expression. Hepatology 1994, 19, 962–972. [Google Scholar] [CrossRef]
- Xue, F.; Takahara, T.; Yata, Y.; Kuwabara, Y.; Shinno, E.; Nonome, K.; Minemura, M.; Takahara, S.; Li, X.; Yamato, E.; et al. Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut 2003, 52, 694–700. [Google Scholar] [CrossRef]
- Tashiro, H.; Fudaba, Y.; Itoh, H.; Mizunuma, K.; Ohdan, H.; Itamoto, T.; Asahara, T. Hepatocyte growth factor prevents chronic allograft dysfunction in liver-transplanted rats. Transplantation 2003, 76, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Inoue, T.; Oda, M.; Kwon, A.H.; Kamiyama, Y.; Okumura, T.; Naka, D.; Kawaguchi, T.; Kitamura, N.; Miyazawa, K. Exogenously administered HGF activator augments liver regeneration through the production of biologically active HGF. Biochem. Biophys. Res. Commun. 2002, 290, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gan, G.; Wang, X.; Xu, T.; Xie, W. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy 2019, 15, 1258–1279. [Google Scholar] [CrossRef]
- Wang, H.; Rao, B.; Lou, J.; Li, J.; Liu, Z.; Li, A.; Cui, G.; Ren, Z.; Yu, Z. The Function of the HGF/c-Met Axis in Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2020, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hu, C.T.; Cheng, C.C.; Lee, M.C.; Pan, S.M.; Lin, T.Y.; Wu, W.S. Oxidation of heat shock protein 60 and protein disulfide isomerase activates ERK and migration of human hepatocellular carcinoma HepG2. Oncotarget 2016, 7, 11067–11082. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.; Shi, X.; Wang, H.; Cui, C.; Xiao, F.; Wu, C.T.; Guo, X.; Wang, L. SENP1 regulates hepatocyte growth factor-induced migration and epithelial-mesenchymal transition of hepatocellular carcinoma. Tumor Biol. 2016, 37, 7741–7748. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hanazawa, T.; Nohata, N.; Kikkawa, N.; Enokida, H.; Yoshino, H.; Yamasaki, T.; Hidaka, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget 2012, 3, 1386–1400. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, X.H.; Liu, H.H.; Ma, M.; Chen, J.; Chen, J.; Gao, D.M.; Cui, J.F.; Chen, R.X. Activated hepatic stellate cells promote progression of post-heat residual hepatocellular carcinoma from autophagic survival to proliferation. Int. J. Hyperth. 2019, 36, 253–263. [Google Scholar] [CrossRef]
- He, M.; Peng, A.; Huang, X.Z.; Shi, D.C.; Wang, J.C.; Zhao, Q.; Lin, H.; Kuang, D.M.; Ke, P.F.; Lao, X.M. Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. OncoImmunology 2016, 5, e1219828. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Charteris, D.G.; Sethi, C.S.; Fisher, S.K. Animal models of retinal detachment and reattachment: Identifying cellular events that may affect visual recovery. Eye 2002, 16, 375–387. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. MicroRNA-101-3p suppresses proliferation and migration in hepatocellular carcinoma by targeting the HGF/c-Met pathway. Investig. New Drugs 2020, 38, 60–69. [Google Scholar] [CrossRef]
- Yamada, N.; Matsushima-Nishiwaki, R.; Kozawa, O. Quercetin suppresses the migration of hepatocellular carcinoma cells stimulated by hepatocyte growth factor or transforming growth factor-α: Attenuation of AKT signaling pathway. Arch. Biochem. Biophys. 2020, 682, 108296. [Google Scholar] [CrossRef]
- Kiseleva, Y.V.; Antonyan, S.Z.; Zharikova, T.S.; Zharikov, Y.O.; Tupikin, K.A.; Kalinin, D.V. Molecular pathways of liver regeneration: A comprehensive review. World J. Hepatol. 2021, 13, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Franco-Gou, R.; Peralta, C.; Massip-Salcedo, M.; Xaus, C.; Serafín, A.; Roselló-Catafau, J. Protection of reduced-size liver for transplantation. Am. J. Transplant. 2004, 4, 1408–1420. [Google Scholar] [CrossRef]
- Lindroos, P.M.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 1991, 13, 743–750. [Google Scholar] [CrossRef]
- Ishiki, Y.; Ohnishi, H.; Muto, Y.; Matsumoto, K.; Nakamura, T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology 1992, 16, 1227–1235. [Google Scholar] [CrossRef]
- Adamek, B.; Zalewska-Ziob, M.; Strzelczyk, J.K.; Kasperczyk, J.; Wolkowska-Pokrywa, K.; Spausta, G.; Hudziec, E.; Wiczkowski, A.; Swietochowska, E.; Kukla, M.; et al. Hepatocyte growth factor and epidermal growth factor activity during later stages of rat liver regeneration upon interferon α-2b influence. Clin. Exp. Hepatol. 2017, 3, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Khosravi, A.; Torabian, P.; Gholipoor, N.; Samaei, N.M. Association of the epidermal growth factor gene +61A>G polymorphism with hepatocellular carcinoma in an Iranian population. Gastroenterol. Hepatol. Bed Bench 2017, 10, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, I.; Ella, K.A.; El Shaarawy, A.; Elshayb, E.; El-Rebey, H.S.; El Hoseeny, M.M.; Naguib, M.; Nada, A. Genetic polymorphism of epidermal growth factor gene as a predictor of hepatocellular carcinoma in hepatitis c cirrhotic patients. Asian Pac. J. Cancer Prev. 2020, 21, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, X.; Wang, L.; Zhu, B.; Wang, X.; Xia, J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell. Mol. Med. 2014, 18, 218–230. [Google Scholar] [CrossRef]
- Niu, J.; Li, W.; Liang, C.; Wang, X.; Yao, X.; Yang, R.H.; Zhang, Z.S.; Liu, H.F.; Liu, F.Y.; Pei, S.H.; et al. EGF promotes DKK1 transcription in hepatocellular carcinoma by enhancing the phosphorylation and acetylation of histone H3. Sci. Signal. 2020, 13. [Google Scholar] [CrossRef]
- Liang, C.; Niu, J.; Wang, X.; Zhang, Z.S.; Yang, R.H.; Yao, X.; Liu, F.Y.; Li, W.Q.; Pei, S.H.; Sun, H.; et al. P300-dependent acetylation of histone H3 is required for epidermal growth factor receptor-mediated high-mobility group protein A2 transcription in hepatocellular carcinoma. Cancer Sci. 2021, 112, 679–690. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Yao, X.; Yang, R.H.; Zhang, Z.S.; Liu, F.Y.; Li, W.Q.; Pei, S.H.; Ma, J.; Xie, S.Q.; et al. PKM2-Induced the Phosphorylation of Histone H3 Contributes to EGF-Mediated PD-L1 Transcription in HCC. Front. Pharmacol. 2020, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, D.; Ning, F.; Du, J.; Wang, H. EGF is highly expressed in hepatocellular carcinoma (HCC) and promotes motility of HCC cells via fibronectin. J. Cell. Biochem. 2018, 119, 4170–4183. [Google Scholar] [CrossRef]
- Liu, Z.C.; Ning, F.; Wang, H.F.; Chen, D.Y.; Cai, Y.N.; Sheng, H.Y.; Lash, G.E.; Liu, L.; Du, J. Epidermal growth factor and tumor necrosis factor α cooperatively promote the motility of hepatocellular carcinoma cell lines via synergistic induction of fibronectin by NF-κB/p65. Biochim. Et Biophys. Acta Gen. Subj. 2017, 1861, 2568–2582. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Kim, H.; Kim, Y.J.; Jeon, B.N.; Kang, H.B.; Ko, H. Catechol inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition and stem cell-like properties in hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 7620. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Kim, H.; Ko, H. Delphinidin inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma cells. J. Cell. Biochem. 2019, 120, 9887–9899. [Google Scholar] [CrossRef]
- Li, X.; Tsauo, J.; Geng, C.; Zhao, H.; Lei, X.; Li, X. Ginsenoside Rg3 decreases NHE1 expression via inhibiting EGF-EGFR-ERK1/2-HIF-1 α pathway in hepatocellular carcinoma: A novel antitumor mechanism. Am. J. Chin. Med. 2018, 46, 1915–1931. [Google Scholar] [CrossRef]
- Si, H.; Genna, B.; Zhuang, X.; Wang, J.; Burenbatu, B.; Feng, Q.; Wang, H. DaHuangWan targets EGF signaling to inhibit the proliferation of hepatoma cells. PLoS ONE 2020, 15, e0231466. [Google Scholar] [CrossRef]
- Kiso, S.; Kawata, S.; Tamura, S.; Higashiyama, S.; Ito, N.; Tsushima, H.; Taniguchi, N.; Matsuzawa, Y. Role of heparin-binding epidermal growth factor-like growth factor as a hepatotrophic factor in rat liver regeneration after partial hepatectomy. Hepatology 1995, 22, 1584–1590. [Google Scholar] [CrossRef]
- Deng, M.; Huang, H.; Dirsch, O.; Dahmen, U. Effect and risk of AEE788, a dual tyrosine kinase inhibitor, on regeneration in a rat liver resection model. Eur. Surg. Res. 2010, 44, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Young, W.P.; Younes, M.N.; Jasser, S.A.; Yigitbasi, O.G.; Zhou, G.; Bucana, C.D.; Bekele, B.N.; Myers, J.N. AEE788, a dual tyrosine kinase receptor inhibitor, induces endothelial cell apoptosis in human cutaneous squamous cell carcinoma xenografts in nude mice. Clin. Cancer Res. 2005, 11, 1963–1973. [Google Scholar] [CrossRef]
- Younes, M.N.; Park, Y.W.; Yazici, Y.D.; Gu, M.; Santillan, A.A.; Nong, X.; El-Naggar, A.K.; Myes, J.N. Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary edenoid cystic carcinoma in an orthotopic nude mouse model. Mol. Cancer Ther. 2006, 5, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Kiso, S.; Kawata, S.; Tamura, S.; Inui, Y.; Yoshida, Y.; Sawai, Y.; Umeki, S.; Ito, N.; Yamada, A.; Miyagawa, J.I.; et al. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology 2003, 124, 701–707. [Google Scholar] [CrossRef]
- Glanemann, M.; Shi, B.; El-Zidy, N.; Gaebelein, G.; Kronbach, Z.; Neuhaus, P.; Nussler, A.K. Subcutaneous administration of epidermal growth factor: A true treatment option in case of postoperative liver failure? Int. J. Surg. 2009, 7, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Wagner, B.; Sibilia, M. The EGF receptor is required for efficient liver regeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 17081–17086. [Google Scholar] [CrossRef]
- Liu, Q.; Rehman, H.; Krishnasamy, Y.; Haque, K.; Schnellmann, R.G.; Lemasters, J.J.; Zhong, Z. Amphiregulin stimulates liver regeneration after small-for-size mouse liver transplantation. Am. J. Transplant. 2012, 12, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mercado, A.I.; Negrete-Sánchez, E.; Gulfo, J.; Ávalos De León, C.G.; Casillas-Ramírez, A.; Cornide-Petronio, M.E.; Bujaldon, E.; Rotondo, F.; Gracia-Sancho, J.; Jiménez-Castro, M.B.; et al. EGF-GH axis in rat steatotic and non-steatotic liver transplantation from brain-dead donors. Transplantation 2019, 103, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Fujita, J.; Marino, M.W.; Wada, H.; Jungbluth, A.A.; Mackrell, P.J.; Rivadeneira, D.E.; Stapleton, P.P.; Daly, J.M. Effect of TNF gene depletion on liver regeneration after partial hepatectomy in mice. Surgery 2001, 129, 48–54. [Google Scholar] [CrossRef]
- Ledda-Columbano, G.M.; Curto, M.; Piga, R.; Zedda, A.I.; Menegazzi, M.; Sartori, C.; Shinozuka, H.; Bluethmann, H.; Poli, V.; Ciliberto, G.; et al. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene 1998, 17, 1039–1044. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.X.; Li, C.H.; Zhang, A.Q.; Jiang, S.; Lai, Y.H.; Ge, X.L.; Pan, K.; Dong, J.H. mTOR-Dependent Suppression of Remnant Liver Regeneration in Liver Failure After Massive Liver Resection in Rats. Dig. Dis. Sci. 2015, 60, 2718–2729. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Liu, B.; Lu, J.; Li, H.; Zhu, H.; Gao, H.; Zhou, X.; Chang, H. Insulin-like growth factor-1 induces epithelial-mesenchymal transition in hepatocellular carcinoma by activating survivin. Oncol. Rep. 2018, 40, 952–958. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.C.; Deng, M.; Jiang, H.Y.; Guo, L.H.; Zhou, W.J.; Ruan, B. Serum insulin-like growth factor-1 and its binding protein 3 as prognostic factors for the incidence, progression, and outcome of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 81098–81108. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wang, X.; Wang, H. Esculetin induces apoptosis of SMMC-7721 cells through IGF-1/PI3K/Akt-mediated mitochondrial pathways. Can. J. Physiol. Pharmacol. 2017, 95, 787–794. [Google Scholar] [CrossRef]
- Yao, Y.; Mao, W.; Dong, M.; Yang, D.; Li, W.; Chen, Y. Serum Insulin-Like Growth Factor-1 (IGF-1): A Novel Prognostic Factor for Early Recurrence of Hepatocellular Carcinoma (HCC). Clin. Lab. 2017, 63, 261–270. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic acid inhibits proliferation and invasion of hepatocellular carcinoma cells SMMC 7721 via PI3K/AKT/mTOR signal pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Wang, B.; Sun, Q.; He, Z.; Hong, J.; Kuehn, F.; Liu, E.; Zhang, Z. IGF-1 induces the epithelial-mesenchymal transition via Stat5 in hepatocellular carcinoma. Oncotarget 2017, 8, 111922–111930. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, Z.; Chen, C.; Liu, Y.; Yuan, D.; Hei, Z.; Luo, G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021, 272, 119119. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Wendum, D.; Cadoret, A.; Rey, C.; Leneuve, P.; Blaise, A.; Housset, C.; Tronche, F.; Le Bouc, Y.; Holzenberger, M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J. 2006, 20, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Rajkumar, K.; Murphy, L.J. Hepatic regeneration in insulin-like growth factor binding protein-1 transgenic mice. J. Hepatol. 1999, 30, 674–680. [Google Scholar] [CrossRef]
- Castro, G.R.A.; Coelho, J.C.U.; Parolin, M.B.; Matias, J.E.F.; de Freitas, A.C.T. Insulin-like growth factor i correlates with MELD and returns to normal level after liver transplantation. Ann. Transplant. 2013, 18, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, D.; Mocchegiani, F.; Palmonella, G.; Coletta, M.; Brugia, M.; Montalti, R.; Fava, G.; Taccaliti, A.; Risaliti, A.; Vivarelli, M. Postoperative insulin-like growth factor 1 levels reflect the graft’s function and predict survival after liver transplantation. PLoS ONE 2015, 10, e0133153. [Google Scholar] [CrossRef] [PubMed]
- Salso, A.; Tisone, G.; Tariciotti, L.; Lenci, I.; Manzia, T.M.; Baiocchi, L. Relationship between GH/IGF-1 Axis, graft recovery, and early survival in patients undergoing liver transplantation. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Szili, B.; Görög, D.; Gerlei, Z.; Gyori, G.; Lakatos, P.; Takács, I. Rapid height growth after liver transplantation in adulthood. Growth Horm. IGF Res. 2016, 29, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pagès, G.; Pouysségur, J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene—A concert of activating factors. Cardiovasc. Res. 2005, 65, 564–573. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef]
- Arii, S. Role of vascular endothelial growth factor on the invasive potential of hepatocellular carcinoma. J. Hepatol. 2004, 41, 333–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zhu, Y.; Kadel, D.; Sun, H.; Chen, J.; Luo, Q.; Sun, H.; Yang, L.; Yang, J.; et al. The dual blockade of MET and VEGFR2 signaling demonstrates pronounced inhibition on tumor growth and metastasis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 93. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Wan, X.; Zhang, Y.; Zhu, R.; Liu, Z.; Ji, D.; Zhang, H.; Wu, F.; Tian, H.; et al. Repression of Human Hepatocellular Carcinoma Growth by Regulating Met/EGFR/VEGFR-Akt/NF-κB Pathways with Theanine and Its Derivative, (R)-2-(6,8-Dibromo-2-oxo-2H-chromene-3-carboxamido)-5-(ethylamino)-5-oxopentanoic Ethyl Ester (DTBrC). J. Agric. Food Chem. 2016, 64, 7002–7013. [Google Scholar] [CrossRef]
- Lee, C.; Whang, Y.M.; Campbell, P.; Mulcrone, P.L.; Elefteriou, F.; Cho, S.W.; Park, S.I. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett. 2018, 414, 205–213. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.; Li, W.; Liu, T.; Deng, G.; Liu, W.; Liu, H.; Gao, F. Deguelin suppresses angiogenesis in human hepatocellular carcinoma by targeting HGF-c-Met pathway. Oncotarget 2018, 9, 152–166. [Google Scholar] [CrossRef]
- Bockhorn, M.; Goralski, M.; Prokofiev, D.; Dammann, P.; Grünewald, P.; Trippler, M.; Biglarnia, A.; Kamler, M.; Niehues, E.M.; Frilling, A.; et al. VEGF is Important for Early Liver Regeneration After Partial Hepatectomy. J. Surg. Res. 2007, 138, 291–299. [Google Scholar] [CrossRef]
- Bujaldon, E.; Cornide-Petronio, M.E.; Gulfo, J.; Rotondo, F.; Ávalos de León, C.; Negrete-Sánchez, E.; Gracia-Sancho, J.; Novials, A.; Jiménez-Castro, M.B.; Peralta Uroz, C. Relevance of VEGFA in rat livers subjected to partial hepatectomy under ischemia-reperfusion. J. Mol. Med. 2019, 97, 1299–1314. [Google Scholar] [CrossRef]
- Gu, Y.; Sowa, J.P.; Paul, A.; Gerken, G.; Schlaak, J.F. Vascular endothelial growth factor improves liver regeneration and survival after 90% hepatectomy in a rat model of diet-induced steatosis. Digestion 2013, 88, 235–242. [Google Scholar] [CrossRef]
- Dong, N.; Shi, X.; Wang, S.; Gao, Y.; Kuang, Z.; Xie, Q.; Li, Y.; Deng, H.; Wu, Y.; Li, M.; et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br. J. Cancer 2019, 121, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.O.N.; Tong, M.; Chung, K.P.S.; Zhou, L.; Che, N.; Tang, K.H.; Ding, J.; Lau, E.Y.T.; Ng, I.O.L.; Ma, S.; et al. Overriding Adaptive Resistance to Sorafenib Through Combination Therapy With Src Homology 2 Domain–Containing Phosphatase 2 Blockade in Hepatocellular Carcinoma. Hepatology 2020, 72, 155–168. [Google Scholar] [CrossRef]
- Deng, Y.R.; Liu, W.B.; Lian, Z.X.; Li, X.; Hou, X. Sorafenib inhibits macrophage-mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget 2016, 7, 38292–38305. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, J.; Zhang, Y.; Cai, H.; Chen, X.; Sun, D. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS Open Bio 2019, 9, 335–347. [Google Scholar] [CrossRef]
- Yuan, S.; Gong, Y.; Chen, R.; Du, J.; Zhang, H.; Chen, T. Chinese herbal formula QHF inhibits hepatocellular carcinoma metastasis via HGF/c-Met signaling pathway. Biomed. Pharmacother. 2020, 132, 110867. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Deng, G.; Liu, W.; Zhou, K.; Li, M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol. Rep. 2017, 37, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7. [Google Scholar] [CrossRef]
- D’Angelo, A.; Sobhani, N.; Bagby, S.; Casadei-Gardini, A.; Roviello, G. Cabozantinib as a second-line treatment option in hepatocellular carcinoma. Expert Rev. Clin. Pharmacol. 2020, 13, 623–629. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Cabozantinib: A Review in Advanced Hepatocellular Carcinoma. Target. Oncol. 2019, 14, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Morimoto, M.; Moriguchi, M.; Izumi, N.; Takayama, T.; Yoshiji, H.; Hino, K.; Oikawa, T.; Chiba, T.; Motomura, K.; et al. A randomized, double-blind, placebo-controlled, phase 3 study of tivantinib in Japanese patients with MET-high hepatocellular carcinoma. Cancer Sci. 2020, 111, 3759–3769. [Google Scholar] [CrossRef]
- Best, J.; Schotten, C.; Lohmann, G.; Gerken, G.; Dechêne, A. Tivantinib for the treatment of hepatocellular carcinoma. Expert Opin. Pharmacother. 2017, 18, 727–733. [Google Scholar] [CrossRef]
- Kobayashi, K.; Higai, K.; Mukozu, T.; Matsui, D.; Amanuma, M.; Yoshimine, N.; Ogino, Y.; Matsui, T.; Wakui, N.; Shinohara, M.; et al. Tivantinib decreases hepatocyte growth factor-induced BCRP expression in hepatocellular carcinoma HepG2 cells. Biol. Pharm. Bull. 2020, 43, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Rota Caremoli, E.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Falchook, G.S.; Kurzrock, R.; Amin, H.M.; Xiong, W.; Fu, S.; Piha-Paul, S.A.; Janku, F.; Eskandari, G.; Catenacci, D.V.; Klevesath, M.; et al. First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin. Cancer Res. 2020, 26, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Yamazaki, K.; Tsushima, T.; Naito, T.; Matsubara, N.; Watanabe, M.; Sarholz, B.; Johne, A.; Doi, T. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn. J. Clin. Oncol. 2020, 50, 859–866. [Google Scholar] [CrossRef]
- Ryoo, B.Y.; Cheng, A.L.; Ren, Z.; Kim, T.Y.; Pan, H.; Rau, K.M.; Choi, H.J.; Park, J.W.; Kim, J.H.; Yen, C.J.; et al. Randomised Phase 1b/2 trial of tepotinib vs sorafenib in Asian patients with advanced hepatocellular carcinoma with MET overexpression. Br. J. Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Sueangoen, N.; Tantiwetrueangdet, A.; Panvichian, R. HCC-derived EGFR mutants are functioning, EGF-dependent, and erlotinib-resistant. Cell Biosci. 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, H.; Li, M.; Wu, H.; Guo, Y.; Chen, J.; Shan, J.; Chen, X.; Shen, J.; Ma, Q.; et al. KIAA1199 promotes sorafenib tolerance and the metastasis of hepatocellular carcinoma by activating the EGF/EGFR-dependent epithelial-mesenchymal transition program. Cancer Lett. 2019, 454, 78–89. [Google Scholar] [CrossRef]
- Guardiola, S.; Varese, M.; Sánchez-Navarro, M.; Giralt, E. A Third Shot at EGFR: New Opportunities in Cancer Therapy. Trends Pharmacol. Sci. 2019, 40, 941–955. [Google Scholar] [CrossRef]
- Huether, A.; Höfner, M.; Sutter, A.P.; Baradari, V.; Schuppan, D.; Scherübl, H. Signaling pathway involved in the inhibition of epidermal growth factor receptor by erlotinib in hepatocellular cancer. World J. Gastroenterol. 2006, 12, 5160–5167. [Google Scholar] [PubMed]
- Huether, A.; Höpfner, M.; Sutter, A.P.; Schuppan, D.; Scherübl, H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J. Hepatol. 2005, 43, 661–669. [Google Scholar] [CrossRef]
- Zhang, J.; Zong, Y.; Xu, G.Z.; Xing, K. Erlotinib for advanced hepatocellular carcinoma a systematic review of phase II/III clinical trials. Saudi Med. J. 2016, 37, 1184–1190. [Google Scholar] [CrossRef]
- Montemurro, F.; Valabrega, G.; Aglietta, M. Lapatinib: A dual inhibitor of EGFR and HER2 tyrosine kinase activity. Expert Opin. Biol. Ther. 2007, 7, 257–268. [Google Scholar] [CrossRef]
- S Dhillon, A.W.J. Lapatinib. Drugs 2007, 67, 2101–2108. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chi, C.W.; Su, W.C.; Huang, H.L. Lapatinib induces autophagic cell death and inhibits growth of human hepatocellular carcinoma. Oncotarget 2014, 5, 4845–4854. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Markowitz, J.; Prescott, N.; Sadee, W.; Heerema, N.; Wei, L.; Dai, Z.; Papp, A.; Campbell, A.; Culler, K.; et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin. Cancer Res. 2009, 15, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Belani, C.P.; Singh, D.A.; Tanaka, M.; Lenz, H.J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; MacK, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Ding, X.; Wang, Y. Afatinib, an EGFR inhibitor, decreases EMT and tumorigenesis of Huh-7 cells by regulating the ERK-VEGF/MMP9 signaling pathway. Mol. Med. Rep. 2019, 20, 3317–3325. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.H.; Bagalagel, A.; Noor, A.O.; Almasri, D.M.; Diri, R. The therapeutic effects of nicotinamide in hepatocellular carcinoma through blocking IGF-1 and effecting the balance between Nrf2 and PKB. Biomed. Pharmacother. 2019, 112, 108653. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015, 16, 426–435. [Google Scholar] [CrossRef]
- Kruger, D.T.; Alexi, X.; Opdam, M.; Schuurman, K.; Voorwerk, L.; Sanders, J.; van der Noort, V.; Boven, E.; Zwart, W.; Linn, S.C. IGF-1R pathway activation as putative biomarker for linsitinib therapy to revert tamoxifen resistance in ER-positive breast cancer. Int. J. Cancer 2020, 146, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Bedi, S.; Khan, S.A.; AbuKhader, M.M.; Alam, P.; Siddiqui, N.A.; Husain, A. A comprehensive review on Brigatinib—A wonder drug for targeted cancer therapy in non-small cell lung cancer. Saudi Pharm. J. 2018, 26, 755–763. [Google Scholar] [CrossRef]
- Spencer, S.A.; Riley, A.C.; Matthew, A.; Di Pasqua, A.J. Brigatinib: Novel ALK Inhibitor for Non–Small-Cell Lung Cancer. Ann. Pharmacother. 2019, 53, 621–626. [Google Scholar] [CrossRef]
- Borowiak, M.; Garratt, A.N.; Wüstefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, P.; Lopez-Talavera, J.C.; Petersen, B.; Monga, S.P.S.; Michalopoulos, G.K. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology 2001, 34, 688–693. [Google Scholar] [CrossRef]
- Bilezikçi, B.; Demirhan, B.; Emiroǧlu, R.; Haberal, M. Hepatocyte growth factor in hepatic allograft biopsies: An immunohistochemical study. Transplant. Proc. 2003, 35, 3022–3023. [Google Scholar] [CrossRef]
- Efimova, E.A.; Glanemann, M.; Nussler, A.K.; Schumacher, G.; Settmacher, U.; Jonas, S.; Nussler, N.; Neuhaus, P. Changes in serum levels of growth factors in healthy individuals after living related liver donation. Transplant. Proc. 2005, 37, 1074–1075. [Google Scholar] [CrossRef]
- Zhuang, L.; Wen, T.; Xu, M.; Yang, J.; Wang, W.; Wu, H.; Zeng, Y.; Yan, L.; Wei, Y.; Li, B. Sorafenib combined with hepatectomy in patients with intermediate-stage and advanced hepatocellular carcinoma. Clin. Res. 2017, 13, 1383–1393. [Google Scholar] [CrossRef]
- Xia, F.; Wu, L.; Lau, W.; Huan, H.; Wen, X.; Ma, K.; Li, X.; Bie, P. Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J. Gastroenterol. 2016, 22, 5384–5392. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zheng, Y.U.N.; He, W.E.I.; Li, Q.; Shen, J. Sorafenib therapy following resection prolongs disease-free survival in patients with advanced hepatocellular carcinoma at a high risk of recurrence. Oncol. Lett. 2017, 13, 984–992. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Zhang, Z.; Zhou, Y.; Yang, J.; Hu, K.; Wang, Z. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? OncoTarget Ther. 2019, 12, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Xu, S.; Zhang, J.; Ran, X.; Bai, L.; Tang, H. Efficacy of sorafenib in patients with hepatocellular carcinoma after resection: A meta-analysis. Oncotarget 2017, 8, 109723–109731. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Zheng, S.; Wang, Y.; Xiang, X.; Cheng, Q.; Zhu, J. The efficacy of sorafenib in preventing hepatocellular carcinoma recurrence after resection: A systematic review and meta-analysis. Span. J. Gastroenterol. 2020, 112, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.J.I.N.; Lee, J.; Sohn, I.; Mao, M.A.O.; Kai, W.; Park, C.; Lim, H.O.Y. A Survey of c-MET Expression and Amplification in 287 Patients with Hepatocellular Carcinoma. Anticancer Res. 2013, 33, 5179–5186. [Google Scholar]
- Granito, A.; Guidetti, E.; Gramantieri, L. c-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 29–38. [Google Scholar]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Muscari, F.; Guellec, S.L.; Pariente, A.; Otal, P.; Suc, B.; Reports, C. Case Report Liver Resection after Downstaging Hepatocellular Carcinoma with Sorafenib. Int. J. Hepatol. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bertacco, A.; Vitale, A.; Mescoli, C.; Cillo, U. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Future Med. 2020, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
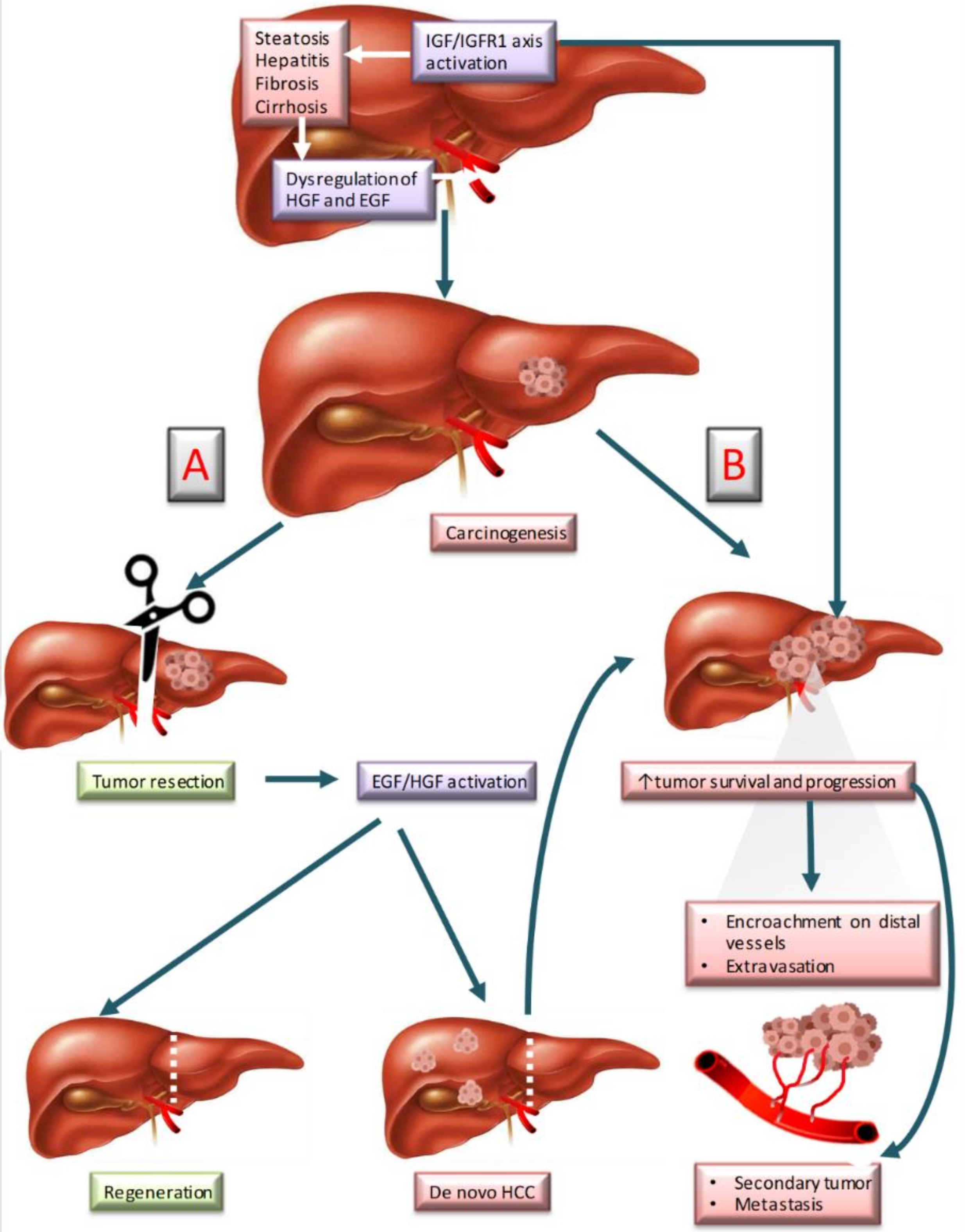

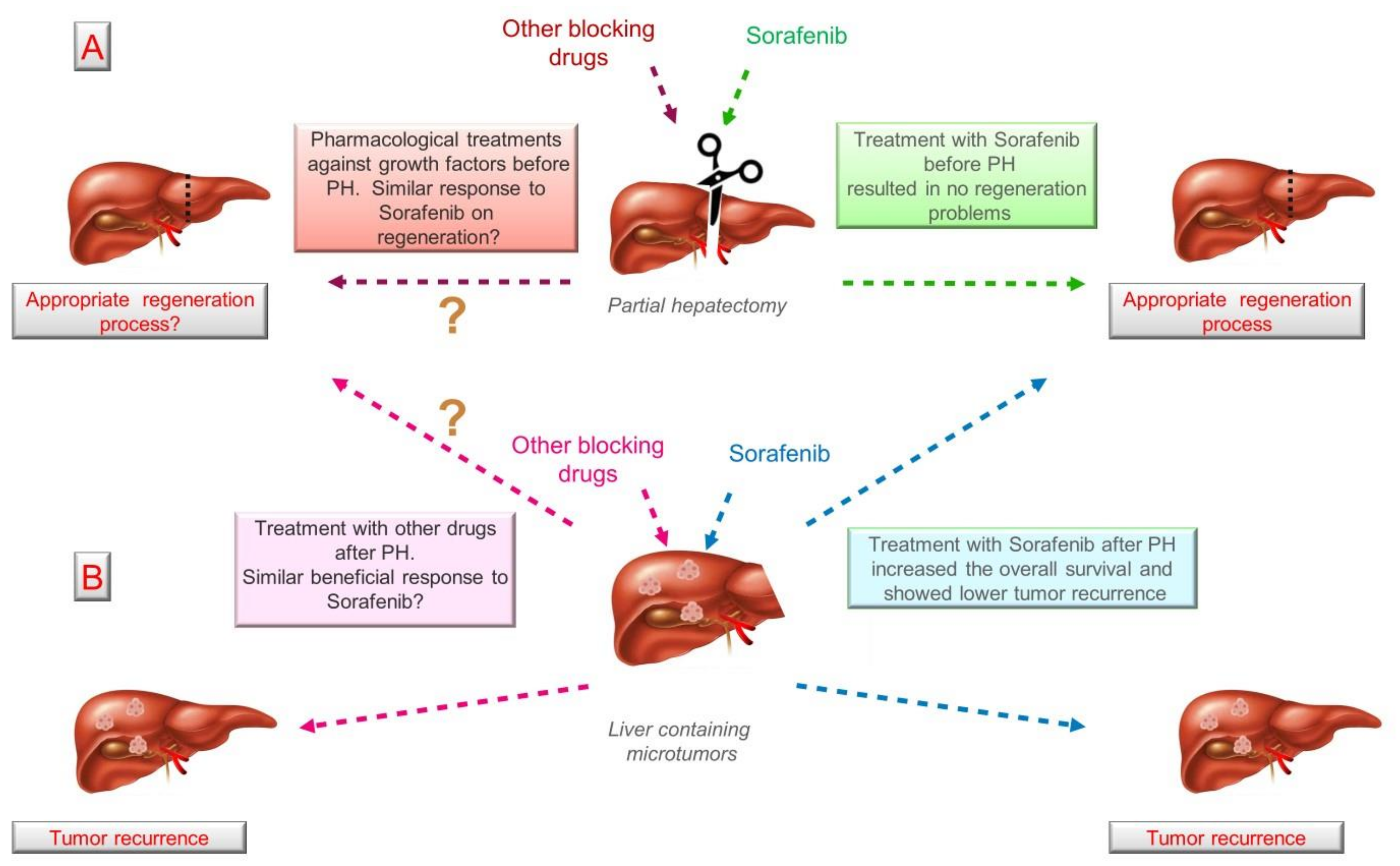
| Growth Factor | Essential to Liver Regeneration | Inductor of Tumorigenesis | Mechanism (s) Reported Inducing Tumorigenesis | Drug(s) Used to Block the Receptor (Approved or Under Testing in Clinical Trials) | Mechanism of Action |
|---|---|---|---|---|---|
| HGF | Yes [30,31,32] | Yes, overexpression of c-MET [35] | ROS, migration and invasion of tumor [36] | Sorafenib Cabozantinib Tivantinib Tepotinib | Multikinase inhibitor [96] Inhibitor of multiple receptor tyrosine kinases [103] A selective blocker of c-Met? [108] High selective MET inhibitor [110] |
| EGF | Yes [65,66] | Yes, overexpression of EGFR [49] | Activation of EGF-EGFR signaling, overexpressionn of DKK1, HMGA2 [53]; PD-L1 [54]; Phosphorylation of p65 [55] | Gefitinib Erlotinib Lapatinib Afatinib | Reversible inhibitor of EGFR-TK [115] EGRF inhibitor [115] Reversible inhibitor of EGFR [119] Irreversible EGFR inhibitor [124] |
| IGF-1 | Yes [79,80] | Yes, up-regulation of IGF-1 gene [75] | Activation of EMT pathways [74,75,76] | Nicotinamide Linsitinib and Brigatinib | Inhibitor of IGF-1 [125] Inhibitors of IGF-1 [126,127,128,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Mercado, A.I.; Caballeria-Casals, A.; Rojano-Alfonso, C.; Chávez-Reyes, J.; Micó-Carnero, M.; Sanchez-Gonzalez, A.; Casillas-Ramírez, A.; Gracia-Sancho, J.; Peralta, C. Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection. Biomedicines 2021, 9, 1158. https://doi.org/10.3390/biomedicines9091158
Álvarez-Mercado AI, Caballeria-Casals A, Rojano-Alfonso C, Chávez-Reyes J, Micó-Carnero M, Sanchez-Gonzalez A, Casillas-Ramírez A, Gracia-Sancho J, Peralta C. Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection. Biomedicines. 2021; 9(9):1158. https://doi.org/10.3390/biomedicines9091158
Chicago/Turabian StyleÁlvarez-Mercado, Ana I., Albert Caballeria-Casals, Carlos Rojano-Alfonso, Jesús Chávez-Reyes, Marc Micó-Carnero, Alfredo Sanchez-Gonzalez, Araní Casillas-Ramírez, Jordi Gracia-Sancho, and Carmen Peralta. 2021. "Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection" Biomedicines 9, no. 9: 1158. https://doi.org/10.3390/biomedicines9091158
APA StyleÁlvarez-Mercado, A. I., Caballeria-Casals, A., Rojano-Alfonso, C., Chávez-Reyes, J., Micó-Carnero, M., Sanchez-Gonzalez, A., Casillas-Ramírez, A., Gracia-Sancho, J., & Peralta, C. (2021). Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection. Biomedicines, 9(9), 1158. https://doi.org/10.3390/biomedicines9091158









